Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides
Abstract
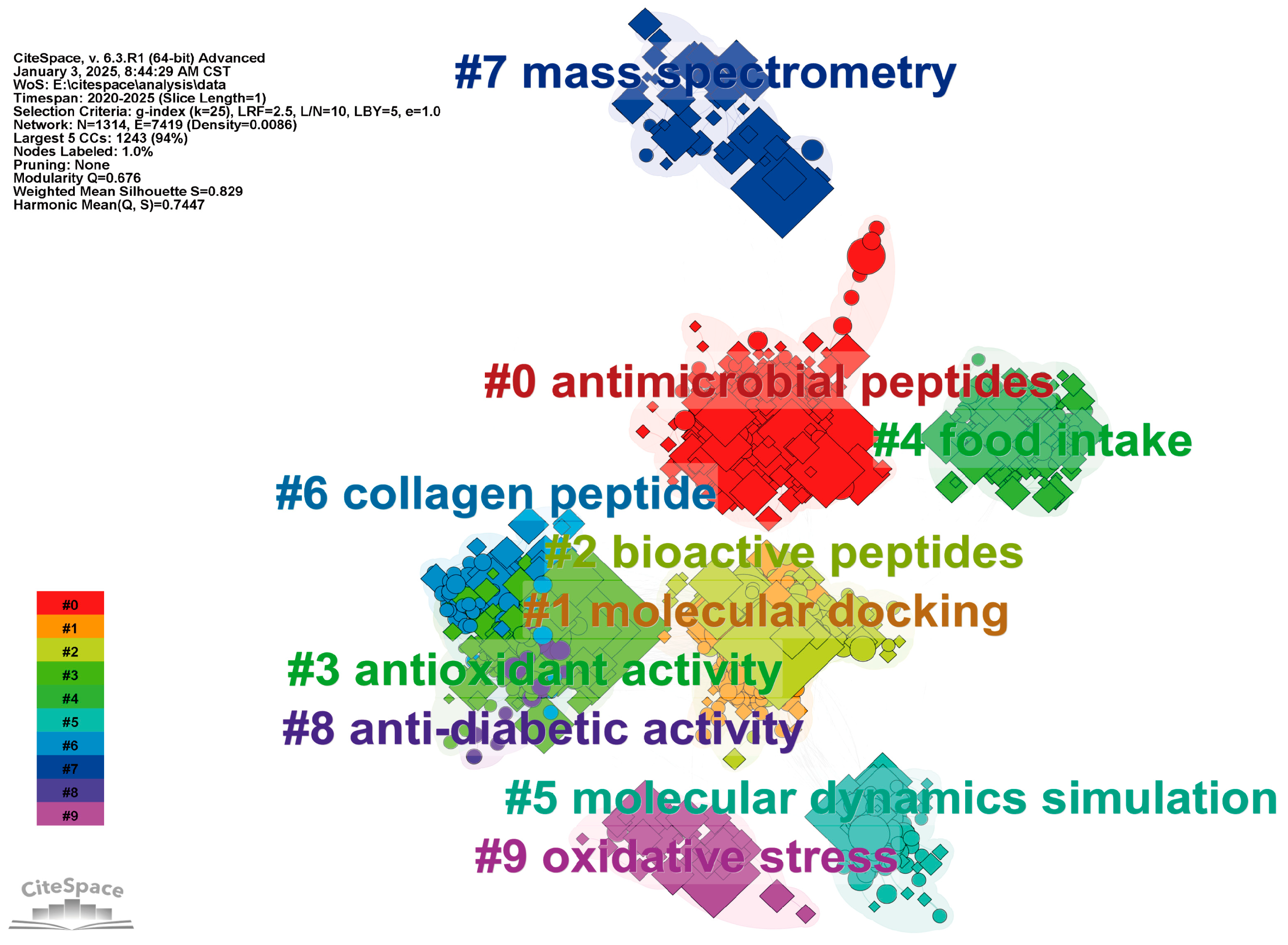
1. Introduction
2. Research Progress
3. Main Source and Function of Bioactive Peptides
3.1. Animal Protein
3.1.1. Aquatic Food
3.1.2. Terrestrial Food
3.2. Plant Protein
4. Receptor Protein
4.1. Keap 1
4.2. Toll-like Receptor 4 (TLR4)
4.3. 5-Hydroxytryptamine (5-HT) Receptor
4.4. Angiotensin I-Converting Enzyme (ACE)
4.5. Tyrosinase
4.6. Xanthine Oxidoreductase (XOR)
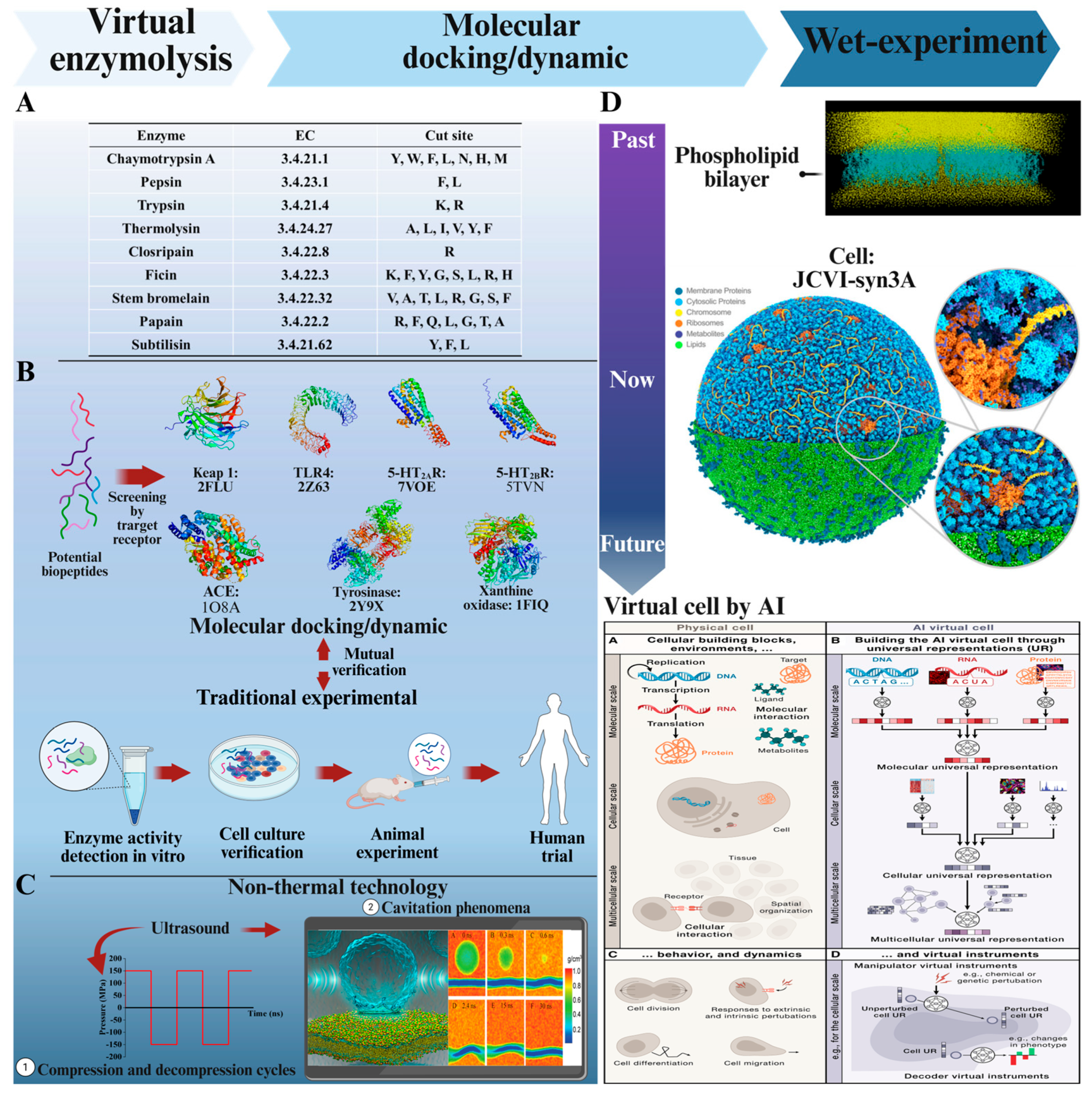
5. Computer-Based Screening of Active Peptides
5.1. Virtual Enzymolysis
5.2. Molecular Docking/Dynamics Simulation Combined with Wet-Lab Experiments
| Protein Sources | Peptides Sequence | Bioactive | Molecular Simulation | Wet-Lab Experiment | References |
|---|---|---|---|---|---|
| Porcine visceral proteins | 3–6 amino acid residue | Prevent xanthine oxidase (XO)-mediated hyperuricemia | Docking receptor: XO (PDB ID: 3NVY) | XO inhibitory activity in vitro | [36] |
| Rapeseed proteins | FQW, FRW, and CPF | angiotensin I-converting enzyme (ACE) inhibitory | Docking receptor: ACE (PDB: 1O8A) | ACE inhibitory activity in vitro | [57] |
| Porcine bone collagen | GPGPM, RGPPGPM, PSGGF, VGGF, FSGL, PGSPGPGPR, AGPGPM and GPTGF | Anti-inflammatory | Docking receptor: calcium-sensing receptor (PDB: 7DD7) | (1) Scavenge 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) radical ability (2) Fe2+ chelating activity (3) Albumin inhibitory activity (4) NO inhibitory activity (5) Cytotoxicity test (6) Determination of inflammatory cytokines | [87] |
