Longan Polysaccharide as Adjuvant for Cyclophosphamide-Induced Side Effects in Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Animals and Treatments
2.3. Intestinal Immunomodulatory Effect In Vitro
2.4. Statistical Analysis
3. Results
3.1. LP Enhances Systemic Immunity of CTX-Treated Mice
3.1.1. LP Mitigates Weight Loss in CTX-Treated Mice
3.1.2. LP Promotes Serum Immunoglobulin Levels in CTX-Treated Mice
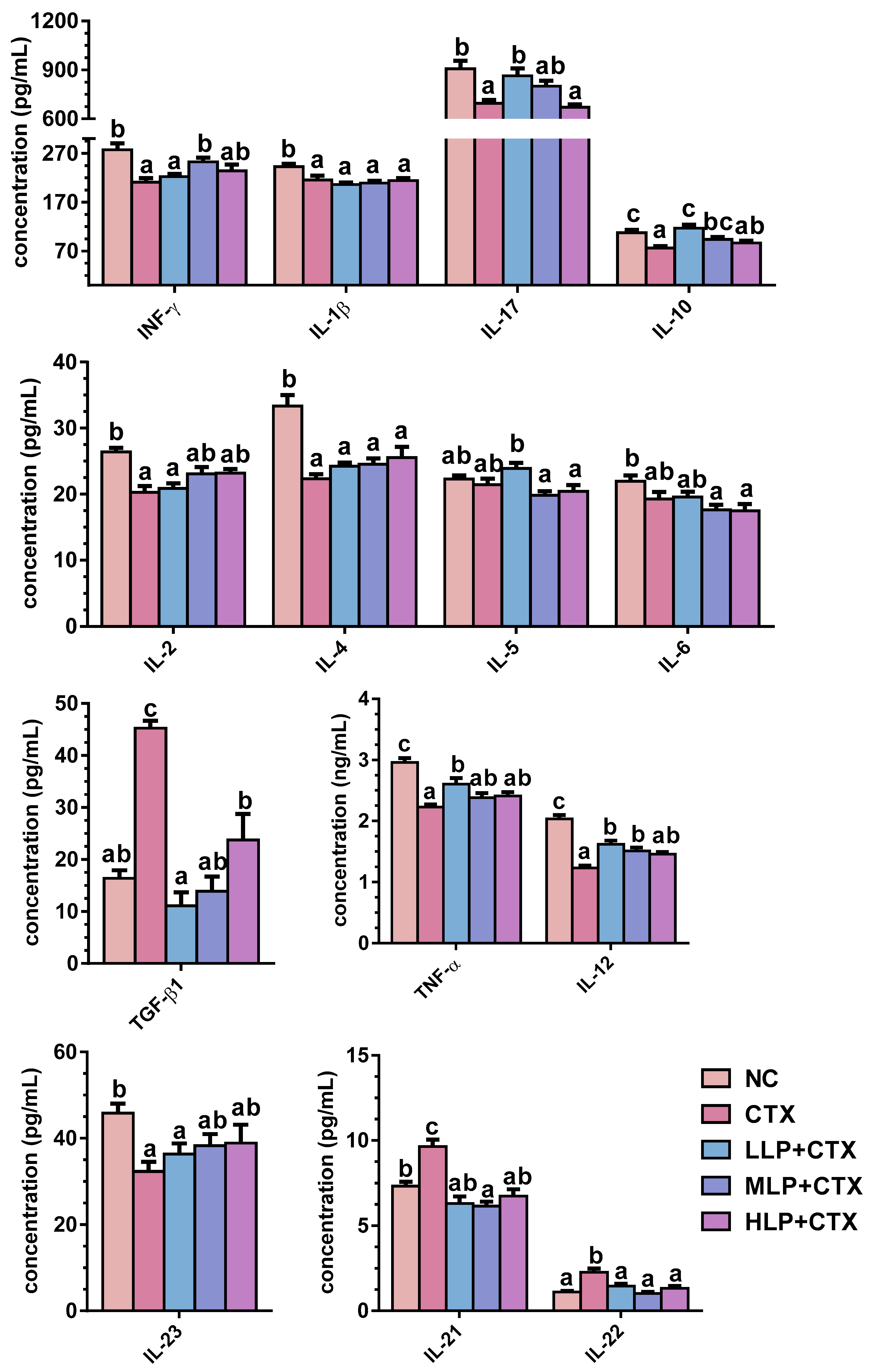
3.1.3. LP Regulates Serum Cytokine Profiles in CTX-Treated Mice
3.2. Antioxidant Effect of LP in CTX-Treated Mice
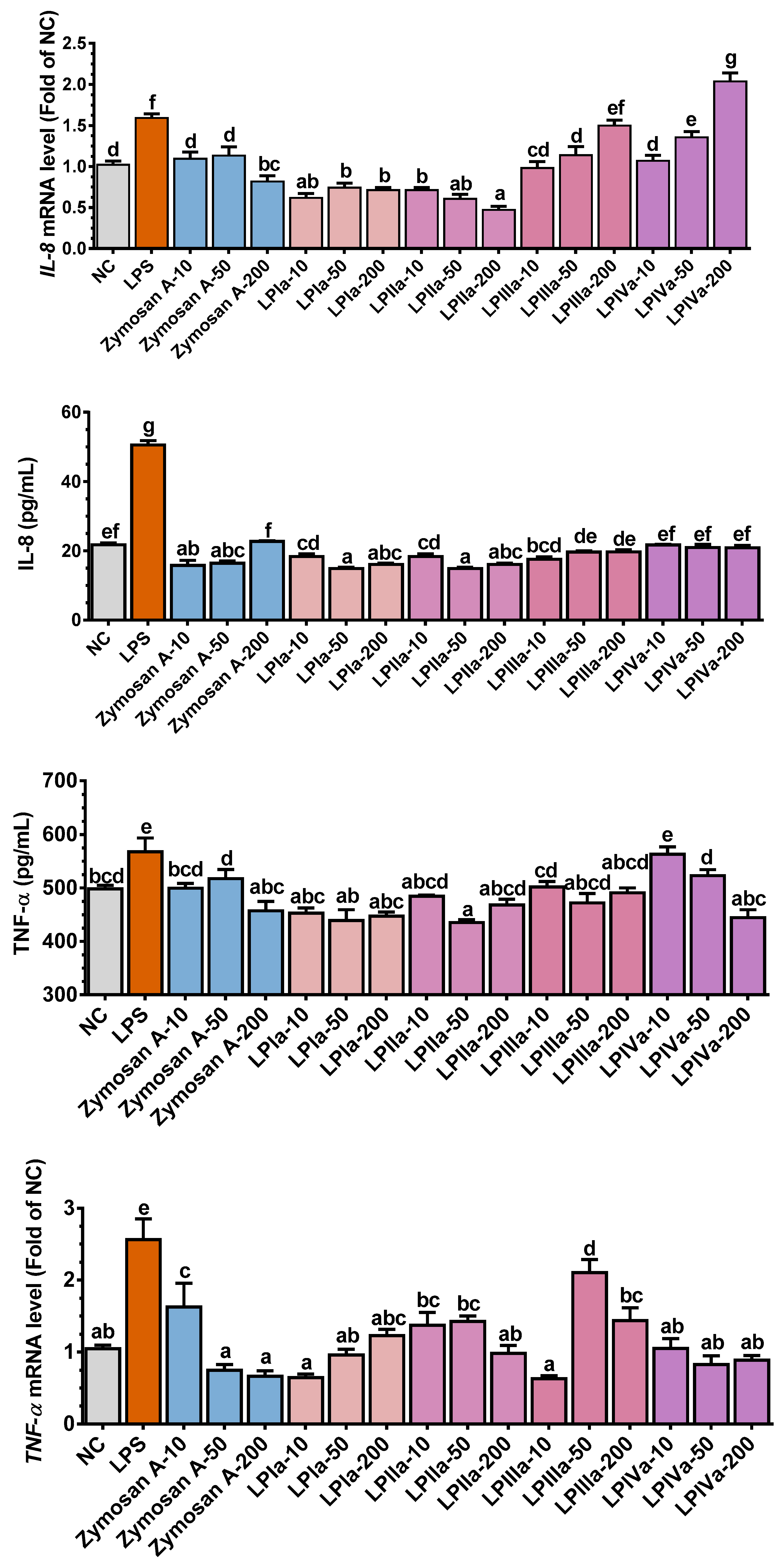
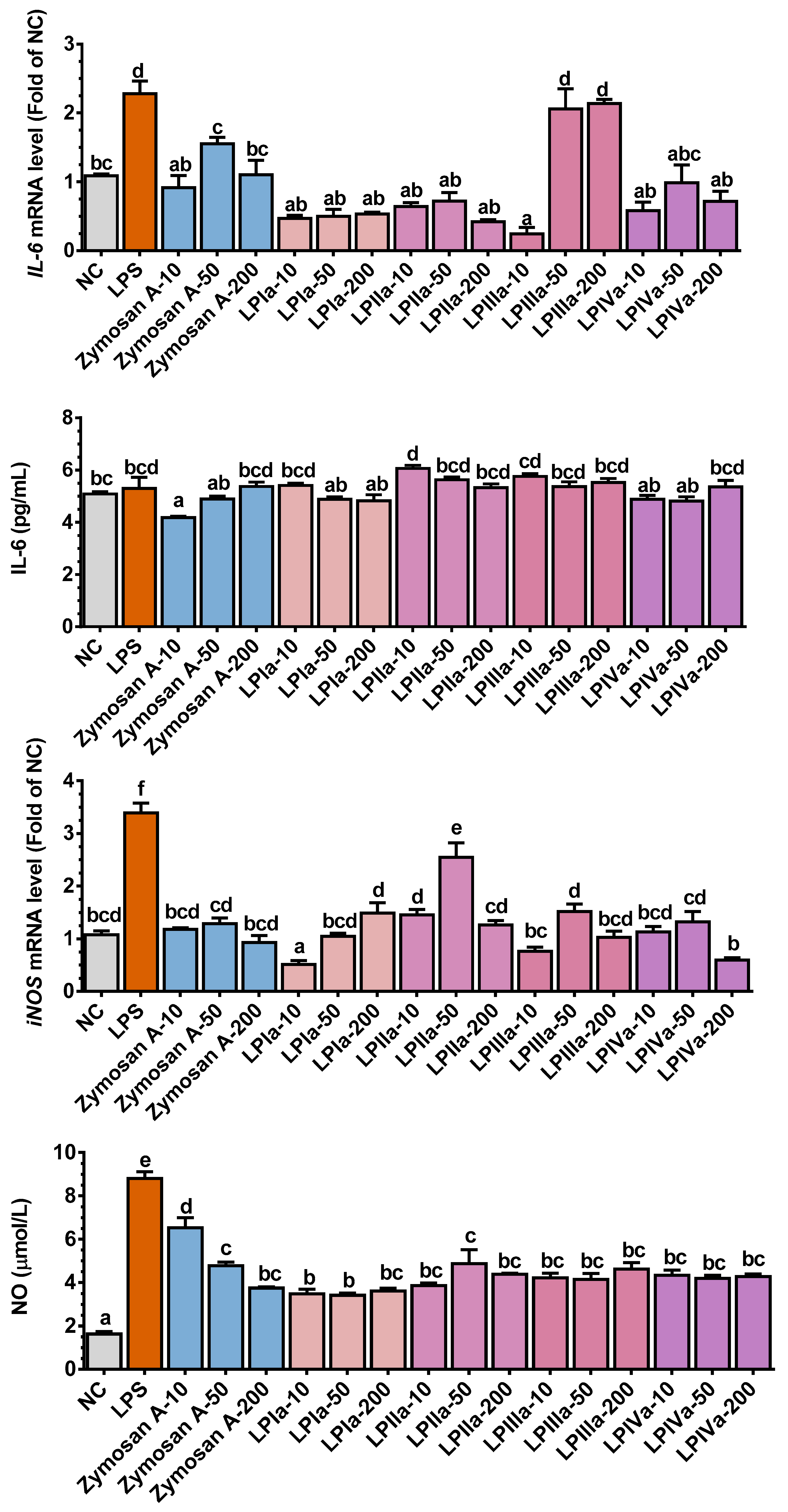
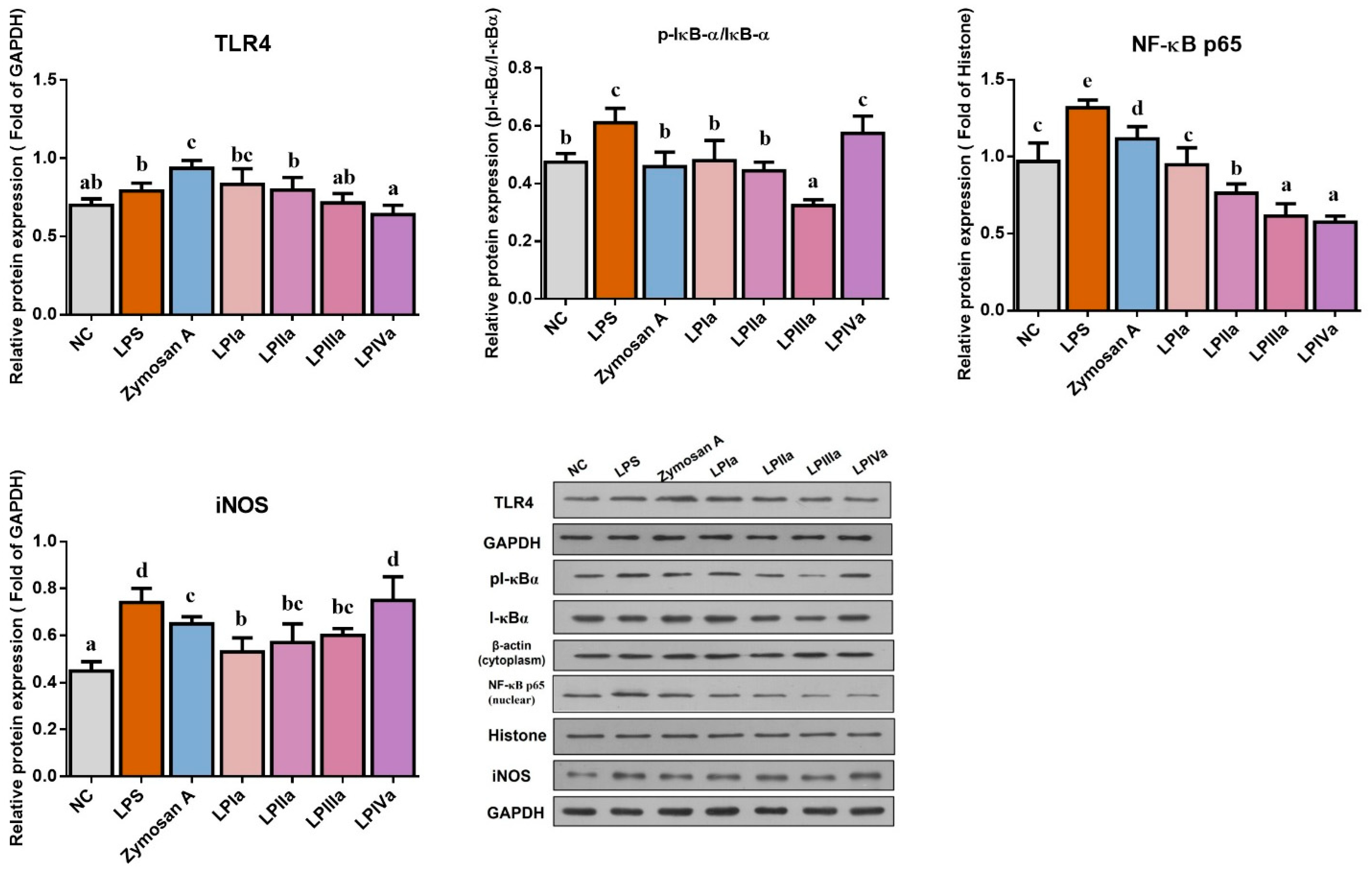
3.3. Anti-Inflammatory Effect of Four LP Fractions in Caco-2/RAW264.7 Coculture Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korkmaz, A.; Topal, T.; Oter, S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol. Toxicol. 2007, 23, 303–312. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Merwid-Lad, A.; Ksiadzyna, D.; Halon, A.; Chlebda-Sieragowska, E.; Trocha, M.; Szandruk, M.; Sozanski, T.; Magdalan, J.; Kopacz, M.; Kuzniar, A.; et al. Impact of morin-5′-sulfonic acid sodium salt on cyclophosphamide-induced gastrointestinal toxicity in rats. Pharmacol. Rep. PR 2015, 67, 1259–1263. [Google Scholar] [CrossRef]
- Arijs, I.; Hertogh, G.D.; Machiels, K.; Steen, K.V.; Lemaire, K.; Schraenen, A.; Lommel, L.V.; Quintens, R.; Assche, G.V.; Vermeire, S. Mucosal Gene Expression of Cell Adhesion Molecules, Chemokines, and Chemokine Receptors in Patients with Inflammatory Bowel Disease Before and After Infliximab Treatment. Am. J. Gastroenterol. 2011, 106, 748–761. [Google Scholar] [CrossRef]
