Oral Administration of an Opuntia ficus-indica Fruit Extract Induces Changes in Gut Microbiota Composition: Relationship with Its Anti-Obesity and Anti-Steatotic Effects in Rats Fed a High-Fat High-Fructose Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Opuntia fichus-indica Var. colorada Extract
2.2. Animals, Diets and Experimental Design
2.3. Determination of Liver Triglyceride Content and Serum Biochemical Parameters
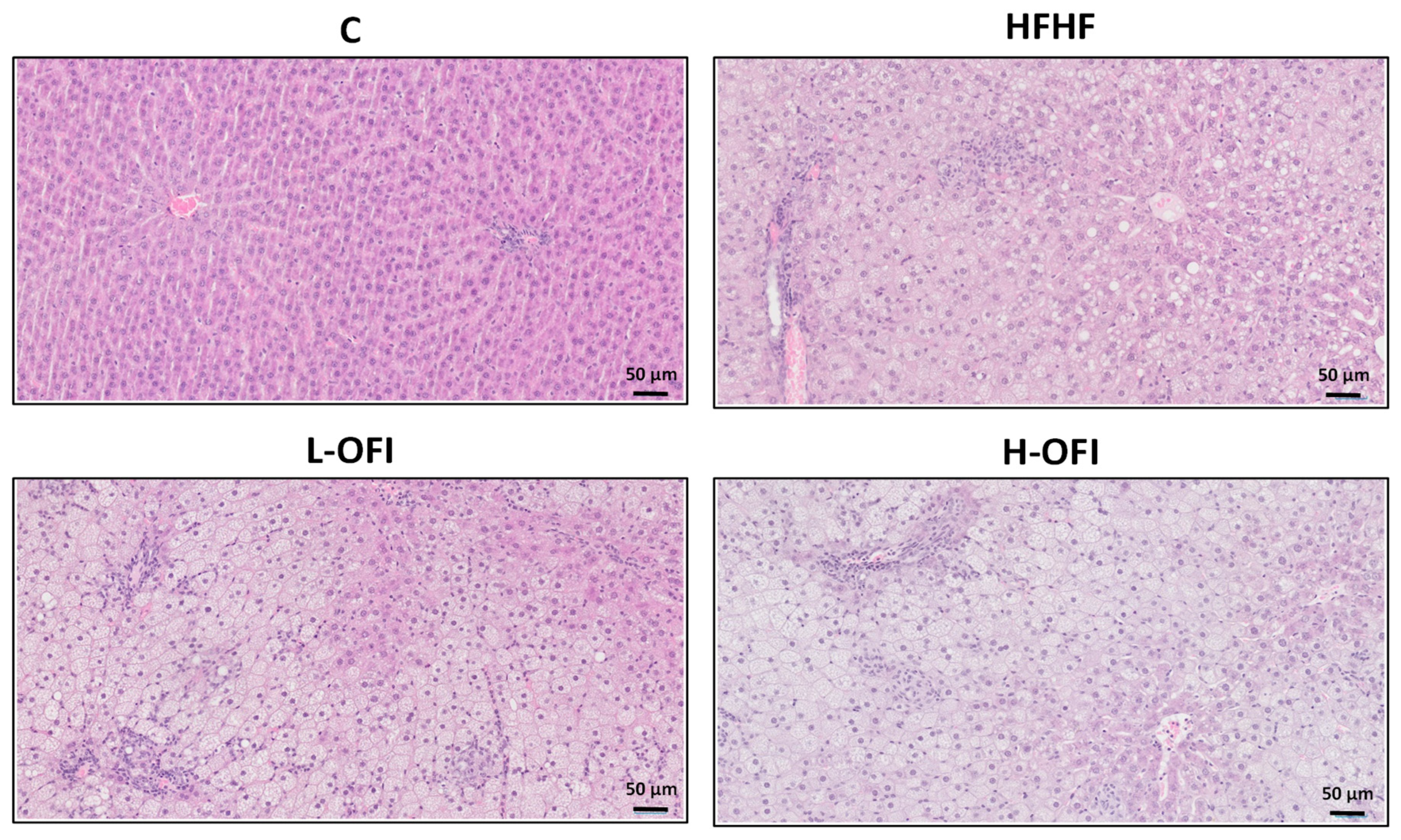
2.4. Liver Histological Analysis
2.5. Fecal DNA Extraction and 16S rRNA Gene Amplification for Microbiota Composition Analysis
2.6. Short-Chain Fatty Acid (SCFA) Analysis
2.7. Statistical Analysis
3. Results
3.1. Body Weight, Adipose Tissue Weights, Liver Weight, Hepatic Triglyceride Content and Serum Parameters
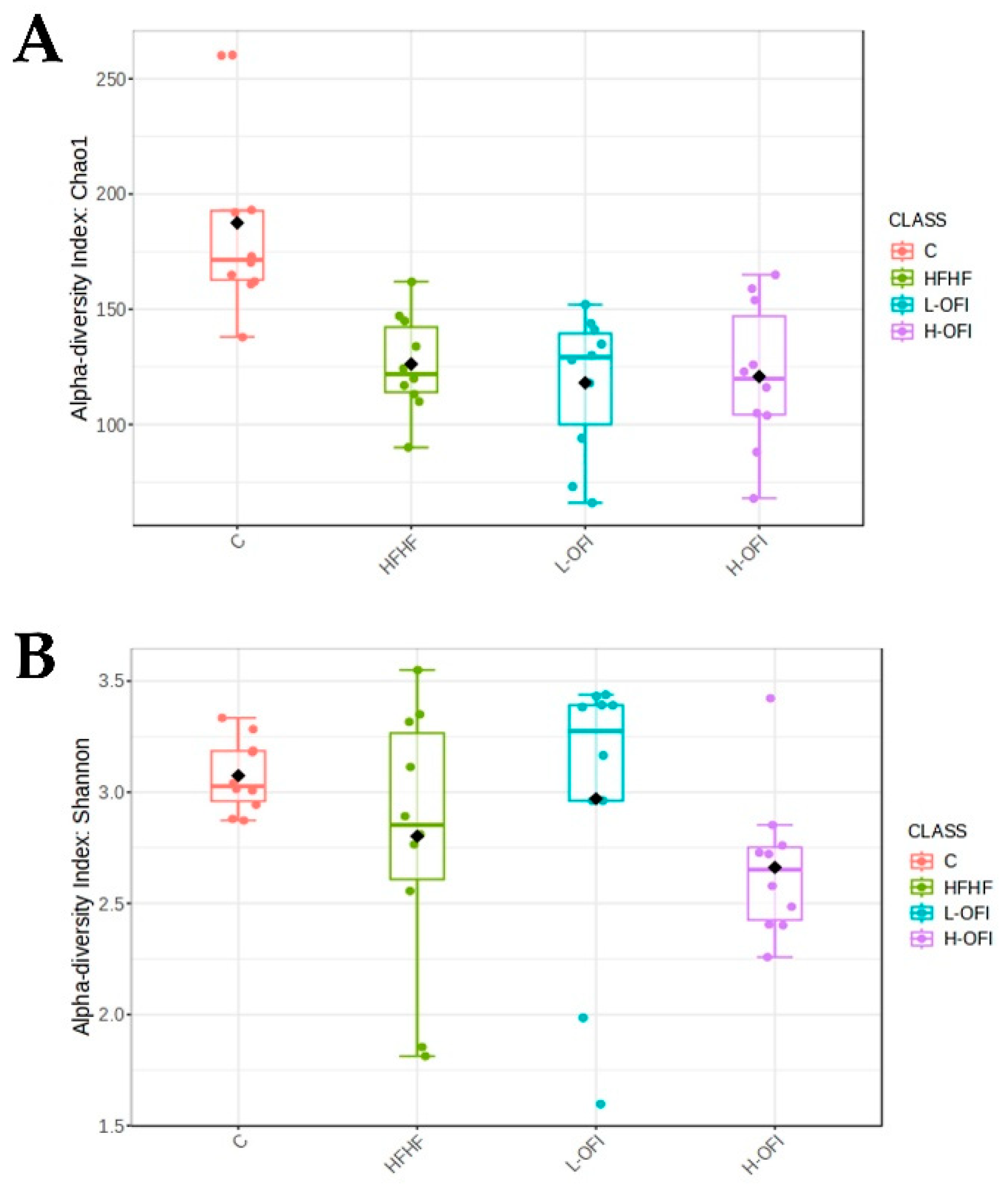
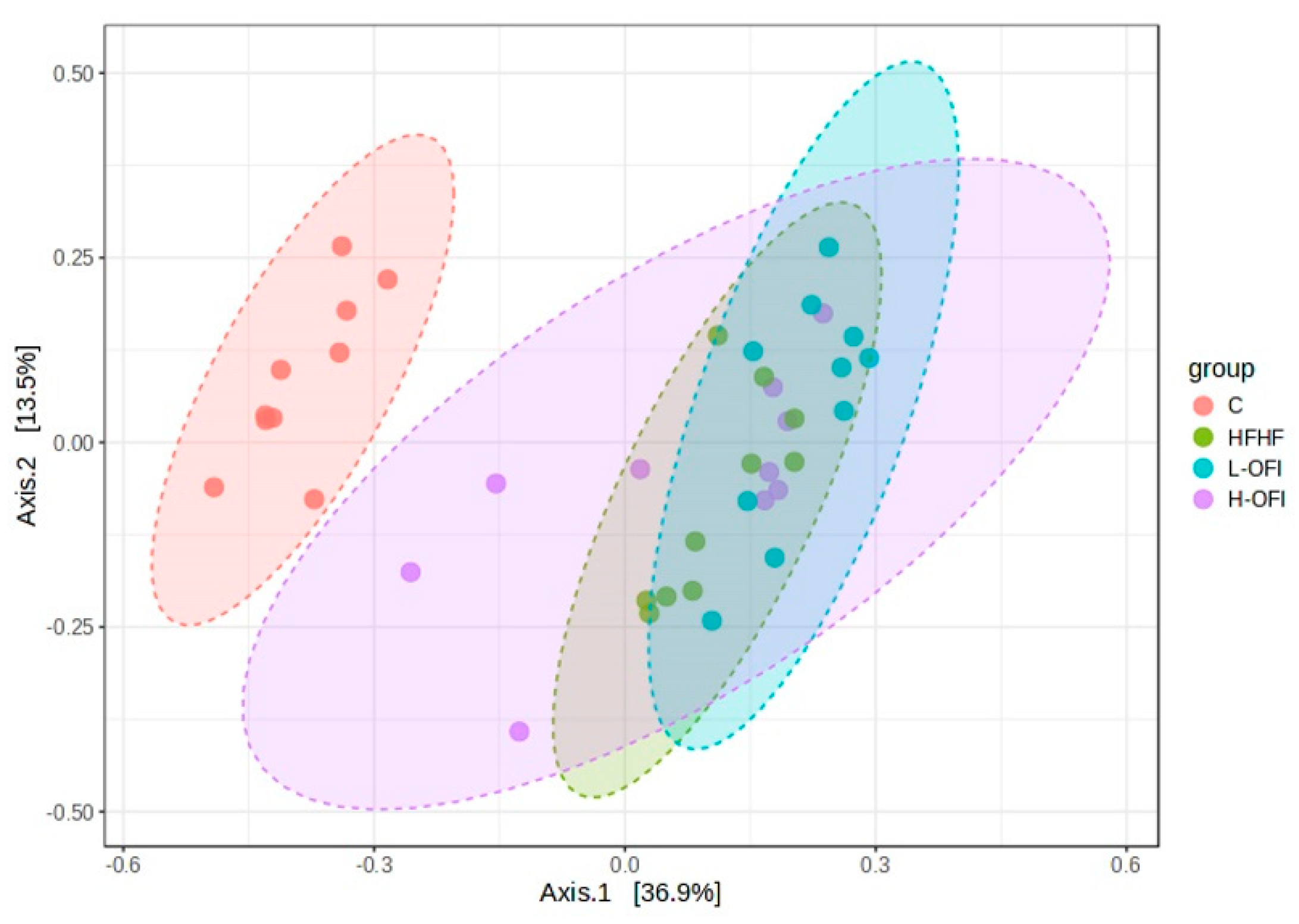
3.2. Microbiota Composition
3.3. Fecal Short-Chain Fatty Acid (SCFA) Content
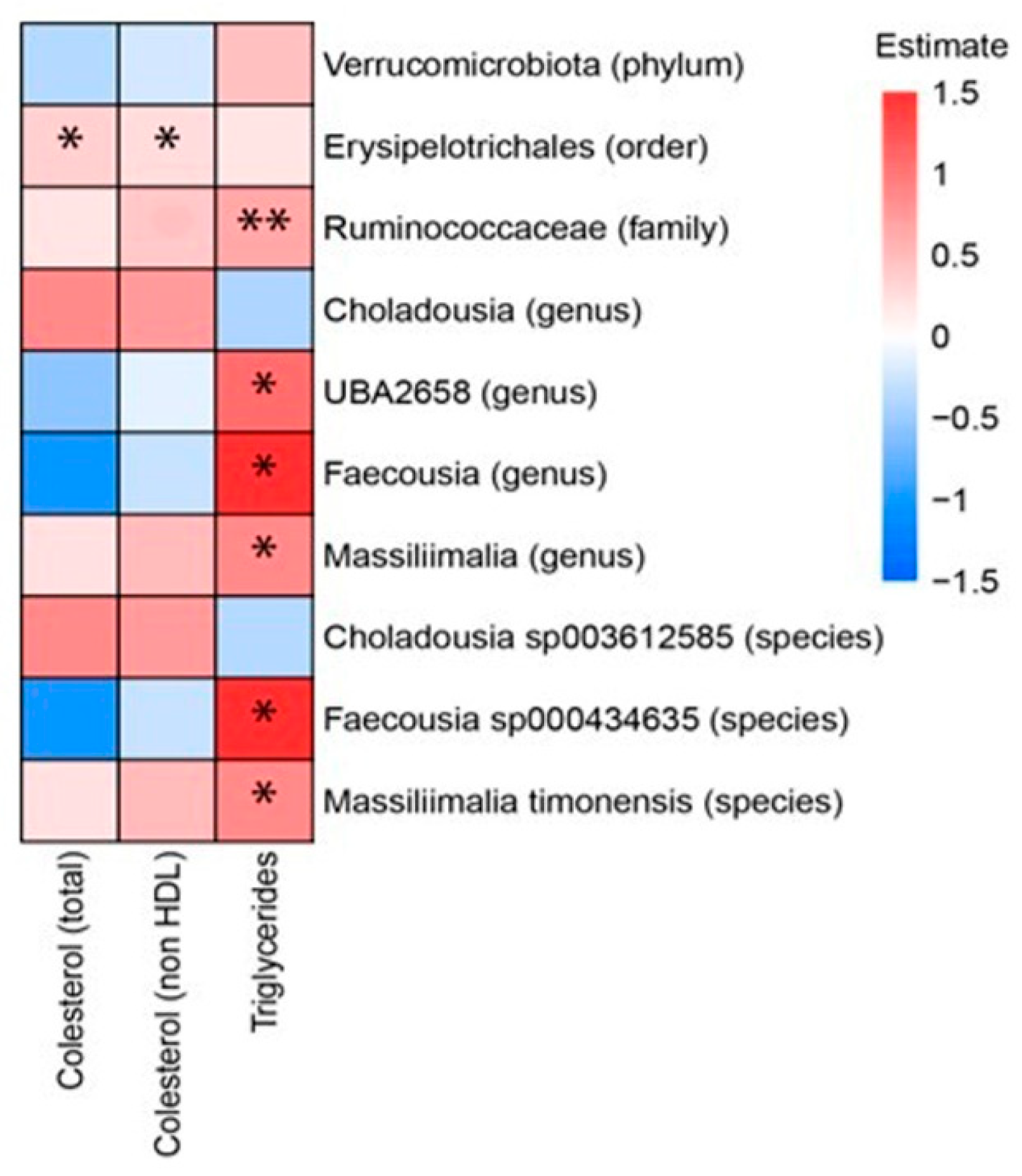
3.4. Correlations Between Microbiota and General Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H. World Obesity Atlas 2023 Report; World Obesity Federation: London, UK, 2023. [Google Scholar]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.; Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 2021, 32, 500–514. [Google Scholar] [CrossRef]
- Golshany, H.; Helmy, S.A.; Morsy, N.F.S.; Kamal, A.; Yu, Q.; Fan, L. The gut microbiome across the lifespan: How diet modulates our microbial ecosystem from infancy to the elderly. Int. J. Food Sci. Nutr. 2025, 76, 95–121. [Google Scholar] [CrossRef]
- Stefano, J.T.; Duarte, S.M.B.; Ribeiro Leite Altikes, R.G.; Oliveira, C.P. Non-pharmacological management options for MAFLD: A practical guide. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231160394. [Google Scholar] [CrossRef] [PubMed]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; Elattar, G.M.; ElRay, A.A.; Eltalkawy, M.D. Oral microbiota and liver diseases. Clin. Nutr. ESPEN 2023, 54, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Lang, S.; Martin, A.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.J.G.T.; Krawczyk, M.; Nowag, A.; Scholz, C.J.; Kretzschmar, A.; et al. Phenotyping non-alcoholic fatty liver disease by the gut microbiota: Ready for prime time? J. Gastroenterol. Hepatol. 2020, 35, 1969–1977. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Niederreiter, L.; Adolph, T.E.; Tilg, H. Food, microbiome and colorectal cancer. Dig. Liver Dis. 2018, 50, 647–652. [Google Scholar] [CrossRef]