| Porcine offals (heart, liver and lung) and one muscle the Longissimus Dorsi | \ | ACE inhibitory | Virtual enzymatic hydrolysis by BIOPEP (proteinase K, papain, subtilisin, bromelain, and ficin) | (1) ACE inhibitory activity in vitro (2) Determination of oxygen radical absorbance capacity (3) Determination of the inhibitory activity of dipeptidyl peptidase 4 | [35] |
| Swim bladders of monkfish (Lophius litulon) | SEGPK, FDGPY and SPGPW | ACE inhibitory | Docking receptor: ACE | (1) ACE inhibitory activity in vitro (2) Effects on human umbilical vein endothelial cells | [38] |
| Bovine milk proteins: α-lactalbumin (P00711), β-lactoglobulin (P02754), β-casein (P02666), α-S1-casein (P02662), α-S2-casein (P02663), κ-casein (P02668), lactoferrin (P24627) | DGG, DGGM | ACE and XO inhibitory | (1) Virtual enzymatic hydrolysis by ExPASy Peptide Cutter (pepsin, trypsin, and chymotrypsin) (2) Docking receptors: ACE (PDB ID: 1O8A) and XO (PDB ID: 1FIQ) (3) Molecular dynamics simulation | (1) Determination of antioxidant activities (2) 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (3) ABTS radical scavenging activity (4) Hydroxyl radical scavenging activity (5) ACE inhibitory activity in vitro (6) XO inhibitory activity in vitro | [72] |