- Zheng, Y.; Zong, Z.M.; Chen, S.L.; Chen, A.H.; Wei, X.Y. Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2017, 95, 1216–1222. [Google Scholar] [CrossRef]
- Zhu, G.; Jiang, Y.; Yao, Y.; Wu, N.; Luo, J.; Hu, M.; Tu, Y.; Xu, M. Ovotransferrin ameliorates the dysbiosis of immunomodulatory function and intestinal microbiota induced by cyclophosphamide. Food Funct. 2019, 10, 1109–1122. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.-P.; Wang, J.-Q.; Liu, X.-Z.; Yin, P.-F.; Huang, D.-F.; Li, W.-J.; Gong, D.-M.; Xie, M.-Y. Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 2014, 64, 395–401. [Google Scholar] [CrossRef]
- Zuo, T.; Zhao, R.; Lu, S.; Zhang, N.; Zhang, Q.; Xue, C. Novel dietary polysaccharide SIP promotes intestinal secretory immunoglobulin A secretion in mice under chemotherapy. J. Funct. Foods 2017, 37, 379–389. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2018, 9, 937–950. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, P.; Chen, C.; Huang, C.; Lin, S.; Zheng, B.; Zhang, Y. Structural properties and prebiotic activities of fractionated lotus seed resistant starches. Food Chem. 2018, 251, 33–40. [Google Scholar] [CrossRef]
- Xiong, F.; Li, H.Y.; Yao, H.L.; Ou, Y.H.; Chan, A.S.C.; Wang, S.P.; Li, H.J.; Lan, W.J. A galacturonic acid-rich polysaccharide from Citrus medica ‘fingered’ alleviated the dextran sulfate sodium-induced ulcerative colitis. Int. J. Biol. Macromol. 2025, 294, 139506. [Google Scholar] [CrossRef]
