Nutritional Value of Brewer’s Spent Grain and Consumer Acceptance of Its Value-Added Food Products
Abstract
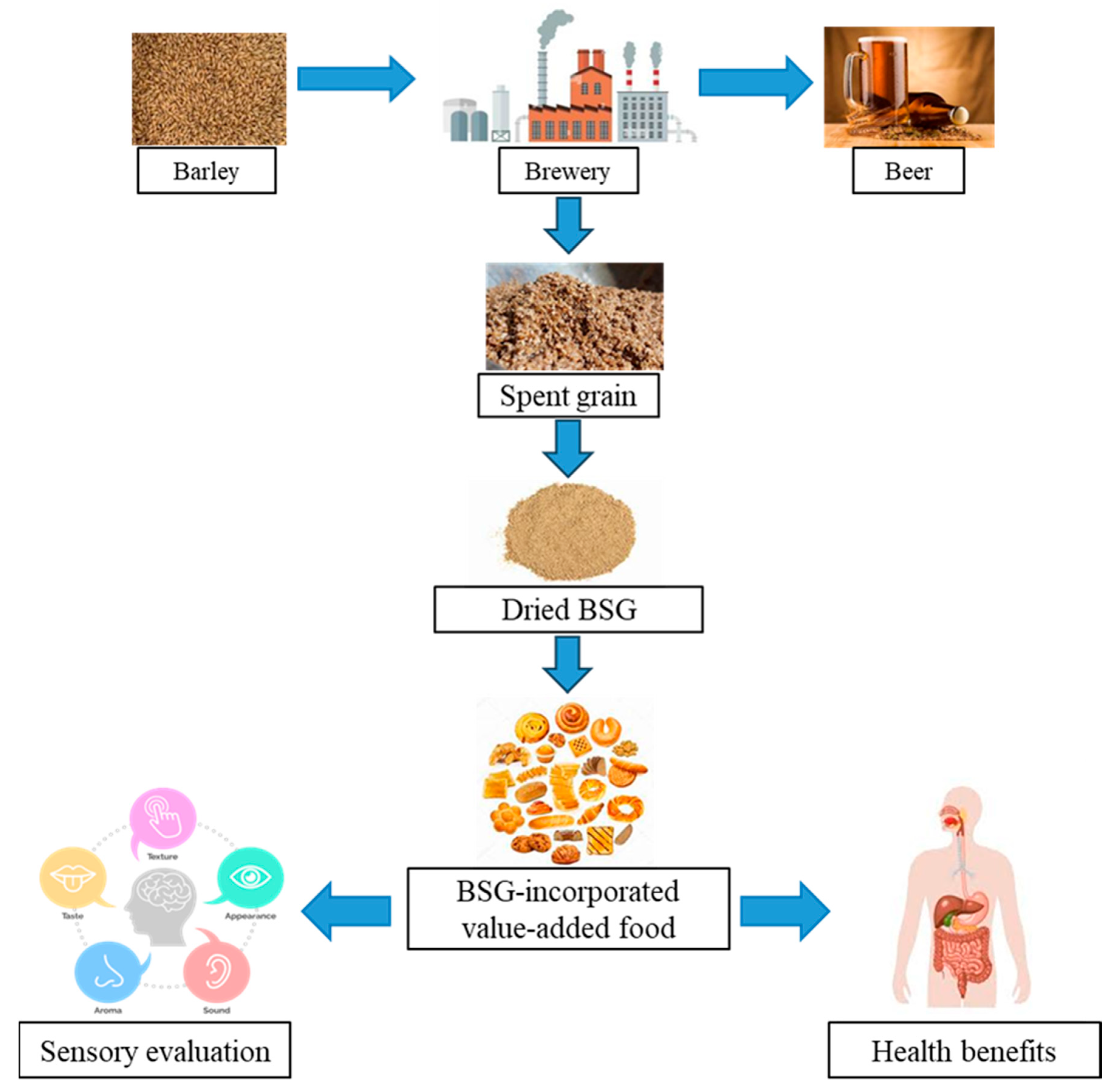
1. Introduction
Methodology
2. Nutritional Profile of BSG
Comparative Analysis with Other Grains
3. Functional and Bioactive Constituents of BSG
3.1. Dietary Fiber
3.2. Assessment of the Potential Health Benefits of BSG Using Human Clinical Trials
3.3. Contaminants of BSG, Potential Risks, Mitigation Strategies, and Regulatory Reference
4. Functional Integration of BSG in Food Products
4.1. BSG as a Functional Food Ingredient in Baked Food
4.1.1. Bread
4.1.2. Breadsticks
4.1.3. Cookies and Shortbread
4.1.4. Muffins
4.1.5. Wafers
4.1.6. Snacks
4.1.7. Pasta
4.1.8. Yoghurt
4.2. Use of BSG in Drinks and Beverages
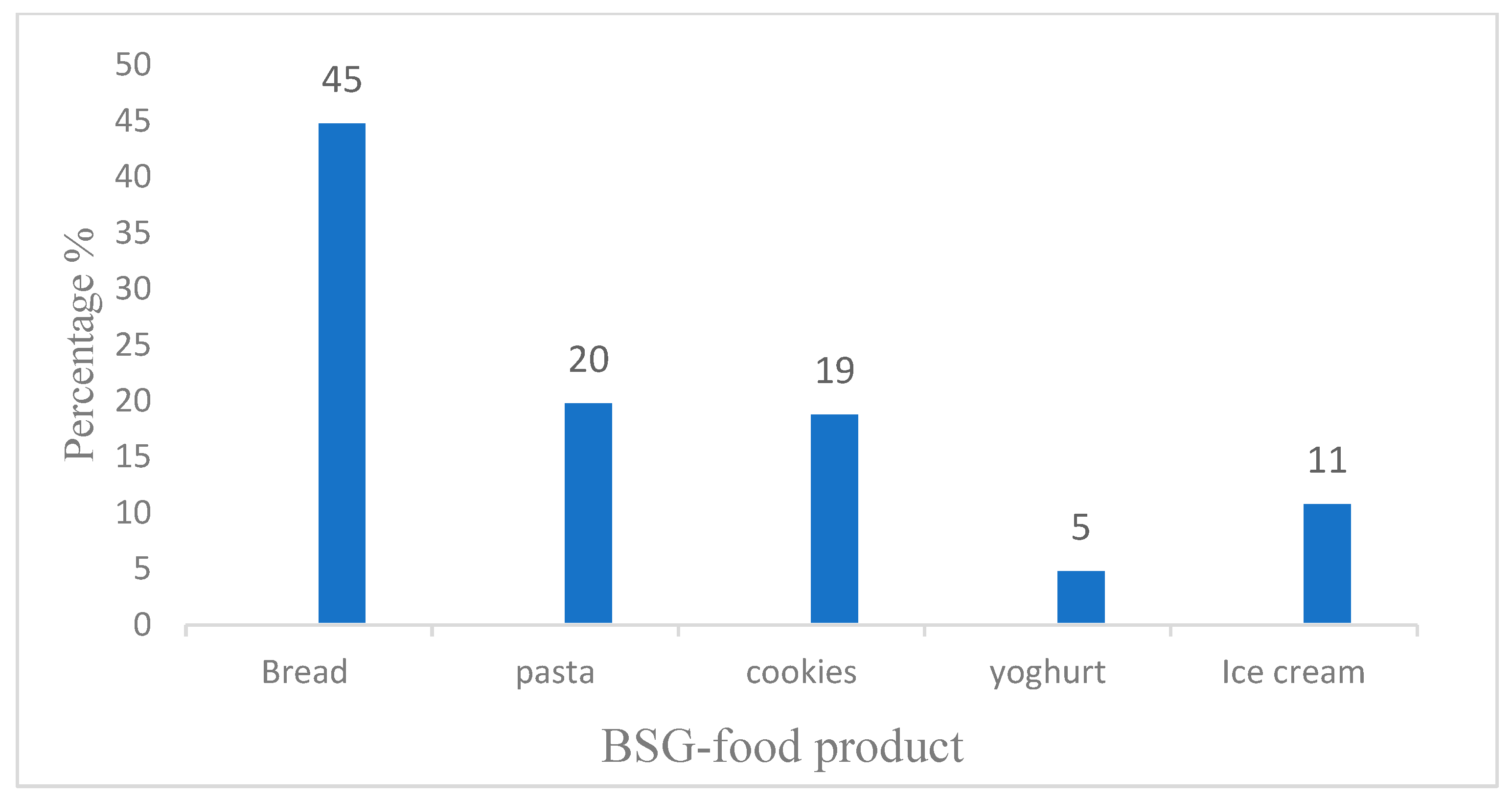
5. Consumer Acceptance of BSG-Incorporated Food Products
5.1. Factors Influencing Consumer Choice
5.1.1. Taste/Aroma
5.1.2. Color/Appearance
5.1.3. Texture
6. Recommendations and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chetrariu, A.; Dabija, A. Spent Grain: A Functional Ingredient for Food Applications. Foods 2023, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process Optimization for the Development of a Functional Beverage Based on Lactic Acid Fermentation of Oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- Statista Beer Production Worldwide in 2020, by Region (In Million Hectoliters). 2021. Available online: https://www.statista.com/statistics/270270/worldwide-beer-production-by-region/ (accessed on 3 July 2025).
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and Protein Recovery from Brewer’s Spent Grain Using Hydrothermal Pretreatment and Their Conversion to Edible Filamentous Fungi—A Brewery Biorefinery Concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential Applications of Brewery Spent Grain: Critical an Overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Mudura, E.; Dulf, F.V.; Vodnar, D.C.; Tofană, M.; Salanță, L.C. Exploitation of Brewing Industry Wastes to Produce Functional Ingredients. In Brewing Technology; InTech: London, UK, 2017. [Google Scholar]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Ajibola, O.; Wu, J. Food-Based Uses of Brewers Spent Grains: Current Applications and Future Possibilities. Food Biosci. 2023, 54, 102774. [Google Scholar] [CrossRef]
- Nyhan, L.; Sahin, A.W.; Schmitz, H.H.; Siegel, J.B.; Arendt, E.K. Brewers’ Spent Grain: An Unprecedented Opportunity to Develop Sustainable Plant-Based Nutrition Ingredients Addressing Global Malnutrition Challenges. J. Agric. Food Chem. 2023, 71, 10543–10564. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Du, L.; Olszewski, M.P.; Meza Zavala, M.F.; Alhnidi, M.J.; Kruse, A. Effect of Protein during Hydrothermal Carbonization of Brewer’s Spent Grain. Bioresour. Technol. 2019, 293, 122117. [Google Scholar] [CrossRef]
- Chin, Y.L.; Keppler, J.K.; Dinani, S.T.; Chen, W.N.; Boom, R. Brewers’ Spent Grain Proteins: The Extraction Method Determines the Functional Properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103666. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. Brewers’ Spent Grain in Food Systems: Processing and Final Products Quality as a Function of Fiber Modification Treatment. J. Food Sci. 2021, 86, 1532–1551. [Google Scholar] [CrossRef] [PubMed]
