Effects of Rhizopus oligosporus-Mediated Solid-State Fermentation on the Protein Profile and α-Glucosidase Inhibitory Activity of Selenium-Biofortified Soybean Tempeh
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Strains
2.2. Schematic Overview of the Experimental Program
2.3. Se-Enriched Culture and Growth Monitoring of R. oligosporus in Plate Assay
2.3.1. Selenium Preparation and Media Formulation
2.3.2. Fungal Cultivation and Growth Analysis
2.4. Solid Fermentation
2.4.1. Preparation of Selenium-Enriched Soybean Tempeh
2.4.2. Inoculum Preparation of Rhizopus oligosporus
2.5. Dry Matter Content
2.6. Determination of Amino Acids
2.7. Determination of Protein
2.8. Determination of Total Selenium
2.9. Analysis of Selenium Speciation
2.10. In Vitro Digestion Model for α-Glucosidase Inhibitory Activity Testing of Tempeh
2.11. Statistical Analysis
3. Results
3.1. Se-Enriched Growth of R. oligosporus in the Plate
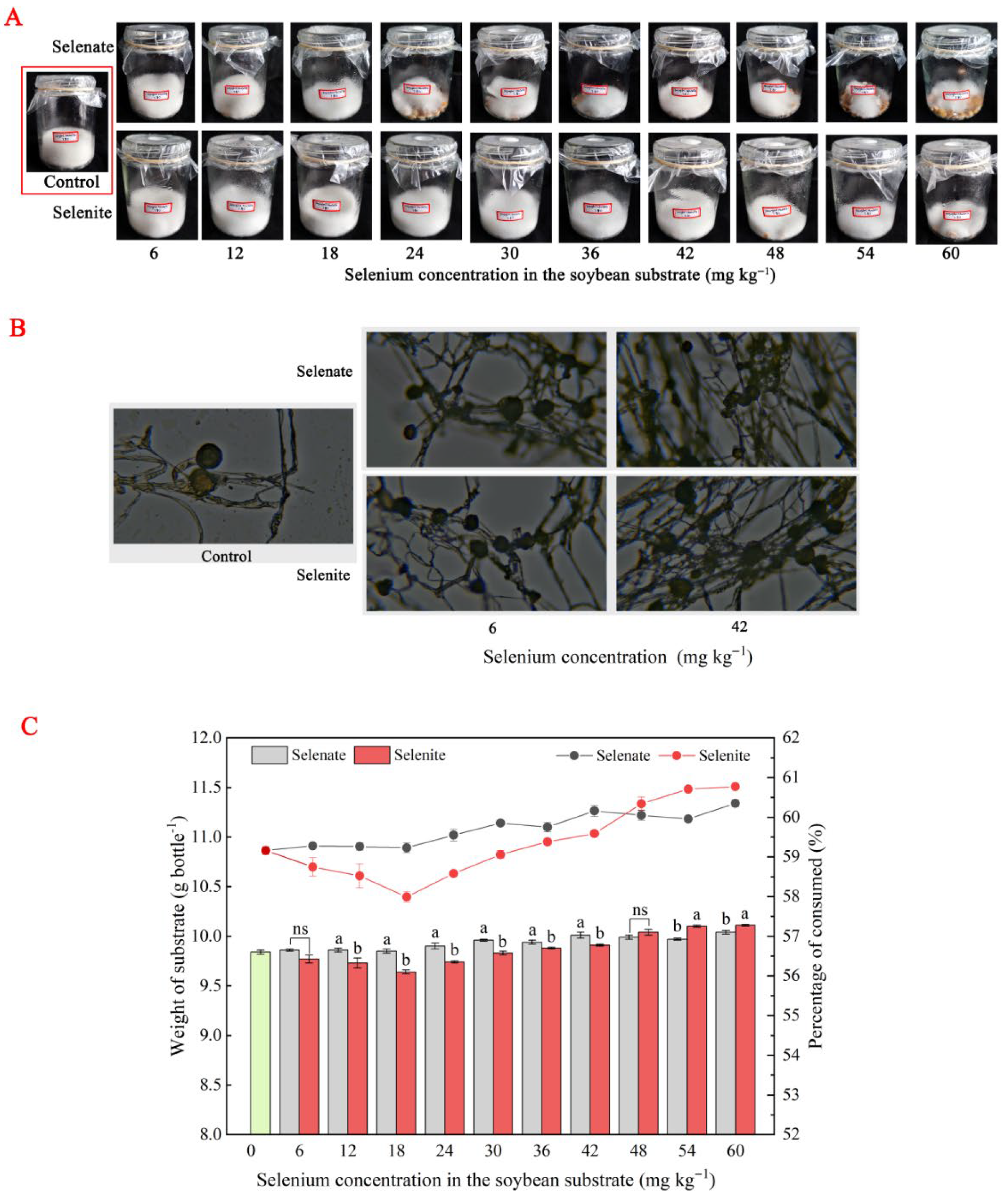
3.2. Se-Enriched Growth of R. oligosporus in Soybean Tempeh
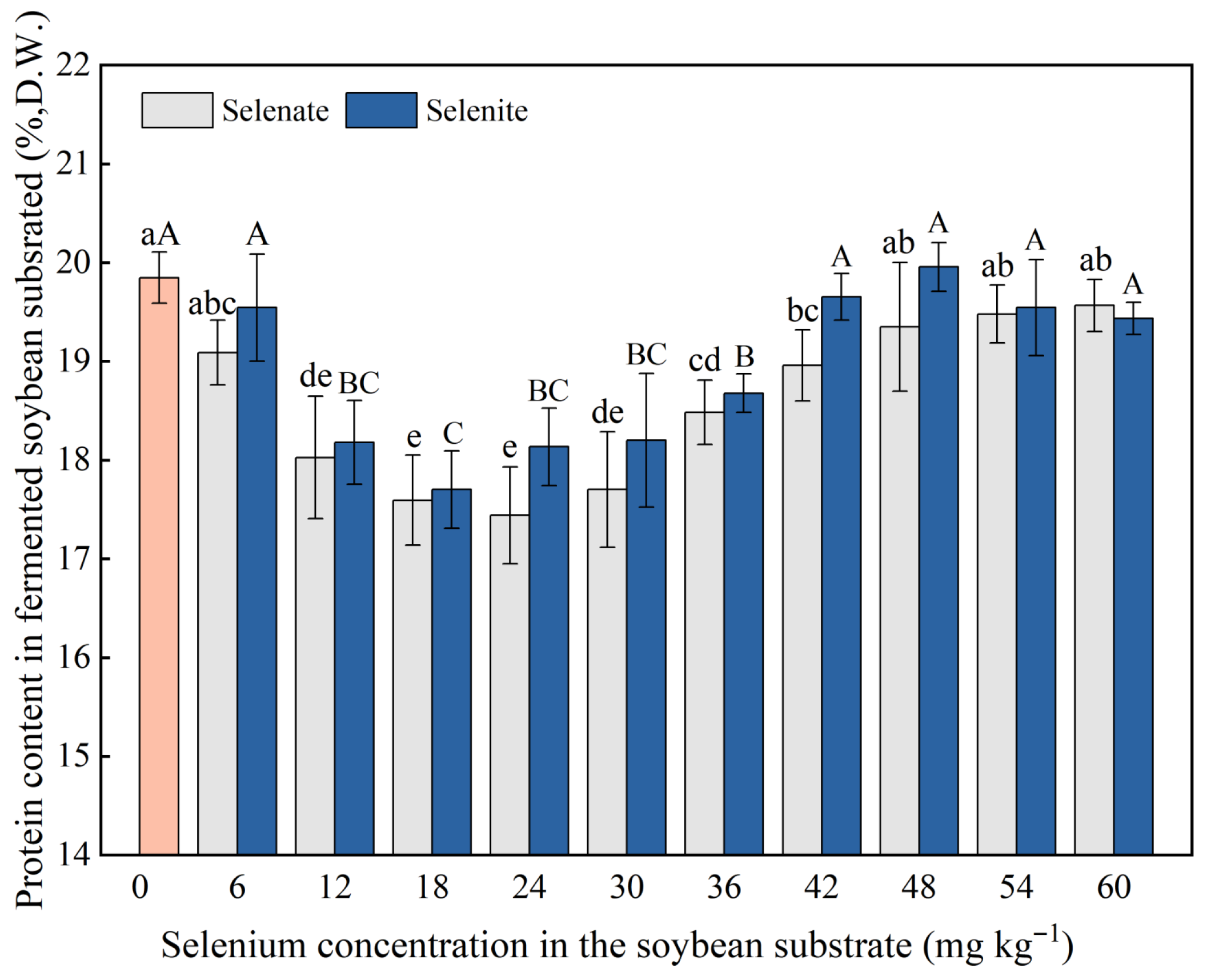
3.3. Effects of Se on the Protein and Amino Acid Contents in Soybean Tempeh
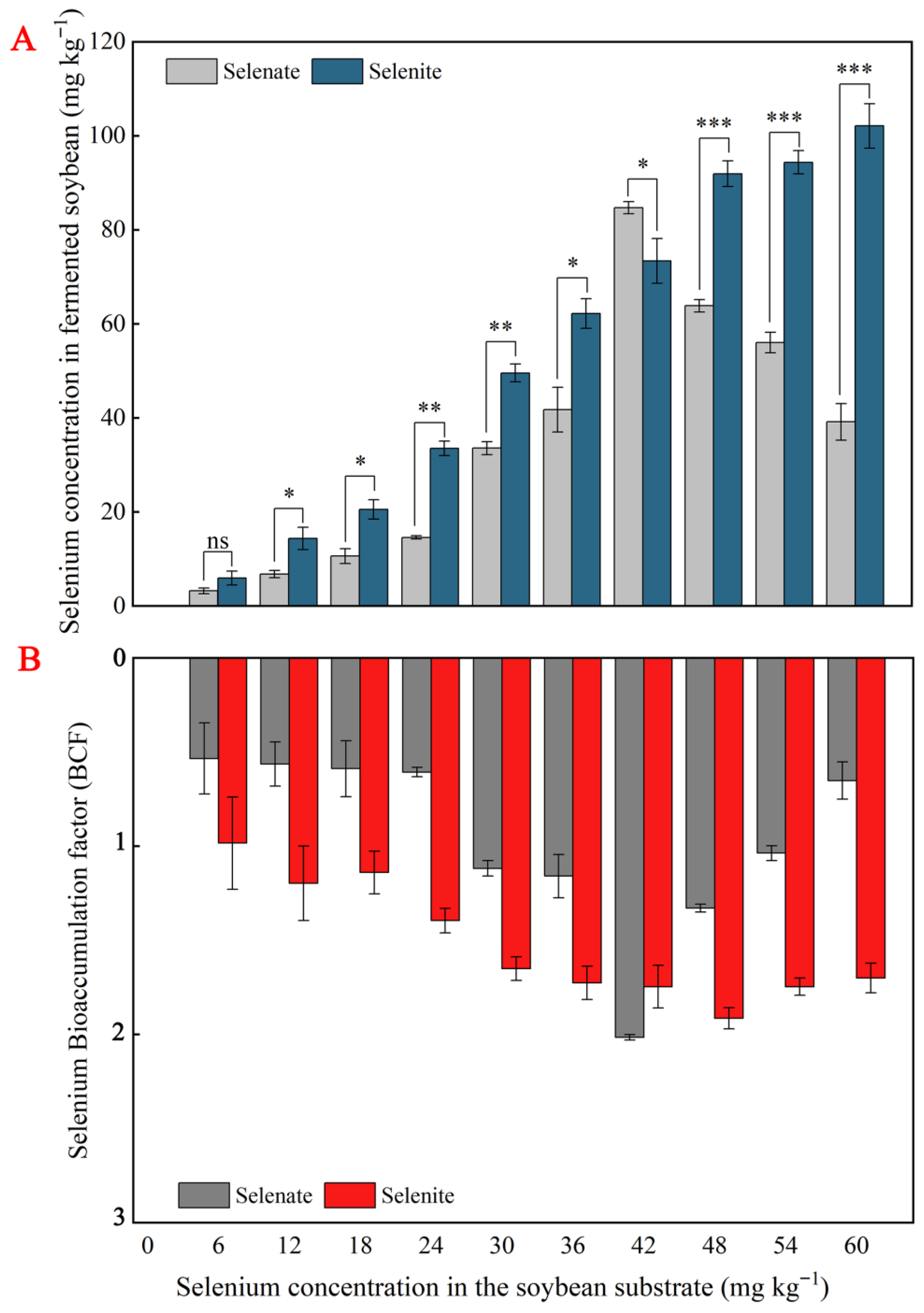
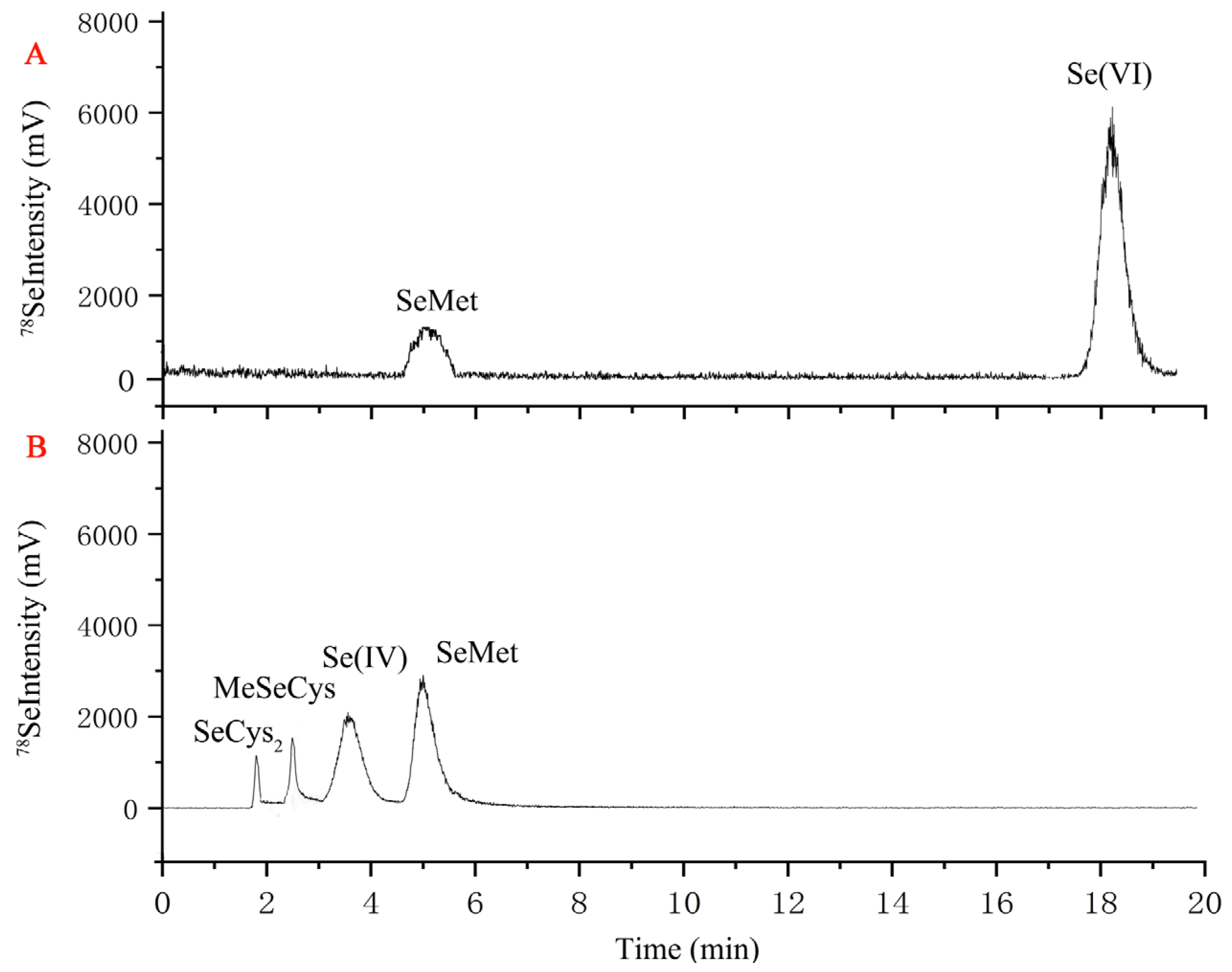
3.4. Effects of Se-Enriched SSF on the Se Content and Species
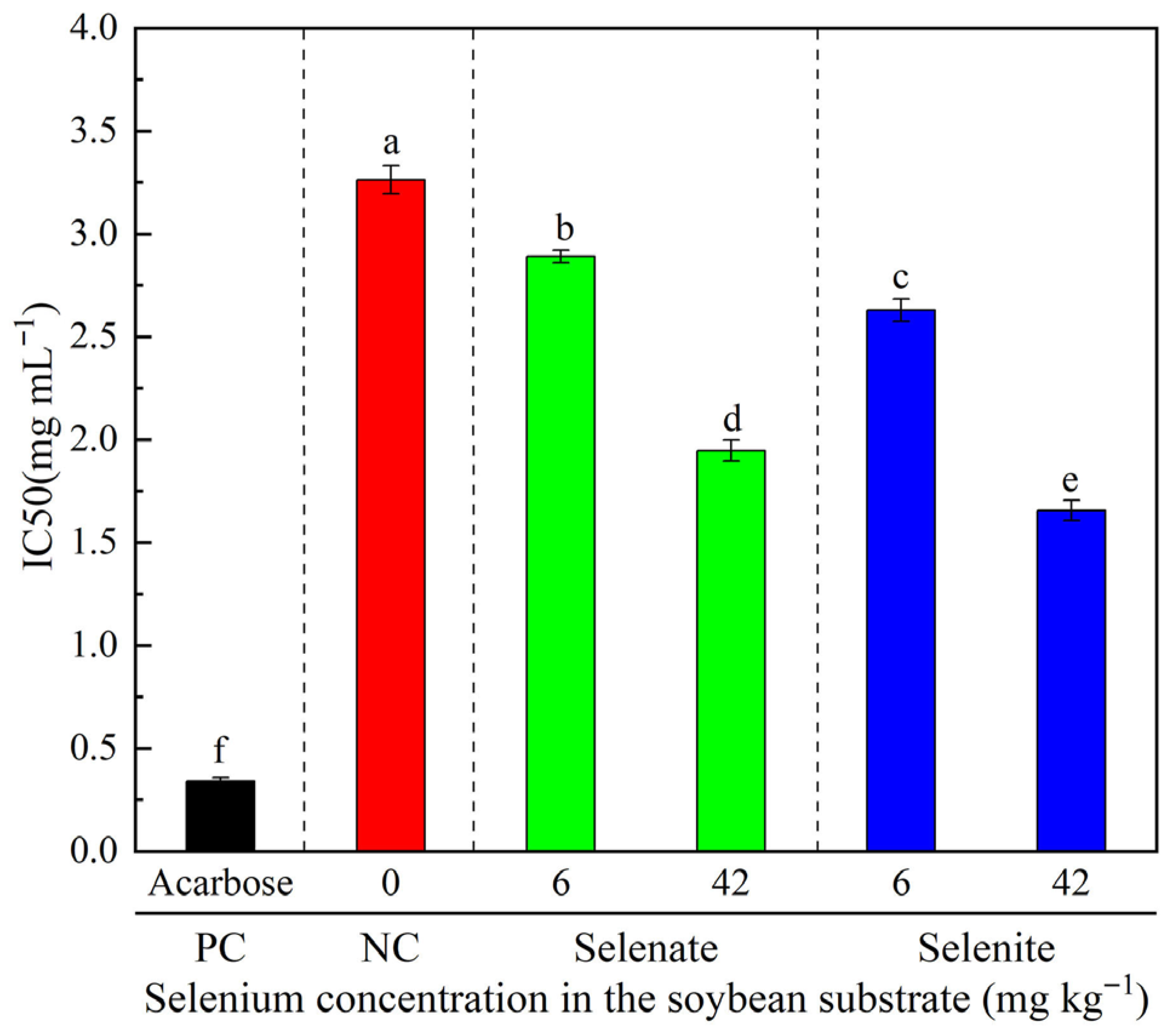
3.5. α-Glucosidase Inhibitory Activity of Selenium-Enriched Tempeh
4. Discussion
4.1. Selenium Supplementation Significantly Alters the Growth and Morphology of R. oligosporus