- Wang, H.; Bi, H.; Gao, T.; Zhao, B.; Ni, W.; Liu, J. A homogalacturonan from Hippophae rhamnoides L. Berries enhance immunomodulatory activity through TLR4/MyD88 pathway mediated activation of macrophages. Int. J. Biol. Macromol. 2018, 107, 1039–1045. [Google Scholar] [CrossRef]
- Zeng, F.; Li, Y.; Zhang, X.; Shen, L.; Zhao, X.; Beta, T.; Li, B.; Chen, R.; Huang, W. Immune regulation and inflammation inhibition of Arctium lappa L. polysaccharides by TLR4/NF-κB signaling pathway in cells. Int. J. Biol. Macromol. 2024, 254, 127700. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jia, X.; Huang, F.; Zhang, R.; Dong, L.; Liu, L.; Zhang, M. Structural elucidation, anti-inflammatory activity and intestinal barrier protection of longan pulp polysaccharide LPIIa. Carbohydr. Polym. 2020, 246, 116532. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.-W.; Liao, S.-T.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; Tang, X.-J.; Zhang, Y. Structural features and immunomodulatory activities of polysaccharides of longan pulp. Carbohydr. Polym. 2012, 87, 636–643. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.; Yang, Y.; Wen, Y.; Liu, S.; Li, C.; Hu, Z.; Cheng, X.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, F.; Zhang, R.; Ma, Q.; Dong, L.; Su, D.; Chi, J.; Zhang, M. Longan pulp polysaccharide protects against cyclophosphamide-induced immunosuppression in mice by promoting intestinal secretory IgA synthesis. Food Funct. 