- Bazsefidpar, N.; Ghandehari Yazdi, A.P.; Karimi, A.; Yahyavi, M.; Amini, M.; Ahmadi Gavlighi, H.; Simal-Gandara, J. Brewers Spent Grain Protein Hydrolysate as a Functional Ingredient for Muffins: Antioxidant, Antidiabetic, and Sensory Evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Falck, P.; Sardari, R.R.R.; Mathew, S.; Grey, C.; Karlsson, E.N.; Adlercreutz, P. Valorization of Brewer’s Spent Grain to Prebiotic Oligosaccharide: Production, Xylanase Catalyzed Hydrolysis, in-Vitro Evaluation with Probiotic Strains and in a Batch Human Fecal Fermentation Model. J. Biotechnol. 2018, 268, 61–70. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.-B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical Composition, Fermentable Sugars, Free Amino Acids, Phenolics, and Minerals in Brewers’ Spent Grains Obtained from Craft Brewing Operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Gupta, S.; Jaiswal, A.K.; Abu-Ghannam, N. Optimization of Fermentation Conditions for the Utilization of Brewing Waste to Develop a Nutraceutical Rich Liquid Product. Ind. Crops Prod. 2013, 44, 272–282. [Google Scholar] [CrossRef]
- Balogun, A.O.; Sotoudehnia, F.; Mcdonald, A. Thermo-Kinetic, Spectroscopic Study of Brewer’s Spent Grains and Characterisation of Their Pyrolysis Products. J. Anal. Appl. Pyrolysis 2017, 127, 8–16. [Google Scholar] [CrossRef]
- Sibhatu, H.K.; Anuradha Jabasingh, S.; Yimam, A.; Ahmed, S. Ferulic Acid Production from Brewery Spent Grains, an Agro-Industrial Waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- Nigam, P.S. An Overview: Recycling of Solid Barley Waste Generated as a by-Product in Distillery and Brewery. Waste Manag. 2017, 62, 255–261. [Google Scholar] [CrossRef]
- Assefa Yohannes, J.A. Lactic Acid Production from Brewer’s Spent Grain by Lactobacillus Plantarum ATCC 8014. J. Sci. Ind. Res. 2020, 79, 610–613. [Google Scholar] [CrossRef]
- Nazzaro, J.; Martin, D.S.; Perez-Vendrell, A.M.; Padrell, L.; Iñarra, B.; Orive, M.; Estévez, A. Apparent Digestibility Coefficients of Brewer’s by-Products Used in Feeds for Rainbow Trout (Oncorhynchus mykiss) and Gilthead Seabream (Sparus aurata). Aquaculture 2021, 530, 735796. [Google Scholar] [CrossRef]
- Giacobbe, S.; Piscitelli, A.; Raganati, F.; Lettera, V.; Sannia, G.; Marzocchella, A.; Pezzella, C. Butanol Production from Laccase-Pretreated Brewer’s Spent Grain. Biotechnol. Biofuels 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, A.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Kawa-Rygielska, J. Baking Properties of Flour and Nutritional Value of Rye Bread with Brewer’s Spent Grain. LWT 2021, 150, 111955. [Google Scholar] [CrossRef]
- Bravi, E.; De Francesco, G.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing By-Product Upcycling Potential: Nutritionally Valuable Compounds and Antioxidant Activity Evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical Composition of Lipids in Brewer’s Spent Grain: A Promising Source of Valuable Phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus Subtilis WX-17. Fermentation 2019, 5, 52. [Google Scholar] [CrossRef]
- Almeida, A.; Geraldo, M.; Ribeiro, L.; Da Silva, M.; Maciel, M.V.O.B.; Haminiuk, C. Bioactive Compounds from Brewer’s Spent Grain: Phenolic Compounds, Fatty Acids and in Vitro Antioxidant Capacity. Acta Sci. Technol. 2017, 39, 269–277. [Google Scholar] [CrossRef]
- Chetrariu, A.; Ursachi, V.F.; Dabija, A. Evaluation of the Fatty Acids and Amino Acid Profiles in Spent Grain from Brewing and Malt Whisky. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2022, 23, 167–177. [Google Scholar]