| Pacific white shrimp and swimming crab | 17 novel XO inhibitory peptides (AEAQMWR exhibited the greatest XO inhibitory activity in vitro) | Anti-hyperuricemic activity | Docking receptor: XO (PDB ID: 1N5X) | (1) Assessment of XO inhibitory activity of sample in vitro (2) HK-2 cells experiment (3) Analysis of inflammation levels (IL-6, IL-1β, and TNF-α) | [28] |
| Hemp (Cannabis sativa L.) protein | DDNPRRF, SRRFHLA, RNIFKGF, VREPVFSF, QADIFNPR and SAERGFLY | Immunomodulatory activity | (1) Docking receptor: TLR4/MD2 complex (PDB: 3VQ2) (2) In silico analysis (Peptide Ranker, AnOxPePred 1.0, PreAIP, PlifePred, HLP) | (1) CACO-2 cells viability assay (2) Analysis of inflammation levels (TNFα, IL-1β, IL-4, IL-6, IL-10, and TLR4) | [65] |
| Bovine hemoglobin | ARRF and ARNF | Antioxidant and anti-inflammatory | (1) Virtual enzymatic hydrolysis by ExPASy Peptide Cutter (trypsin, pepsin, papain) (2) Prediction of peptides (PeptideRanker, ToxinPred, INNOVAGEN, A11erTOP) (3) Docking receptors: TLR4 (PDB ID: 2Z63) and Keap1 (PDB ID: 2FLU) (4) Molecular dynamic simulation of peptides and receptors | (1) RAW264.7 cells experiment (2) Determination of the contents of NO, TNF-α, IL-6, and PGE2 (3) Etermination of ROS content, SOD, MDA, and GSH-Px levels | [63] |
| Hairtail (Trichiurus japonicus) | VVFEVFW | Antidepressant | (1) Docking receptor: monoamine oxidase A (MAO-A) (PBD ID: 2Z5X) | (1) MAO-A inhibition assay | [22] |