2020, 11, 2738–2748. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Wen, Y.; Liu, S.; Li, C.; Yang, R.; Li, W. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol. Nutr. Food Res. 2017, 61, 1700466. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Li, X.; Zhang, R.; Huang, F.; Fan, B.; Tong, L.; Wang, F.; Zhang, M. Longan pulp polysaccharides regulate gut microbiota and metabolites to protect intestinal epithelial barrier. Food Chem. 2023, 422, 136225. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Juzwa, W.; Dembczynski, R.; Schmidt, M. A Gastrointestinally Digested Ribes nigrum L. Fruit Extract Inhibits Inflammatory Response in a Co-culture Model of Intestinal Caco-2 Cells and RAW264.7 Macrophages. J. Agric. Food Chem. 2016, 64, 7710–7721. [Google Scholar] [CrossRef]
- Sahan-Firat, S.; Temiz-Resitoglu, M.; Guden, D.S.; Senol, S.P.; Sari, A.N.; Cil, M.; Unsal, D.; Korkmaz, B.; Tunctan, B.; Malik, K.U.; et al. NF-kappaB activation mediates LPS-or zymosan-induced hypotension and inflammation reversed by BAY61-3606, a selective Syk inhibitor, in rat models of septic and non-septic shock. Clin. Exp. Pharmacol. Physiol. 2019, 46, 173–182. [Google Scholar] [CrossRef]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-kappaB and AP-1 activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef]