- Jaeger, A.; Sahin, A.; Nyhan, L.; Zannini, E.; Arendt, E. Functional Properties of Brewer’s Spent Grain Protein Isolate: The Missing Piece in the Plant Protein Portfolio. Foods 2023, 12, 798. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of Extraction Solvents on the Recovery of Antioxidant Phenolic Compounds from Brewer’s Spent Grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Nagy, V.; Diósi, G. Using Brewer’s Spent Grain as a Byproduct of the Brewing Industry in the Bakery Industry. Élelmiszervizsgálati Közlemények 2021, 67, 3339–3350. [Google Scholar] [CrossRef]
- Teresa, B.-L.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the Bioactive Potential of Brewers Spent Grain Ohmic Extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Recovery of Polyphenols from Brewer’s Spent Grains. Antioxidants 2019, 8, 380. [Google Scholar] [CrossRef]
- Fu, Q.Y.; Yu, X.C.; Li, L.; Liu, G.Q.; Li, B. Antioxidant Activities of Soluble Dietary Fiber Extracted from Brewers’ Spent Grain. Adv. Mat. Res. 2011, 233–235, 2824–2827. [Google Scholar] [CrossRef]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of Brewers’ Spent Grain for the Production of Laccase and Polyphenols. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants 2019, 8, 584. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Brewer’s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Neugart, S.; Piggott, C.O.; Connolly, A.; Jansen, M.A.K.; Krumbein, A.; Schreiner, M.; FitzGerald, R.J.; O’Brien, N.M. The Hydroxycinnamic Acid Content of Barley and Brewers’ Spent Grain (BSG) and the Potential to Incorporate Phenolic Extracts of BSG as Antioxidants into Fruit Beverages. Food Chem. 2013, 141, 2567–2574. [Google Scholar] [CrossRef]
- Adamu, A.S.; Ajayi, M.G.; Oyetunde, J.G. Inorganic and Proximate Nutritional Composition of Common Beans in Nigeria. Eur. J. Pure Appl. Chem. 2016, 3, 25–28. [Google Scholar]
- Arendt, E.; Zannini, E. 7. Oats. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013; Volume 1, pp. e243–e283. ISBN 9780857094131. [Google Scholar]
- Thai, S.; Avena-Bustillos, R.J.; Alves, P.; Pan, J.; Osorio-Ruiz, A.; Miller, J.; Tam, C.; Rolston, M.R.; Teran-Cabanillas, E.; Yokoyama, W.H.; et al. Influence of Drying Methods on Health Indicators of Brewers Spent Grain for Potential Upcycling into Food Products. Appl. Food Res. 2022, 2, 100052. [Google Scholar] [CrossRef]
- Baiano, A.; la Gatta, B.; Rutigliano, M.; Fiore, A. Functional Bread Produced in a Circular Economy Perspective: The Use of Brewers’ Spent Grain. Foods 2023, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Lockert, E.A.; Cammer, A.L.; Whiting, S.J. Effects of Flax Fiber on Laxation and Glycemic Response in Healthy Volunteers. J. Med. Food 2005, 8, 508–511. [Google Scholar] [CrossRef]
- Chawla, S.P.; Kanatt, S.R.; Sharma, A.K. Chitosan. In Polysaccharides; Springer International Publishing: Mumbai, India, 2014; pp. 1–24. [Google Scholar]
- Berer, K.; Martínez, I.; Walker, A.; Kunkel, B.; Schmitt-Kopplin, P.; Walter, J.; Krishnamoorthy, G. Dietary Non-Fermentable Fiber Prevents Autoimmune Neurological Disease by Changing Gut Metabolic and Immune Status. Sci. Rep. 2018, 8, 10431. [Google Scholar] [CrossRef]
- Steiner, J.; Procopio, S.; Becker, T. Brewer’s Spent Grain: Source of Value-Added Polysaccharides for the Food Industry in Reference to the Health Claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- Meuser, F.; Suckow, P. Non-Starch Polysaccharides. Chemistry and Physics of Baking: Materials, Processes, and Products. R. Soc. Chem. 1986, 42–62. [Google Scholar]
- Pool-Zobel, B.L. Inulin-Type Fructans and Reduction in Colon Cancer Risk: Review of Experimental and Human Data. Br. J. Nutr. 2005, 93, S73–S90. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Wang, C. In Vitro Fermentation of Xylooligosaccharides from Wheat Bran Insoluble Dietary Fiber by Bifidobacteria. Carbohydr. Polym. 2010, 82, 419–423. [Google Scholar] [CrossRef]