- Lavallee, C.M.; Bruno, A.; Ma, C.; Raman, M. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: A Narrative Review. Nutrients 2022, 14, 4655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Do, T.; Marshall, L.J.; Boesch, C. Effect of two-week red beetroot juice consumption on modulation of gut microbiota in healthy human volunteers—A pilot study. Food Chem. 2023, 406, 134989. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Gomez-Garcia, I.; Fernandez-Quintela, A.; Gonzalez, M.; Gomez-Zorita, S.; Muguerza, B.; Trepiana, J.; Portillo, M.P. Usefulness of Opuntia spp. on the Management of Obesity and Its Metabolic Co-Morbidities. Nutrients 2024, 16, 1282. [Google Scholar] [CrossRef]
- Besne-Eseverri, I.; Trepiana, J.; Gomez-Zorita, S.; Antunes-Ricardo, M.; Cano, M.P.; Portillo, M.P. Beneficial Effects of Opuntia spp. on Liver Health. Antioxidants 2023, 12, 1174. [Google Scholar] [CrossRef]
- Rodrigues, C.; Paula, C.D.d.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Besne-Eseverri, I.; Trepiana, J.; Eseberri, I.; Gomez-Maqueo, A.; Cano, M.P.; Tome-Carneiro, J.; Davalos, A.; Portillo, M.P. Anti-steatotic effect of Opuntia ficus-indica extracts rich in betalains and phenolics from fruit peel and pulp of different varieties in in vitro models. J. Physiol. Biochem. 2025. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delgado, G.L.; Rodríguez-Rodríguez, E.M.; Ríos, D.; Cano, M.P.; Lobo, M.G. Morphological Characterization of Opuntia Accessions from Tenerife (Canary Islands, Spain) Using UPOV Descriptors. Horticulturae 2024, 10, 662. [Google Scholar] [CrossRef]
- Gomez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Marcos-Zambrano, L.J.; Gomez-Zorita, S.; Fernandez-Quintela, A.; Carrillo de Santa Pau, E.; Martinez, J.A.; Portillo, M.P. Gut Microbiota Induced by Pterostilbene and Resveratrol in High-Fat-High-Fructose Fed Rats: Putative Role in Steatohepatitis Onset. Nutrients 2021, 13, 1738. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. Clin. Exp. 2024, 100, 100737. [Google Scholar] [CrossRef] [PubMed]