- Qiao, D.; He, X.; Wei, C.; Xia, L.; Bao, L. Effects of Hyriopsis cumingii Polysaccharides on Mice Immunologic Receptor, Transcription Factor, and Cytokine. J. Food Sci. 2016, 81, H1288–H1294. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Kim, K.H.; Kim, H.S.; Lee, K.H.; Shin, E.; Do, S.G.; Jo, T.H.; Park, Y.I.; Lee, C.K. Processed Aloe vera gel ameliorates cyclophosphamide-induced immunotoxicity. Int. J. Mol. Sci. 2014, 15, 19342–19354. [Google Scholar] [CrossRef]
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat. Rev. 2016, 42, 3–9. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H.; Li, H.; Lai, F.; Li, X.; Tang, Y.; Min, T.; Wu, H. Antioxidant Mechanism of Betaine without Free Radical Scavenging Ability. J. Agric. Food Chem. 2016, 64, 7921–7930. [Google Scholar] [CrossRef]
- Bennett, S.J.; Griffiths, H.R. Regulation of T-Cell Functions by Oxidative Stress. In Studies on Arthritis and Joint Disorders; Humana Press: New York, NY, USA, 2013; pp. 33–48. [Google Scholar] [CrossRef]
- Fujio, K.; Komai, T.; Inoue, M.; Morita, K.; Okamura, T.; Yamamoto, K. Revisiting the regulatory roles of the TGF-beta family of cytokines. Autoimmun. Rev. 2016, 15, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Russler-Germain, E.V.; Rengarajan, S.; Hsieh, C.S. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017, 10, 1375–1386. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.-X.; Chen, S.-R.; Deng, Y.; Zhao, J.; Wang, Y.; Li, S.-P. Non-starch polysaccharide from Chinese yam activated RAW 264.7 macrophages through the Toll-like receptor 4 (TLR4)-NF-κB signaling pathway. J. Funct. Foods 2017, 37, 491–500. [Google Scholar] [CrossRef]