- EFSA Panel (a) Scientific Opinion on the Substantiation of Health Claims Related to Arabinoxylan Produced from Wheat Endosperm and Reduction of Post-Prandial Glycaemic Responses (ID 830) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2205. [CrossRef]
- Niemi, P.; Martins, D.; Buchert, J.; Faulds, C.B. Pre-Hydrolysis with Carbohydrases Facilitates the Release of Protein from Brewer’s Spent Grain. Bioresour. Technol. 2013, 136, 529–534. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Phenolic Extracts of Brewers’ Spent Grain (BSG) as Functional Ingredients—Assessment of Their DNA Protective Effect against Oxidant-Induced DNA Single Strand Breaks in U937 Cells. Food Chem. 2012, 134, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s Spent Grain from Different Types of Malt: Evaluation of the Antioxidant Activity and Identification of the Major Phenolic Compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Faulds, C.; Sancho, A.I.; Bartolom, B. Mono- and Dimeric Ferulic Acid Release from Brewer’s Spent Grain by Fungal Feruloyl Esterases. Appl. Microbiol. Biotechnol. 2002, 60, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and P-Coumaric Acids Extraction by Alkaline Hydrolysis of Brewer’s Spent Grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Ummels, M.; JanssenDuijghuijsen, L.; Mes, J.J.; van der Aa, C.; Wehrens, R.; Esser, D. Evaluating Brewers’ Spent Grain Protein Isolate Postprandial Amino Acid Uptake Kinetics: A Randomized, Cross-Over, Double-Blind Controlled Study. Nutrients 2023, 15, 3196. [Google Scholar] [CrossRef]
- Schmidt-Combest, S.; Warren, C.; Grams, M.; Wang, W.; Miketinas, D.; Patterson, M. Evaluation of Brewers’ Spent Grain on Cardiovascular Disease Risk Factors in Adults: Lessons Learned from a Pilot Study. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100367. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Hazards Associated with Animal Feed; Joint FAO/WHO Expert Meeting FAO Headquarters: Rome, Italy, 2019. [Google Scholar]
- Penagos-Tabares, F.; Sulyok, M.; Nagl, V.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Mixtures of Mycotoxins, Phytoestrogens and Pesticides Co-Occurring in Wet Spent Brewery Grains (BSG) Intended for Dairy Cattle Feeding in Austria. Food Addit. Contam. Part A 2022, 39, 1855–1877. [Google Scholar] [CrossRef] [PubMed]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; Sabuncuoğlu, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin Mixtures in Food and Feed: Holistic, Innovative, Flexible Risk Assessment Modelling Approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected Shifts in Fusarium Species’ Composition on Cereal Grain in Northern Europe Due to Climatic Change. Food Addit. Contam. Part A 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Rivera-Becerril, F.; van Tuinen, D.; Chatagnier, O.; Rouard, N.; Béguet, J.; Kuszala, C.; Soulas, G.; Gianinazzi-Pearson, V.; Martin-Laurent, F. Impact of a Pesticide Cocktail (Fenhexamid, Folpel, Deltamethrin) on the Abundance of Glomeromycota in Two Agricultural Soils. Sci. Total Environ. 2017, 577, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ji, J.; Wang, J.; Sun, X. Co-Contamination and Interaction of Fungal Toxins and Other Environmental Toxins. Trends Food Sci. Technol. 2020, 103, 162–178. [Google Scholar] [CrossRef]
- European Union Recommendation 2006/576/EC OJEC L229/7. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 15 August 2025).