- Besne-Eseverri, I.; Martin, M.A.; Lobo, G.; Cano, M.P.; Portillo, M.P.; Trepiana, J. Antioxidant and Anti-Inflammatory Effects of Opuntia Extracts on a Model of Diet-Induced Steatosis. Antioxidants 2024, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Lindor, K.D.; Angulo, P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 1925–1931. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarlata, G.G.M.; Scarpellini, E.; Boccuto, L.; Spagnuolo, R.; Tilocca, B.; Roncada, P.; Luzza, F. Metabolic-Dysfunction-Associated Fatty Liver Disease and Gut Microbiota: From Fatty Liver to Dysmetabolic Syndrome. Medicina 2023, 59, 594. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Aguirre, L.; Milton-Laskibar, I.; Fernandez-Quintela, A.; Trepiana, J.; Kajarabille, N.; Mosqueda-Solis, A.; Gonzalez, M.; Portillo, M.P. Relationship between Changes in Microbiota and Liver Steatosis Induced by High-Fat Feeding-A Review of Rodent Models. Nutrients 2019, 11, 2156. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Zhou, Z.; Gao, T.; Zhao, R.; Chen, L. Polysaccharides from Lactarius volemus Fr. ameliorate high-fat and high-fructose diet induced metabolic disorders and intestinal barrier dysfunction. Int. J. Biol. Macromol. 2025, 287, 138341. [Google Scholar] [CrossRef] [PubMed]
- Milton-Laskibar, I.; Marcos-Zambrano, L.J.; Gomez-Zorita, S.; Carrillo de Santa Pau, E.; Fernandez-Quintela, A.; Martinez, J.A.; Portillo, M.P. Involvement of microbiota and short-chain fatty acids on non-alcoholic steatohepatitis when induced by feeding a hypercaloric diet rich in saturated fat and fructose. Gut Microbiome 2022, 3, e5. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Bai, Y.; Zhi, F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem. Biophys. Res. Commun. 2019, 509, 767–772. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Wan, Y.; Li, D. High-fat, low-carbohydrate diet was associated with unfavourable impact on colonic luminal microenvironment. Gut 2020, 69, 1717. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.; Chen, X.; Wang, J.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal. Transduct Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Liu, X.; Jin, M.; Yang, X.; Hu, X.; Li, C.; Zhao, K. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food Funct. 2020, 11, 6971–6986. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum Involves in the Modulation of Intestinal ANGPTLT4 Expression in Mice Treated by High-Fat Diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Himbert, C.; Stephens, W.Z.; Gigic, B.; Hardikar, S.; Holowatyj, A.N.; Lin, T.; Ose, J.; Swanson, E.; Ashworth, A.; Warby, C.A.; et al. Differences in the gut microbiome by physical activity and BMI among colorectal cancer patients. Am. J. Cancer Res. 2022, 12, 4789–4801. [Google Scholar]
- Onate, F.P.; Chamignon, C.; Burz, S.D.; Lapaque, N.; Monnoye, M.; Philippe, C.; Bredel, M.; Chene, L.; Farin, W.; Paillarse, J.; et al. Adlercreutzia equolifaciens Is an Anti-Inflammatory Commensal Bacterium with Decreased Abundance in Gut Microbiota of Patients with Metabolic Liver Disease. Int. J. Mol. Sci. 2023, 24, 12232. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandona, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, Q.; Xu, H.; Liu, H.; Tan, B.; Deng, H.; Chen, Y.; Wang, R.; Tang, F.; Cheng, X.; et al. Alterations in the gut microbiota community are associated with childhood obesity and precocious puberty. BMC Microbiol. 2024, 24, 311–318. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Abdugheni, R.; Yu, F.; Zhao, Y.; Ma, B.; Yang, Z.; Li, R.; Li, Y.; Maimaitiyiming, Y.; et al. Gut microbiota changes associated with low-carbohydrate diet intervention for obesity. Open Life. Sci. 2024, 19, 20220803. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Bukhari, I.; Zhao, Y.; Huang, H.; Yu, Y.; Sun, X.; Mi, Y.; Mei, L.; Zheng, P. Effects of cholesterol-lowering probiotics on non-alcoholic fatty liver disease in FXR gene knockout mice. Front. Nutr. 2023, 10, 1121203. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Jin, R.; Peng, Y.; Chai, J.; Wei, X.; Zhao, Y.; Deng, F.; Zhao, J.; Li, Y. Exploring the Intestinal Microbial Community of Lantang Pigs through Metagenome-Assembled Genomes and Carbohydrate Degradation Genes. Fermentation 2024, 10, 207. [Google Scholar] [CrossRef]