- Beguin, P.; Errachid, A.; Larondelle, Y.; Schneider, Y.J. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct. 2013, 4, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Yang, X.; Yao, J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, S.; Huang, R.; Liu, X.; Shen, M.; Xie, J. Immunomodulatory activity and mechanism of Chinese yam polysaccharide after sulfated modification. Ind. Crops Prod. 2023, 197, 116549. [Google Scholar] [CrossRef]
- Rosch, C.; Taverne, N.; Venema, K.; Gruppen, H.; Wells, J.M.; Schols, H.A. Effects of in vitro fermentation of barley beta-glucan and sugar beet pectin using human fecal inocula on cytokine expression by dendritic cells. Mol. Nutr. Food Res. 2017, 61, 1600243. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yue, H.; Fei, W.; Hao, Z.; Vos, P.D.; Jia, S. Low-methoxyl lemon pectin attenuates inflammatory responses and improves intestinal barrier integrity in caerulein-induced experimental acute pancreatitis. Mol. Nutr. Food Res. 2017, 61, 1600885. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Feng, X.; Zhang, X.; Zhang, Y.; Gao, C.; Yin, C.; Lu, X.; Zhao, J.; Du, W.; et al. Structural characterization and immune-modulating properties of a novel β-galactomannan extracted from black soybean hulls. Carbohydr. Polym. 2025, 358, 123568. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
| Group | IgA (μg/mL) | IgG1 (μg/mL) | IgG2a (μg/mL) | IgG2b (μg/mL) | IgM (mg/mL) |
|---|---|---|---|---|---|