- Patriarca, A.; Fernández Pinto, V. Prevalence of Mycotoxins in Foods and Decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The Role of Microorganisms on Biotransformation of Brewers’ Spent Grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling Brewers’ Spent Grain for Composition and Microbial Ecology at the Site of Production. LWT—Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Terefe, G. Preservation Techniques and Their Effect on Nutritional Values and Microbial Population of Brewer’s Spent Grain: A Review. CABI Agric. Biosci. 2022, 3, 51. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Bartolomé, B.; Santos, M.; Jiménez, J.J.; del Nozal, M.J.; Gómez-Cordovés, C. Pentoses and Hydroxycinnamic Acids in Brewer’s Spent Grain. J. Cereal Sci. 2002, 36, 51–58. [Google Scholar] [CrossRef]
- Faccenda, A.; Zambom, M.A.; Avila, A.S.; Castagnara, D.D.; Dri, R.; Fischer, M.L.; Tinini, R.C.R.; Dessbesell, J.G.; Almeida, A.-R.E.; Almeida, K.V. Influence of the Storage Period on the Nutritional and Microbiological Value of Sun-Dried Brewer’s Grains. Rev. Colomb. De Cienc. Pecu. 2020, 34, 254–266. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; İbanoǧlu, S. The Recycling of Brewer’s Processing by-Product into Ready-to-Eat Snacks Using Extrusion Technology. J. Cereal Sci. 2008, 47, 469–479. [Google Scholar] [CrossRef]
- Aprodu, I.; Simion, A.; Banu, I. Valorisation of the Brewers’ Spent Grain Through Sourdough Bread Making. Int. J. Food Eng. 2017, 13, 20170195. [Google Scholar] [CrossRef]
- Czubaszek, A.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Kawa-Rygielska, J. Effect of Added Brewer’s Spent Grain on the Baking Value of Flour and the Quality of Wheat Bread. Molecules 2022, 27, 1624. [Google Scholar] [CrossRef] [PubMed]
- Ktenioudaki, A.; Chaurin, V.; Reis, S.F.; Gallagher, E. Brewer’s Spent Grain as a Functional Ingredient for Breadsticks. Int. J. Food Sci. Technol. 2012, 47, 1765–1771. [Google Scholar] [CrossRef]
- Ajanaku, O.; Dawodu, F.; AJANAKU, O.; Nwinyi, O. Functional and Nutritional Properties of Spent Grain Enhanced Cookies. Am. J. Food Technol. 2011, 6, 763–771. [Google Scholar] [CrossRef]
- Petrovic, J.; Pajin, B.; Tanackov-Kocic, S.; Pejin, J.; Fistes, A.; Bojanic, N.; Loncarevic, I. Quality Properties of Cookies Supplemented with Fresh Brewer’s Spent Grain. Food Feed. Res. 2017, 44, 57–63. [Google Scholar] [CrossRef]
- Okpala, L.C.; Ofoedu, P.I. Quality Characteristics of Cookies Produced from Sweet Potato and Wheat Flour Blend Fortified with Brewer’s Spent Grain Flour. Curr. Res. Nutr. Food Sci. J. 2018, 6, 113–119. [Google Scholar] [CrossRef]
- Heredia-Sandoval, N.G.; Granados-Nevárez, M.d.C.; Calderón de la Barca, A.M.; Vásquez-Lara, F.; Malunga, L.N.; Apea-Bah, F.B.; Beta, T.; Islas-Rubio, A.R. Phenolic Acids, Antioxidant Capacity, and Estimated Glycemic Index of Cookies Added with Brewer’s Spent Grain. Plant Foods Hum. Nutr. 2020, 75, 41–47. [Google Scholar] [CrossRef]
- Sileoni, V.; Alfeo, V.; Bravi, E.; Belardi, I.; Marconi, O. Upcycling of a By-Product of the Brewing Production Chain as an Ingredient in the Formulation of Functional Shortbreads. J. Funct. Foods 2022, 98, 105292. [Google Scholar] [CrossRef]
- Shih, Y.-T.; Wang, W.; Hasenbeck, A.; Stone, D.; Zhao, Y. Investigation of Physicochemical, Nutritional, and Sensory Qualities of Muffins Incorporated with Dried Brewer’s Spent Grain Flours as a Source of Dietary Fiber and Protein. J. Food Sci. 2020, 85, 3943–3953. [Google Scholar] [CrossRef]
- Cermeño, M.; Dermiki, M.; Kleekayai, T.; Cope, L.; McManus, R.; Ryan, C.; Felix, M.; Flynn, C.; FitzGerald, R.J. Effect of Enzymatically Hydrolysed Brewers’ Spent Grain Supplementation on the Rheological, Textural and Sensory Properties of Muffins. Future Foods 2021, 4, 100085. [Google Scholar] [CrossRef]
- Goerlitz, C.D.; Harper, W.J.; Delwiche, J.F. RELATIONSHIP OF WATER ACTIVITY TO CONE CRISPNESS AS ASSESSED BY POSITIONAL RELATIVE RATING. J. Sens. Stud. 2007, 22, 687–694. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Valorisation of Spent Grain from Malt Whisky in the Spelt Pasta Formulation: Modelling and Optimization Study. Appl. Sci. 2022, 12, 1441. [Google Scholar] [CrossRef]
- Ainsworth, P.; İbanoğlu, Ş.; Plunkett, A.; İbanoğlu, E.; Stojceska, V. Effect of Brewers Spent Grain Addition and Screw Speed on the Selected Physical and Nutritional Properties of an Extruded Snack. J. Food Eng. 2007, 81, 702–709. [Google Scholar] [CrossRef]
- Cappa, C.; Alamprese, C. Brewer’s Spent Grain Valorization in Fiber-Enriched Fresh Egg Pasta Production: Modelling and Optimization Study. LWT—Food Sci. Technol. 2017, 82, 464–470. [Google Scholar] [CrossRef]
- Nocente, F.; Taddei, F.; Galassi, E.; Gazza, L. Upcycling of Brewers’ Spent Grain by Production of Dry Pasta with Higher Nutritional Potential. LWT 2019, 114, 108421. [Google Scholar] [CrossRef]
- Sahin, A.W.; Hardiman, K.; Atzler, J.J.; Vogelsang-O’Dwyer, M.; Valdeperez, D.; Münch, S.; Cattaneo, G.; O’Riordan, P.; Arendt, E.K. Rejuvenated Brewer’s Spent Grain: The Impact of Two BSG-Derived Ingredients on Techno-Functional and Nutritional Characteristics of Fibre-Enriched Pasta. Innov. Food Sci. Emerg. Technol. 2021, 68, 102633. [Google Scholar] [CrossRef]