- Afouda, P.; Traore, S.I.; Dione, N.; Andrieu, C.; Tomei, E.; Richez, M.; Di Pinto, F.; Lagier, J.; Dubourg, G.; Raoult, D.; et al. Description and genomic characterization of Massiliimalia massiliensis gen. nov., sp. nov., and Massiliimalia timonensis gen. nov., sp. nov., two new members of the family Ruminococcaceae isolated from the human gut. Antonie Van Leeuwenhoek 2019, 112, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cheng, J.; Jia, W.; Xu, Y. Akkermansia muciniphila Ameliorates Alcoholic Liver Disease in Experimental Mice by Regulating Serum Metabolism and Improving Gut Dysbiosis. Metabolites 2023, 13, 1057. [Google Scholar] [CrossRef]
- Sanchez-Tapia, M.; Aguilar-Lopez, M.; Perez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef] [PubMed]
- Mellai, M.; Allesina, M.; Edoardo, B.; Cascella, F.; Nobile, V.; Spina, A.; Amone, F.; Zaccaria, V.; Insolia, V.; Perri, A.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial: Efficacy of Opuntia ficus-indica Prebiotic Supplementation in Subjects with Gut Dysbiosis. Nutrients 2024, 16, 586. [Google Scholar] [CrossRef]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernandez-Quiroz, F.; Martinez-Castro, N.; Velez-Ixta, J.M.; Guajardo-Lopez, D.; Garcia-Mena, J.; Hernandez-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Amato, A.; Calvi, P.; Giardina, M.; Nuzzo, D.; Picone, P.; Palumbo-Piccionello, A.; Amata, S.; Giardina, I.C.; Massaro, A.; et al. Positive impact of indicaxanthin from Opuntia ficus-indica fruit on high-fat diet-induced neuronal damage and gut microbiota dysbiosis. Neural Regen. Res. 2026, 21, 324–332. [Google Scholar] [CrossRef]
- Liao, H.; Zhao, Y.; Liang, Y.; Zou, K. Flavonoids Derived from Opuntia ficus-indica Fruit Alleviate Renal Injury in Diabetic Nephropathy Mice by Altering Gut Microbiota and Promoting the Production of SCFAs. Nutrients 2025, 17, 1800. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, H.; Shao, S.; Zhao, L.; Zhang, R.; Zhang, S. Anthocyanins from Opuntia ficus-indica Modulate Gut Microbiota Composition and Improve Short-Chain Fatty Acid Production. Biology 2022, 11, 1505. [Google Scholar] [CrossRef]
- Cho, S.; Jung, U.J.; Choi, M. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]

| Compound | Content (µg of Compound/g Dry Weight) |
|---|---|
| Piscidic acid | 2564 ± 108 |
| Indicaxanthin (Bx-proline) | 510 ± 14 |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside (IG2) | 30.4 ± 3.9 |