| NC | 70.52 ± 0.87d | 537.21 ± 33.93b | 48.19 ± 3.00c | 64.48 ± 3.34b | 1.55 ± 0.02c |
| CTX | 39.81 ± 1.21a | 437.52 ± 14.50a | 28.33 ± 1.41a | 45.24 ± 3.38a | 1.24 ± 0.05a |
| LLP + CTX | 50.30 ± 1.19b | 431.63 ± 27.66a | 51.72 ± 1.70c | 46.77 ± 2.21a | 1.39 ± 0.05b |
| MLP + CTX | 51.84 ± 1.99b | 534.59 ± 9.83b | 47.90 ± 2.41c | 66.47 ± 3.99b | 1.56 ± 0.05bc |
| HLP + CTX | 57.92 ± 2.18c | 462.76 ± 33.93ab | 35.16 ± 1.09b | 62.19 ± 2.67b | 1.43 ± 0.02bc |
| Group | SOD (U/mgprot) | MDA (nmol/mgprot) | GSH (μmol/gprot) | NO (μmol/gprot) |
|---|---|---|---|---|
| Liver | ||||
| NC | 21.46 ± 0.30d | 3.8 ± 0.13a | 2.95 ± 0.17b | 0.13 ± 0.01b |
| CTX | 13.00 ± 1.01a | 6.97 ± 0.52c | 2.02 ± 0.13a | 0.05 ± 0.01a |
| LLP + CTX | 14.54 ± 0.43ab | 5.17 ± 0.24b | 2.68 ± 0.08b | 0.06 ± 0.01a |
| MLP + CTX | 16.02 ± 0.38bc | 4.96 ± 0.17b | 2.69 ± 0.13b | 0.10 ± 0.01b |
| HLP + CTX | 17.33 ± 0.22c | 4.16 ± 0.19ab | 2.94 ± 0.10b | 0.13 ± 0.01b |
| Kidney | ||||
| NC | 27.69 ± 0.17c | 6.49 ± 0.07a | 8.82 ± 0.27b | 0.79 ± 0.05d |
| CTX | 21.20 ± 0.93a | 8.74 ± 0.17b | 7.42 ± 0.27a | 0.27 ± 0.02a |
| LLP + CTX | 23.59 ± 0.68b | 6.22 ± 0.28a | 8.21 ± 0.22ab | 0.42 ± 0.05b |
| MLP + CTX | 26.50 ± 0.51c | 6.33 ± 0.18a | 8.57 ± 0.14b | 0.56 ± 0.02c |
| HLP + CTX | 29.07 ± 0.94c | 6.73 ± 0.22a | 8.10 ± 0.15ab | 0.69 ± 0.03d |
| Intestine | ||||
| NC | 36.11 ± 0.35c | 0.71 ± 0.04a | 119.30 ± 4.91c | 0.82 ± 0.04d |
| CTX | 25.84 ± 1.39a | 0.96 ± 0.01c | 77.35 ± 1.92a | 0.24 ± 0.03a |