- Schettino, R.; Verni, M.; Acin-Albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed Brewers’ Spent Grain Improves Nutritional and Antioxidant Properties of Pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef]
- Cuomo, F.; Trivisonno, M.C.; Iacovino, S.; Messia, M.C.; Marconi, E. Sustainable Re-Use of Brewer’s Spent Grain for the Production of High Protein and Fibre Pasta. Foods 2022, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, J.; Butula, N.; Jonuzi, E.; Korzeniowska, M.; Laaksonen, O.; Föste, M.; Kütt, M.-L.; Yang, B. Potential of Brewers’ Spent Grain in Yogurt Fermentation and Evaluation of Its Impact in Rheological Behaviour, Consistency, Microstructural Properties and Acidity Profile during the Refrigerated Storage. Food Hydrocoll. 2022, 125, 107412. [Google Scholar] [CrossRef]
- Naibaho, J.; Jonuzi, E.; Butula, N.; Korzeniowska, M.; Föste, M.; Sinamo, K.N.; Chodaczek, G.; Yang, B. Fortification of Milk-Based Yogurt with Protein Hydrolysates from Brewers’ Spent Grain: Evaluation on Microstructural Properties, Lactic Acid Bacteria Profile, Lactic Acid Forming Capability and Its Physical Behavior. Curr. Res. Food Sci. 2022, 5, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Steinmacher, N.C.; Honna, F.A.; Gasparetto, A.V.; Anibal, D.; Grossmann, M.V.E. Bioconversion of Brewer’s Spent Grains by Reactive Extrusion and Their Application in Bread-Making. LWT—Food Sci. Technol. 2012, 46, 542–547. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Shea, N.; Gallagher, E. Rheological Properties of Wheat Dough Supplemented with Functional By-Products of Food Processing: Brewer’s Spent Grain and Apple Pomace. J. Food Eng. 2013, 116, 362–368. [Google Scholar] [CrossRef]
- Roth, M.; Jekle, M.; Becker, T. Opportunities for Upcycling Cereal Byproducts with Special Focus on Distiller’s Grains. Trends Food Sci. Technol. 2019, 91, 282–293. [Google Scholar] [CrossRef]
- Magabane, I.E. Technologies for Improving the Quality of Bread Doughs Made with Barley Spent Grain and Sorghum; University of Pretoria: Pretoria, South Africa, 2017. [Google Scholar]
- Torbica, A.; Škrobot, D.; Janić Hajnal, E.; Belović, M.; Zhang, N. Sensory and Physico-Chemical Properties of Wholegrain Wheat Bread Prepared with Selected Food by-Products. LWT 2019, 114, 108414. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Alvarez-Jubete, L.; Smyth, T.J.; Kilcawley, K.; Rai, D.K.; Gallagher, E. Application of Bioprocessing Techniques (Sourdough Fermentation and Technological Aids) for Brewer’s Spent Grain Breads. Food Res. Int. 2015, 73, 107–116. [Google Scholar] [CrossRef]
- Odeseye, A.A.; Awonorin, S.O.; Abdussalaam, R.O.; Sanni, L.O.; Olayanju, T.M.A. The Effect of Processing Variables on the Biscuit-Making Potential of Cocoyam-Brewer’s Spent Grain Flour Blends. Croat. J. Food Sci. Technol. 2020, 12, 56–66. [Google Scholar] [CrossRef]
- Combest, S.; Warren, C.; Patterson, M. Upcycling Brewers’ Spent Grain: The Development of Muffins and Biomarker Response After Consuming Muffins for 8-Weeks in Healthy Adults From Randomized-Controlled Trial. Curr. Dev. Nutr. 2020, 4, nzaa052_014. [Google Scholar] [CrossRef]
- Raza, K.; Nadeem, M.; Hussain, S.; Jabbar, S.; Din, A.; Ainee, A.; Qureshi, T. DEVELOPMENT AND PHYSICO-CHEMICAL CHARACTERIZATION OF DATE WAFERS. J. Agric. Res. 2015, 54. [Google Scholar]
- Virdi, A.S.; Mahajan, A.; Devraj, M.; Sanghi, R. Brewers’ Spent Grains: Techno-Functional Challenges and Opportunity in the Valorization for Food Products. LWT 2025, 227, 117785. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Crofton, E.; Scannell, A.G.M.; Hannon, J.A.; Kilcawley, K.N.; Gallagher, E. Sensory Properties and Aromatic Composition of Baked Snacks Containing Brewer’s Spent Grain. J. Cereal Sci. 2013, 57, 384–390. [Google Scholar] [CrossRef]
- Kirjoranta, S.; Tenkanen, M.; Jouppila, K. Effects of Process Parameters on the Properties of Barley Containing Snacks Enriched with Brewer’s Spent Grain. J. Food Sci. Technol. 2016, 53, 775–783. [Google Scholar] [CrossRef]
- Khan, I.; Yousif, A.M.; Johnson, S.K.; Gamlath, S. Effect of Sorghum Flour Addition on In Vitro Starch Digestibility, Cooking Quality, and Consumer Acceptability of Durum Wheat Pasta. J. Food Sci. 2014, 79, 1560–1567. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Bustos, M.C.; Heinzmann Alabí, M.B.; Nicolazzi, M.S.; Penci, M.C.; Ribotta, P.D. Effect of Ingredients on the Quality of Gluten-Free Sorghum Pasta. J. Food Sci. 2017, 82, 2085–2093. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Use of Grape Peels By-Product for Wheat Pasta Manufacturing. Plants 2021, 10, 926. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Spent Grain from Malt Whisky: Assessment of the Phenolic Compounds. Molecules 2021, 26, 3236. [Google Scholar] [CrossRef]
- Kamali Rousta, L.; Pouya Ghandehari Yazdi, A.; Khorasani, S.; Tavakoli, M.; Ahmadi, Z.; Amini, M. Optimization of Novel Multigrain Pasta and Evaluation of Physicochemical Properties: Using D-optimal Mixture Design. Food Sci. Nutr. 2021, 9, 5546–5556. [Google Scholar] [CrossRef]