| Egg white proteins including ovalbumin, ovotransferrin, and ovomucoid | GDVA and DEK | Tyrosinase inhibitory | (1) Virtual enzymatic hydrolysis by ExPASy Peptide Cutter (chymotrypsin, trypsin, pepsin) (2) Prediction of peptides (peptide property calculator, ToxinPred) (3) Docking receptor: tyrosinase (PDB ID: 2Y9X) (4) Molecular dynamic simulation of peptides and receptors | (1) Tyrosinase inhibitory activity in vitro | [73] |
| Oyster protein | ALSGSW, GGYGIF, and MAIGLW | XO inhibitory | (1) Docking receptor: XOD complex (PDB ID: 1N5X) | (1) Determination of inhibitory activity of XO | [75] |
| Kuruma shrimp (Marsupenaeus japonicus) heads | ARL/I (ACE inhibitory) | ACE inhibitory | (1) Docking receptor: ACE (PDB ID: 1O86) | (1) ACE inhibitory activity (2) Determination of ACE inhibition pattern (3) Hemolytic activity assay | [26] |
5.3. Virtual Organelles/Cells Used for Screening Active Peptides
6. Computer-Based Experimental Design for Bioactive Peptide Preparation
6.1. Application of Non-Thermal Processing Techniques in the Preparation of Active Polypeptides
6.2. Non-Thermal Processing Techniques Constructive in MD Simulation
7. Artificial Intelligence Promotes Virtual Screening of Bioactive Peptides
7.1. Simulation and Algorithm Improvement
7.2. Protein Structure Prediction
7.3. Mining of Active Peptides
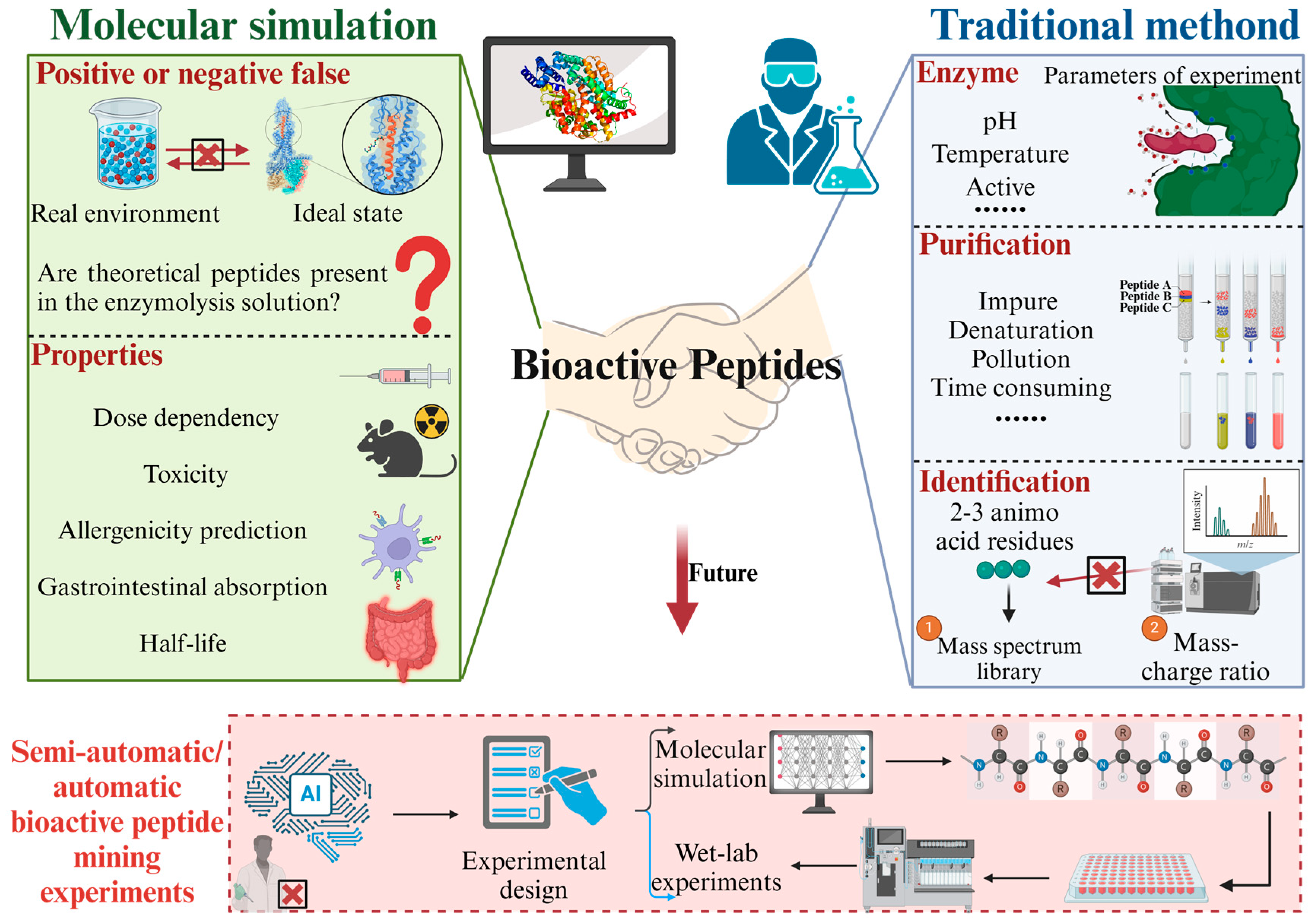
8. Comparison Between Molecular Simulation and Traditional Method of Bioactive Peptide Mining
8.1. Drawback of Traditional Mining Methods of Bioactive Peptides
8.1.1. Types of Enzymes