| Portulacaxanthin III (Bx-glycine) | 30.0 ± 1.6 |
| Vulgaxanthin II (Bx-glutamic acid) | 18.0 ± 2.9 |
| Vulgaxanthin III (Bx-asparagine) | 14.6 ± 0.9 |
| Quercetin glycoside 2 (QG2) | 14.4 ± 3.3 |
| Vulgaxanthin I (Bx-glutamine) | 12.4 ± 0.4 |
| Betanin | traces |
| C | HFHF | |
|---|---|---|
| Total energy (kcal/g) | 3.9 | 4.5 |
| Carbohydrates (energy%) | 63.9 | 40 |
| Fructose (energy%) | - | 10 |
| Proteins (energy%) | 20.3 | 20 |
| Lipids (energy%) | 15.8 | 40 |
| Fiber (amount%) | 8.6 | 6.5 |
| C | HFHF | L-OFI | H-OFI | ANOVA | |
|---|---|---|---|---|---|
| Total body weight (g) | 425.0± 7.4 b | 473.5 ± 10.6 a | 467.0 ± 9.0 a | 469.0 ± 11.0 a | p < 0.05 |
| Visceral WAT weight (g) | 30.8 ± 2.8 c | 43.7 ± 1.4 a | 38.7 ± 2.0 b | 40.7 ± 4.1 ab | p < 0.05 |
| Subcutaneous WAT weight (g) | 13.4 ± 0.7 c | 19.0 ± 0.9 a | 15.5 ± 1.3 bc | 19.9 ± 2.3 ab | p < 0.05 |
| Liver weight (g) | 10.7 ± 0.3 b | 22.0 ± 0.9 a | 20.6 ± 1.0 a | 21.0 ± 1.1 a | p < 0.05 |
| C | HFHF | L-OFI | H-OFI | ANOVA | |
|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 100.4 ± 2.1 a | 93.7 ± 5.1 a | 30.1 ± 3.4 b | 24.2 ± 2.1 b | p < 0.001 |
| Total cholesterol (mg/dL) | 63.6 ± 5.4 b | 123 ± 4.3 a | 132 ± 1.6 a | 77.3 ± 5.3 b | p < 0.001 |
| HDL cholesterol | 13.5 ± 0.7 b | 13.1 ± 0.4 b | 18.3 ± 1.7 a | 22.5 ± 2.0 a | p < 0.05 |
| Non-HDL cholesterol (mg/dL) | 50.1 ± 5.5 b | 109.4 ± 4.6 a | 114.4 ± 2.1 a | 54.2 ± 6.4 b | p < 0.001 |
| ALT (U/L) | 14.1 ± 0.7 b | 100.1 ± 14.2 a | 103.4 ± 13.5 a | 97.0 ± 15.1 a | p < 0.05 |
| AST (U/L) | 75.8 ± 3.7 b | 118.2 ± 10.8 a | 112.5 ± 3.7 a | 93.4 ± 7.0 c | p < 0.05 |
| Log2FC | St. Error | p-Value | FDR | |
|---|---|---|---|---|
| SPECIES | ||||
| Choladousia sp003612585 | 2.35 | 0.62 | 0.000353 | 0.00267 |
| Cutibacterium acnes | 2.58 | 0.836 | 0.00309 | 0.0173 |
| Adlercreutzia muris | 2.14 | 0.797 | 0.00955 | 0.0448 |
| Massiliimalia timonensis | −1.9 | 0.715 | 0.01 | 0.0458 |
| Akkermansia_muciniphila_D_776786 | −2.31 | 0.89 | 0.0117 | 0.0526 |
| Faecousia_sp000434635 | −4.15 | 1.65 | 0.0148 | 0.0622 |
| GENUS | ||||
| g__UBA2658 | −3.88 | 0.861 | 0.0000314 | 0.000356 |
| g__Choladousia | 2.35 | 0.62 | 0.000353 | 0.00284 |
| g__Cutibacterium | 2.58 | 0.836 | 0.00309 | 0.0174 |