4.2. Selenium Fortification Differentially Enhanced Tempeh Quality
4.3. Se-Fermented Tempeh Significantly Enhanced α-Glucosidase Inhibitory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Liu, H.L.; Wang, X.Q.; Zhang, B.M.; Han, Z.; Wang, W.; Chi, Q.; Zhou, J.; Nie, L.; Xu, S.; Liu, D.; et al. Concentration and distribution of selenium in soils of mainland China, and implications for human health. J. Geochem. Explor. 2021, 220, 106654. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Nambi, V.E.; Chandrasekar, V.; Karthikeyan, S. Value addition of grains using solid state fermentation. Nutr. Food Sci. Int. J. 2017, 3, 555619. [Google Scholar] [CrossRef]
- Sakekar, A.A.; Gaikwad, S.R.; Punekar, N.S. Protein expression and secretion by filamentous fungi. J. Biosci. 2021, 46, 5. [Google Scholar] [CrossRef]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Tohin, J.M.; White, C.; Gadd, G.M. Metal accumulation by fungi: Applications in environmental biotechnology. J. Ind. Microbiol. 1994, 13, 126–130. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Zhao, G.; Guo, Y. Selenium enriched Hypsizygus marmoreus, a potential food supplement with improved Se bioavailability. LWT-Food Sci. Technol. 2021, 140, 110819. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Liu, D.; Zhang, C.; Wu, W.; Yi, H.; Zhang, J. Biotransformation of inorganic selenium into selenium nanoparticles and organic selenium by Lactiplantibacillus plantarum CXG4. Food Biosci. 2025, 65, 106060. [Google Scholar] [CrossRef]