| LLP + CTX | 27.73 ± 0.45ab | 0.86 ± 0.02b | 83.13 ± 2.89a | 0.42 ± 0.04b |
| MLP + CTX | 33.65 ± 1.20c | 0.80 ± 0.02b | 94.22 ± 3.00b | 0.63 ± 0.02c |
| HLP + CTX | 30.06 ± 0.65b | 0.66 ± 0.01a | 110.81 ± 3.69c | 0.77 ± 0.02d |
| Serum | (U/mL) | (nmol/mL) | (μmol/mL) | (μmol/mL) |
| NC | 93.63 ± 1.20c | 6.86 ± 0.14a | 32.97 ± 1.28c | 6.16 ± 0.12d |
| CTX | 80.96 ± 1.76a | 10.28 ± 0.55b | 20.86 ± 1.35a | 2.08 ± 0.15a |
| LLP + CTX | 87.62 ± 0.79b | 8.23 ± 0.27a | 22.71 ± 1.25a | 2.91 ± 0.22b |
| MLP + CTX | 97.16 ± 1.06cd | 7.76 ± 0.16a | 25.81 ± 0.85ab | 5.42 ± 0.20c |
| HLP + CTX | 100.01 ± 1.48d | 8.08 ± 0.37a | 30.30 ± 2.17bc | 6.28 ± 0.09d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Fan, B.; Wang, F.; Zhang, M. Longan Polysaccharide as Adjuvant for Cyclophosphamide-Induced Side Effects in Murine Model. Foods 2025, 14, 2901. https://doi.org/10.3390/foods14162901
Bai Y, Fan B, Wang F, Zhang M. Longan Polysaccharide as Adjuvant for Cyclophosphamide-Induced Side Effects in Murine Model. Foods. 2025; 14(16):2901. https://doi.org/10.3390/foods14162901
Chicago/Turabian StyleBai, Yajuan, Bei Fan, Fengzhong Wang, and Mingwei Zhang. 2025. "Longan Polysaccharide as Adjuvant for Cyclophosphamide-Induced Side Effects in Murine Model" Foods 14, no. 16: 2901. https://doi.org/10.3390/foods14162901
APA StyleBai, Y., Fan, B., Wang, F., & Zhang, M. (2025). Longan Polysaccharide as Adjuvant for Cyclophosphamide-Induced Side Effects in Murine Model. Foods, 14(16), 2901. https://doi.org/10.3390/foods14162901