- Spinelli, S.; Padalino, L.; Costa, C.; Del Nobile, M.A.; Conte, A. Food By-Products to Fortified Pasta: A New Approach for Optimization. J. Clean. Prod. 2019, 215, 985–991. [Google Scholar] [CrossRef]
- Bianchi, F.; Tolve, R.; Rainero, G.; Bordiga, M.; Brennan, C.S.; Simonato, B. Technological, Nutritional and Sensory Properties of Pasta Fortified with Agro-industrial By-products: A Review. Int. J. Food Sci. Technol. 2021, 56, 4356–4366. [Google Scholar] [CrossRef]
- Bustos, M.C.; Perez, G.T.; Leon, A.E. Structure and Quality of Pasta Enriched with Functional Ingredients. RSC Adv. 2015, 5, 30780–30792. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic Viability in Yoghurt: A Review of Influential Factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Sitanggang, A.B.; Lu, Y.; Julianti, E. Brewers’ Spent Grain as a Food Ingredient: Techno-Processing Properties, Nutrition, Acceptability, and Market. Trends Food Sci. Technol. 2024, 152, 104685. [Google Scholar] [CrossRef]
- Haruna, M.; Udobi, C.; Ndife, J. Effect of Added Brewers Dry Grain on the Physico-Chemical, Microbial and Sensory Quality of Wheat Bread. Am. J. Food Nutr. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile Profile, Fatty Acids Composition and Total Phenolics Content of Brewers’ Spent Grain by-Product with Potential Use in the Development of New Functional Foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Jozinović, A.; Babić, J.; Miličević, B.; Panak Balentić, J.; Šubarić, D. Resolving the Problem of Poor Expansion in Corn Extrudates Enriched with Food Industry By-Products. Innov. Food Sci. Emerg. Technol. 2018, 47, 517–524. [Google Scholar] [CrossRef]
- Uchegbu, N.N. Consumer Acceptability of Crackers Produced from Blend of Sprouted Pigeon Pea, Unripe Plantain and Brewers’ Spent Grain and Its Hypoglycemic Effect in Diabetic Rats. World Acad. Sci. Eng. Technol. Int. J. Nutr. Food Eng. 2016, 10, 374–378. [Google Scholar]
- Naibaho, J.; Korzeniowska, M.; Julianti, E.; Sebayang, N.S.; Yang, B. Campaign Education and Communication to the Potential Consumers of Brewers’ Spent Grain (BSG)-Added Food Products as Sustainable Foods. Heliyon 2023, 9, e19169. [Google Scholar] [CrossRef]
- Spurling, N.; McMeekin, A.; Shove, E.; Southerton, D.; Welch, D. Interventions in Practice: Re-Framing Policy Approaches to Consumer Behaviour; University of Manchester, Sustainable Practices Research Group: Manchester, UK, 2013. [Google Scholar]
- Crofton, E.C.; Scannell, A.G.M. Snack Foods from Brewing Waste: Consumer-Led Approach to Developing Sustainable Snack Options. Br. Food J. 2020, 122, 3899–3916. [Google Scholar] [CrossRef]
- Roth, M.; Schuster, H.; Kollmannsberger, H.; Jekle, M.; Becker, T. Changes in Aroma Composition and Sensory Properties Provided by Distiller’s Grains Addition to Bakery Products. J. Cereal Sci. 2016, 72, 75–83. [Google Scholar] [CrossRef]
- Abd EL-Moneim, R.; Shamsia, S.; EL-Deeb, A.; Ziena, H. UTILIZATION OF BREWERS SPENT GRAIN (BSG) IN MAKING FUNCTIONAL YOGHURT. J. Food Dairy Sci. 2015, 6, 577–589. [Google Scholar] [CrossRef]
- Abd El-Moneim, R.; Shamsia, S.; EL-Deeb, A.; Ziena, H. Utilization of Brewers Spent Grain (BSG) in Producing Functional Processed Cheese “Block”. J. Food Dairy Sci. 2018, 2018, 103–109. [Google Scholar] [CrossRef]
- Żelaziński, T.; Ekielski, A.; Siwek, A.; Durczak, K. By-Products from Brewery Industry as the Attractive Additives to the Extruded Cereals Food. Carpathian J. Food Sci. Technol. 2018, 10, 83–97. [Google Scholar]
- Thorvaldsson, M. Biotransformation of Brewer’s Spent Grain and Application as a Food Ingredient in Extruded Breakfast Cereals. Master’s Thesis, University of Technology, Gothenburg, Sweden, 2020. [Google Scholar]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From Stale Bread and Brewers Spent Grain to a New Food Source Using Edible Filamentous Fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
| Components | Amount | References |
|---|---|---|
| Moisture (% DW) | 70–80 | [12,13] |
| Carbohydrate (% DW) | 50–60 | [14,15] |
| Sugars (% DW) | ||
| Maltose | 5–20 | [16,17] |
| Glucose | 2.3–23 | [16,17] |
| Maltotriose | 0.7 | [17] |
| Fructose | 0.4 | [17] |
| Galactose | 0.77 | [16] |
| Xylose | 12.96 | [16] |
| Arabinose | 6 | [16] |
| Mannose | 0.94 | [16] |
| Cellulose (% DW) | 15–20 | [18,19,20] |
| Hemicellulose (% DW) | 15–30 | [20,21,22] |
| Protein (% DW) | 15–30 | [13,14,17] |
| Lipids (% DW) | 4–10 | [13,23] |
| Ash (% DW) | 2–4 | [15,20,24] |
| Dietary fiber (% DW) | 30–60 | [13,17,25] |
| Arabinoxylan (% DW) | 10.37 | [6] |
| β-glucans (% DW) | 1 | [26] |
| Fatty acids (% DW) | 2–19 | [27,28] |
| Fatty acid profile (µg/g DW) | ||
| Palmitic acid | 2000–3200 | [27,29,30] |
| Linoleic acid | 500–4270 | [27,29,30] |
| Myristic acid | 25–50 | [27,30] |
| Pentadecylic acid | 10 | [30] |
| Margaric acid | 110–250 | [27,30] |
| Oleic acid | 100–700 | [27,29,30] |
| Stearic acid | 90–650 | [27,29,30] |
| Linolenic | 50–500 | [29,30] |
| Arachidic acid | 25–300 | [27,30] |
| Behenic acid | 250 | [27] |
| Decosadienoic acid (Omega-6) | 1085 | [30] |
| Tricosylic acid | 25.73 | [30] |