- Kora, A.J. Nutritional and antioxidant significance of selenium-enriched mushrooms. Bull. Natl. Res. Cent. 2020, 44, 34. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in foods. Adv. Food Res. 1988, 32, 85–113. [Google Scholar] [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Hoefig, C.S.; Renko, K.; Kohrle, J.; Birringer, M.; Schomburg, L. Comparison of different selenocompounds with respect to nutritional value vs toxicity using liver cells in culture. J. Nutr. Biochem. 2011, 22, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine and selenium yeast: Appropriate forms of selenium for use in infant formulas and nutritional supplements. J. Med. Food 1988, 1, 201–206. [Google Scholar] [CrossRef]
- Thanh, N.V.; Nout, M.J.R. Rhizopus oligosporus biomass, sporangiospore yield and viability as influenced by harvesting age and processing conditions. Food Microbiol. 2002, 19, 91–96. [Google Scholar] [CrossRef]
- Ojeda, L. Official Method of Analysis of AOAC International, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Eveleigh, J.W.; Winter, G.D. Amino Acid Composition Determination. Mol. Biol. Biochem. Biophys. 1970, 8, 91–123. [Google Scholar] [CrossRef]
- Hu, T.; Liu, L.; Chen, S.; Wu, W.; Xiang, C.; Guo, Y. Determination of Selenium Species in Cordyceps militaris by high-performance liquid chromatography coupled to hydride generation atomic fluorescence spectrometry. Anal. Lett. 2018, 51, 2316–2330. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The role of selenium and nano selenium on physiological responses in plant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Castellano, S. Selenium strikes back at fungi. Nat. Microbiol. 2019, 4, 726–727. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, S.; Li, Y.; Xu, S.; Shi, G.; Ding, Z. Effect of selenium on mushroom growth and metabolism: A review. Trends Food Sci. Technol. 2021, 118, 328–340. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Z.; Chen, Q.; Lin, M.; Zheng, Y.; Liu, Y.; Zhao, P.; Zheng, S.; Liu, H.; Rensing, C.; et al. Selenite inhibited cadmium translocation and stimulated root growth of Brassica rapa L.: Regulation of element uptake, polysaccharide synthesis and crosslink, and cell wall enzymes. Environ. Exp. Bot. 2023, 211, 105344. [Google Scholar] [CrossRef]
- González-Salitre, L.; Castañeda-Ovando, A.; Basilio-Cortés, U.A.; García-Contreras, A.d.C.; Serrano, G.M.R.; Cardelle-Cobas, A.; Román-Gutiérrez, A.D.; González-Olivares, L.G. Biogenic production of seleno-amino acids and seleno-nanoparticles by Saccharomyces boulardii. Food Biosci. 2023, 53, 102552. [Google Scholar] [CrossRef]
- Zhu, L.; Long, P.; Hu, M.; Wang, L.; Shao, Y.; Cheng, S.; Dong, X.; He, Y. Insight into selenium biofortification and the selenite metabolic mechanism of Monascus ruber M7. Food Chem. 2024, 455, 139740. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Hui, D.; Zhang, Y.; Yang, M.; Zhang, W.; Zhao, Y.; Zhang, H.; Ji, H.; Zhang, B. Inorganic selenium transformation into organic selenium by Monascus purpureus. Foods 2023, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, M.H.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of selenium nanoparticles by Aspergillus flavus and Candida albicans for antifungal applications. Micro Nano Lett. 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Aghcheh, R.K.; Braus, G.H. Importance of stress response mechanisms in filamentous fungi for agriculture and industry. In Stress Response Mechanisms in Fungi; Skoneczny, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 189–222. [Google Scholar] [CrossRef]
- Sun, P.; Ge, G.; Sun, L.; Du, S.; Liu, Y.; Yan, X.; Zhang, J.; Zhang, Y.; Wang, Z.; Jia, Y. Effects of selenium enrichment on fermentation characteristics, selenium content and microbial community of Alfalfa silage. BMC Plant Biol. 2024, 24, 555. [Google Scholar] [CrossRef]
- Wells, M.; Stolz, J.F. Microbial selenium metabolism: A brief history, biogeochemistry and ecophysiology. FEMS Microbiol. Ecol. 2020, 96, fiaa209. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, J.; Zeng, L.; Chen, L.; Korpelainen, H.; Li, C. Selenium species transforming along soil-plant continuum and their beneficial roles for horticultural crops. Hortic. Res. 2023, 10, uhac270. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2025, 7, 4199–4239. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W.; Zhou, F.; Du, Z.; Xue, M.; Chen, T.; Liang, D. Effect of phosphate and silicate on selenite uptake and phloem-mediated transport in tomato (Solanum lycopersicum L.). Environ. Sci. Pollut. Res. 2019, 26, 20475–20484. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics andexpression of sulfate/selenate transporters in seleniumhyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Hui, G.; Zhang, J.; Li, H.; Wu, W.; Wei, X.; Guo, Y. Selenium biofortification and its effect on multi-element change in Auricularia auricular. Food Chem. 2019, 295, 206–213. [Google Scholar] [CrossRef]
- Alexander, J.; Olsen, A.K. Selenium-a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and selenoproteins: Mechanisms, health functions, and emerging applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Lu, Y.; Wei, Z.; Li, Y.; Li, L.; Pan, X. A review on the in vitro and in vivo screening of α-glucosidase inhibitors. Heliyon 2024, 10, e37467. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewska, I.; Kucharska, A.Z. Inhibitory Potential of Red Cabbage against Digestive Enzymes Linked to Obesity and Type 2 Diabetes. J. Agric. Food Chem. 2019, 65, 7192–7199. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Guo, J.; Zheng, S.; Wang, X.; Li, S. Enzymatic synthesis of organoselenium compounds via C-Se bond formation mediated by sulfur carrier proteins. Nat. Synth. 2024, 3, 477–487. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Dey, S.; Bhattacharyya, N.P.; Mukhopadhyay, D.; Lashuel, H. The role of intrinsically unstructured proteins in neurodegenerative diseases. PLoS ONE 2009, 4, e5566. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Dun, X.; Wang, X.; Wang, H. Research progress of selenium-enriched foods. Nutrients 2023, 15, 4189. [Google Scholar] [CrossRef]