| g__QWKK01 | 2.35 | 0.822 | 0.00591 | 0.0298 |
| g__Eubacterium_R | −5.4 | 1.92 | 0.00683 | 0.0341 |
| g__Adlercreutzia_404257 | 2.22 | 0.804 | 0.00767 | 0.0375 |
| g__Massiliimalia_59888 | −1.9 | 0.715 | 0.01 | 0.0455 |
| g__Evtepia | −3.04 | 1.15 | 0.0102 | 0.0461 |
| g__Akkermansia | −2.31 | 0.89 | 0.0117 | 0.0521 |
| g__Adlercreutzia_404199 | 2.05 | 0.79 | 0.0121 | 0.053 |
| g__UBA1367 | 2.13 | 0.842 | 0.014 | 0.0601 |
| g__Faecousia | −4.15 | 1.65 | 0.0148 | 0.0622 |
| g__Anaerofilum_74150 | 2 | 0.835 | 0.0199 | 0.0778 |
| FAMILY | ||||
| f__Propionibacteriaceae | 2.58 | 0.836 | 0.00309 | 0.0178 |
| f__Eggerthellaceae | 1.97 | 0.671 | 0.00474 | 0.0252 |
| f__Akkermansiaceae | −2.31 | 0.89 | 0.0117 | 0.0555 |
| f__Atopobiaceae | 2.13 | 0.842 | 0.014 | 0.0642 |
| f__Oscillospiraceae_88309 | −2.55 | 1.01 | 0.014 | 0.0642 |
| ORDER | ||||
| o__Propionibacteriales | 2.58 | 0.836 | 0.00309 | 0.0173 |
| o__Coriobacteriales | 1.96 | 0.656 | 0.00413 | 0.0211 |
| o__Verrucomicrobiales | −2.31 | 0.89 | 0.0117 | 0.0523 |
| o__Oscillospirales | −2.48 | 0.963 | 0.0125 | 0.0546 |
| CLASS | ||||
| c__Actinomycetia | 3 | 0.955 | 0.00264 | 0.0136 |
| c__Coriobacteriia | 1.96 | 0.656 | 0.00413 | 0.0186 |
| c__Verrucomicrobiae | −2.31 | 0.89 | 0.0117 | 0.0444 |
| PHYLUM | ||||
| p__Actinobacteriota | 2.01 | 0.624 | 0.00211 | 0.0127 |
| p__Verrucomicrobiota | −2.31 | 0.89 | 0.0117 | 0.0633 |
| Log2FC | St. Error | p-Value | FDR | |
|---|---|---|---|---|
| SPECIES | ||||
| Massiliimalia timonensis | −2.63 | 0.696 | 0.000371 | 0.00352 |
| Bittarella massiliensis | −2.8 | 0.762 | 0.000509 | 0.00462 |
| Adlercreutzia caecicola | 1.16 | 0.393 | 0.00448 | 0.0295 |
| Paramuribaculum intestinale | 1.35 | 0.473 | 0.00588 | 0.0345 |
| Ventrisoma faecale | −1.83 | 0.776 | 0.0216 | 0.0967 |
| GENUS | ||||
| g__Massiliimalia_59888 | −2.63 | 0.696 | 0.000371 | 0.0041 |
| g__Bittarella | −2.8 | 0.762 | 0.000509 | 0.00532 |
| g__Adlercreutzia_404218 | 1.16 | 0.393 | 0.00448 | 0.0287 |
| g__Paramuribaculum | 1.35 | 0.473 | 0.00588 | 0.0344 |
| g__UBA2658 | −2.27 | 0.838 | 0.0089 | 0.0478 |
| g__Muricomes_149725 | −1.88 | 0.706 | 0.00997 | 0.0506 |
| g__Ventrisoma | −1.83 | 0.776 | 0.0216 | 0.0921 |
| FAMILY | ||||
| f__Ruminococcaceae | −1.98 | 0.728 | 0.00873 | 0.0568 |