| Essential amino acids (% DW) | ||
| Valine | 1–4 | [12,31] |
| Isoleucine | 2–10 | [12,31] |
| Leucine | 0.4–7 | [12,31] |
| Methionine | 0.87–3.7 | [12] |
| Threonine | 0.8–4 | [12,31] |
| Lysine | 0.5–6 | [12,31] |
| Histidine | 1.26–5.9 | [12,31] |
| Tryptophan | 1 | [31] |
| Non-essential amino acids (% DW) | ||
| Alanine | 1–4 | [12,31] |
| Arginine | 1–8 | [12,31] |
| Aspartic acid | 2–9 | [12,31] |
| Glutamic acid | 5–15 | [12,31] |
| Proline | 2–10 | [12,31] |
| Serine | 5 | [31] |
| Tyrosine | 8 | [31] |
| Glycine | 1–4 | [31] |
| Cysteine | 0.47 | [12] |
| Minerals (μg/g DW) | ||
| Calcium | 1000–3600 | [29,32] |
| Magnesium | 1900 | [32] |
| Phosphorus | 4000–6000 | [29,32] |
| Sodium | 137 | [32] |
| Potassium | 1570 | [29] |
| Zinc | 67.2 | [29] |
| Manganese | 34.3 | [29] |
| Iron | 210 | [29] |
| Vitamins (μg/g DW) | ||
| B1 | 25 | [33] |
| B2 | 2–25 | [17,33] |
| B6 | 9 | [33] |
| K | 4.5 | [33] |
| Polyphenols (μg/g) | ||
| Vanillin | 27.18 | [34] |
| Catechin | 29–116.04 | [16,34] |
| P-Coumaric | 453–686 | [16,35] |
| Ferulic acid | 10.56–1144 | [16,34] |
| Caffeic acid | 0.28 | [35] |
| 4-Hydroxybenzoic | 14–41.3 | [34,35] |
| Phytosterols (μg/g DW) | ||
| Campesterol | 250 | [27] |
| Stigmasterol | 50 | [27] |
| Sitosterol | 450 | [27] |
| Δ5-Avenasterol | 60 | [27] |
| Δ7-Stigmastenol | 20 | [27] |
| Δ7-Avenasterol | 14 | [27] |
| 24-Methylenecycloartenol | 40 | [27] |
| Campestanol (ergostanol) | 20 | [27] |
| Sitostanol (stigmastanol) | 6 | [27] |
| Lignin (% DW) | 11–15 | [36,37,38] |
| Byproduct | Cereals | Legumes/Pulses | |||||
|---|---|---|---|---|---|---|---|
| Nutrient (% DW) | BSG | Barley | Wheat | Oats | Soybean | Pea | White Beans |
| Carbohydrate | 15 | 60 | 60 | 55 | 20 | 45 | 54.79 |
| Fat | 10 | 5 | 5 | 8 | 15 | 4 | 4.42 |
| Fiber | 40 | 10 | 10 | 10 | 25 | 20 | 3.23 |
| Protein | 30 | 15 | 15 | 25 | 45 | 27 | 26.18 |
| Ash | 3 | 5 | 2 | 2 | 5 | 4 | 7.18 |
| Food Product | Quantity (%) | Characteristics | References |
|---|---|---|---|
| Bread | 10–15% | 1. Enhanced nutritional composition 2. Color changes from light cream to brown 3. Increased water absorption capacity with high amount of spent grain 4. Increased crumb firmness 5. High fiber content 6. Rheological and pasting properties of dough were affected 7. Increased shelf life | [1,48,81,82,83] |
| Bread sticks | 15% | 1. Reduction in loaf volume 2. Less crispy, darker and decreased baking volume 3. 15% BSG increased dietary fiber 4. Increased shelf life | [6,84] |
| Cookies | 10–20 | 1. Reduced bulk density and water absorption 2. Dough forming time and dough stability increased 3. Increased emulsion and oil absorption capacity 4. Increase in the protein and fiber content 5. Total antioxidant activity increases | [85,86,87,88] |
| Short bread | 30% | 1. Reduced carbohydrate content as well as the energy value. 2. Elevated fiber and protein content | [1,89] |
| Muffins Wafers | 15–30% 5–15% | 1. Increased viscosity of the batter increased 2. Reduced muffin hardness 3. Enhances the levels of fat, protein, and total dietary fiber 1. Increase in the gumminess, chewiness, hardness of the product. 2. Increased firmness and cohesiveness. 3. Adhesiveness decreased. | [14,90,91] [92,93] |
| Snacks | 10–15% | 1. Increased phytic acid and resistant starch content of the product 2. β-glucan, polyphenols and flavonoids exhibited increased concentrations 3. Decreased cell size, limited product expansion, and lower bulk density | [33,81,94] |
| Pasta | 5–20% | 1. The increase of the spent grain affects the color of the pasta. 2. A solid structure with higher firmness 3. Decreased cooking loss. 4. Decreased degree of starch gelatinization. 5. Shortened the optimal cooking duration 6. Enhanced nutritional profile | [1,95,96,97,98,99] |
| Yoghurt/Yoghurt fortified with BSGP | 5–10% | 1. Improved quality of yoghurt 2. Increased viscosity and shear stress 3. Shortened fermentation time 4. Improved lactic acid formation 5. Helps in growth and survival of LAB 6. Long shelf life (14 days) | [100,101] |
| Fruit juice and smoothies/beverages | 0–10% | 1. Increased antioxidant activity 2. Increased shelf life and phenolic components | [1,18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eche, V.; Emenike, C.U.; Rupasinghe, H.P.V. Nutritional Value of Brewer’s Spent Grain and Consumer Acceptance of Its Value-Added Food Products. Foods 2025, 14, 2900. https://doi.org/10.3390/foods14162900
Eche V, Emenike CU, Rupasinghe HPV. Nutritional Value of Brewer’s Spent Grain and Consumer Acceptance of Its Value-Added Food Products. Foods. 2025; 14(16):2900. https://doi.org/10.3390/foods14162900
Chicago/Turabian StyleEche, Victoria, C. U. Emenike, and H. P. Vasantha Rupasinghe. 2025. "Nutritional Value of Brewer’s Spent Grain and Consumer Acceptance of Its Value-Added Food Products" Foods 14, no. 16: 2900. https://doi.org/10.3390/foods14162900
APA StyleEche, V., Emenike, C. U., & Rupasinghe, H. P. V. (2025). Nutritional Value of Brewer’s Spent Grain and Consumer Acceptance of Its Value-Added Food Products. Foods, 14(16), 2900. https://doi.org/10.3390/foods14162900