| Amino Acid (%) | Control | Selenate (mg kg−1) | Selenite (mg kg−1) | |||
|---|---|---|---|---|---|---|
| 6 | 42 | 6 | 42 | |||
| Flavor amino acid | Glutamic acid | 6.43 ± 0.09 c | 7.21 ± 0.09 b | 7.54 ± 0.04 a | 6.50 ± 0.01 c | 6.54 ± 0.01 c |
| Aspartic acid | 4.35 ± 0.03 d | 4.60 ± 0.01 b | 4.74 ± 0.03 a | 4.40 ± 0.02 c | 4.41 ± 0.01 c | |
| Phenylalanine | 2.09 ± 0.01 bc | 2.13 ± 0.02 a | 2.07 ± 0.02 c | 2.10 ± 0.01 b | 2.11 ± 0.01 ab | |
| Alanine | 2.47 ± 0.09 a | 2.41 ± 0.02 ab | 2.06 ± 0.01 b | 2.36 ± 0.07 c | 2.17 ± 0.02 d | |
| Glycine | 1.66 ± 0.01 c | 1.69 ± 0.01 b | 1.73 ± 0.01 a | 1.65 ± 0.01 c | 1.63 ± 0.01 d | |
| Tyrosine | 1.58 ± 0.02 a | 1.55 ± 0.04 a | 1.57 ± 0.03 a | 1.57 ± 0.01 a | 1.58 ± 0.01 a | |
| Total | 18.58 ± 0.02 c | 19.59 ± 0.09 b | 19.71 ± 0.09 a | 18.57 ± 0.06 c | 18.43 ± 0.02 d | |
| Essential amino acids | 13.69 ± 0.04 b | 14.71 ± 0.10 a | 14.74 ± 0.10 a | 13.63 ± 0.03 bc | 13.55 ± 0.03 c | |
| Non-essential amino acid | 22.16 ± 0.13 c | 23.74 ± 0.21 b | 24.35 ± 0.03 a | 22.28 ± 0.07 c | 22.20 ± 0.01 c | |
| Free amino acids | 30.44 ± 0.05 c | 32.50 ± 0.21 b | 33.10 ± 0.09 a | 30.49 ± 0.03 c | 30.32 ± 0.04 c | |
| Total amino acids | 35.86 ± 0.09 c | 38.45 ± 0.31 b | 39.09 ± 13 a | 35.92 ± 0.07 c | 35.76 ± 0.04 c | |