| SCFA (µmol SCFA/g Feces) | HFHF | L-OFI | H-OFI | ANOVA |
|---|---|---|---|---|
| Acetic acid | 12.03 ± 1.59 a | 11.87 ± 1.40 a | 9.20 ± 0.63 a | NS |
| Propionic acid | 1.69 ± 0.34 a | 1.51 ± 0.27 a | 1.99 ± 0.28 a | NS |
| Isobutyric acid | 0.42 ± 0.04 a | 0.36 ± 0.02 a | 0.44 ± 0.04 a | NS |
| Butyric acid | 1.01 ± 0.13 a | 0.82 ± 0.17 a | 1.01 ± 0.11 a | NS |
| Isovaleric acid | 0.50 ± 0.03 a | 0.54 ± 0.03 a | 0.49 ± 0.04 a | NS |
| Valeric acid | 0.55 ± 0.12 a | 0.30 ± 0.05 a | 0.43 ± 0.10 a | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-García, I.; Besné-Eseverri, I.; Portillo, M.P.; Fernández-Quintela, A.; Díaz, L.E.; Riezu-Boj, J.I.; Milagro, F.I.; Trepiana, J. Oral Administration of an Opuntia ficus-indica Fruit Extract Induces Changes in Gut Microbiota Composition: Relationship with Its Anti-Obesity and Anti-Steatotic Effects in Rats Fed a High-Fat High-Fructose Diet. Foods 2025, 14, 2891. https://doi.org/10.3390/foods14162891
Gómez-García I, Besné-Eseverri I, Portillo MP, Fernández-Quintela A, Díaz LE, Riezu-Boj JI, Milagro FI, Trepiana J. Oral Administration of an Opuntia ficus-indica Fruit Extract Induces Changes in Gut Microbiota Composition: Relationship with Its Anti-Obesity and Anti-Steatotic Effects in Rats Fed a High-Fat High-Fructose Diet. Foods. 2025; 14(16):2891. https://doi.org/10.3390/foods14162891
Chicago/Turabian StyleGómez-García, Iker, Irene Besné-Eseverri, Maria P. Portillo, Alfredo Fernández-Quintela, Ligia Esperanza Díaz, Jose I. Riezu-Boj, Fermín I. Milagro, and Jenifer Trepiana. 2025. "Oral Administration of an Opuntia ficus-indica Fruit Extract Induces Changes in Gut Microbiota Composition: Relationship with Its Anti-Obesity and Anti-Steatotic Effects in Rats Fed a High-Fat High-Fructose Diet" Foods 14, no. 16: 2891. https://doi.org/10.3390/foods14162891
APA StyleGómez-García, I., Besné-Eseverri, I., Portillo, M. P., Fernández-Quintela, A., Díaz, L. E., Riezu-Boj, J. I., Milagro, F. I., & Trepiana, J. (2025). Oral Administration of an Opuntia ficus-indica Fruit Extract Induces Changes in Gut Microbiota Composition: Relationship with Its Anti-Obesity and Anti-Steatotic Effects in Rats Fed a High-Fat High-Fructose Diet. Foods, 14(16), 2891. https://doi.org/10.3390/foods14162891