| Treatment | Selenium Speciation (%) | ||||
|---|---|---|---|---|---|
| SeCys2 | MeSeCys | Se(IV) | SeMet | Se(VI) | |
| Selenate | / | / | / | 21.4 ± 2.2 | 78.6 ± 5.0 |
| Selenite | 20.4 ± 1.2 | 7.5 ± 0.8 | 15.9 ± 0.3 | 56.2 ± 4.1 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Hu, C.; Li, X.; Shen, R.; Yin, L.; Wu, Q.; Hu, T. Effects of Rhizopus oligosporus-Mediated Solid-State Fermentation on the Protein Profile and α-Glucosidase Inhibitory Activity of Selenium-Biofortified Soybean Tempeh. Foods 2025, 14, 2899. https://doi.org/10.3390/foods14162899
Wang C, Hu C, Li X, Shen R, Yin L, Wu Q, Hu T. Effects of Rhizopus oligosporus-Mediated Solid-State Fermentation on the Protein Profile and α-Glucosidase Inhibitory Activity of Selenium-Biofortified Soybean Tempeh. Foods. 2025; 14(16):2899. https://doi.org/10.3390/foods14162899
Chicago/Turabian StyleWang, Chengying, Changli Hu, Xin Li, Ruizhe Shen, Liwei Yin, Qiguo Wu, and Ting Hu. 2025. "Effects of Rhizopus oligosporus-Mediated Solid-State Fermentation on the Protein Profile and α-Glucosidase Inhibitory Activity of Selenium-Biofortified Soybean Tempeh" Foods 14, no. 16: 2899. https://doi.org/10.3390/foods14162899
APA StyleWang, C., Hu, C., Li, X., Shen, R., Yin, L., Wu, Q., & Hu, T. (2025). Effects of Rhizopus oligosporus-Mediated Solid-State Fermentation on the Protein Profile and α-Glucosidase Inhibitory Activity of Selenium-Biofortified Soybean Tempeh. Foods, 14(16), 2899. https://doi.org/10.3390/foods14162899