8.1.2. Purification of Protein and Bioactive Peptides
8.1.3. Limitations of Mass Spectrometry
8.2. Drawback of Computer Mining Methods of Bioactive Peptides
8.2.1. False Positives or Negatives
8.2.2. Limitations in the Study of Bioactive Peptide Properties
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, Z.J.; Jiang, H.; Sun, J.A.; Zhao, Y.H.; Gao, X.; Mao, X.Z. Research progress in the preparation and structure-activity relationship of bioactive peptides derived from aquatic foods. Trends Food Sci. Technol. 2024, 147, 104443. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Bunne, C.; Roohani, Y.; Rosen, Y.; Gupta, A.; Zhang, X.; Roed, M.; Alexandrov, T.; AlQuraishi, M.; Brennan, P.; Burkhardt, D.B.; et al. How to build the virtual cell with artificial intelligence: Priorities and opportunities. Cell 2024, 187, 7045–7063. [Google Scholar] [CrossRef]
- Bai, G.P.; Pan, Y.L.; Zhang, Y.M.; Li, Y.; Wang, J.P.; Wang, Y.; Teng, W.D.; Jin, G.F.; Geng, F.; Cao, J.X. Research advances of molecular docking and molecular dynamic simulation in recognizing interaction between muscle proteins and exogenous additives. Food Chem. 2023, 429, 136836. [Google Scholar] [CrossRef]
- Jin, Z.; Wei, Z. Molecular simulation for food protein–ligand interactions: A comprehensive review on principles, current applications, and emerging trends. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13280. [Google Scholar] [CrossRef]
- Chang, J.; Wang, H.; Su, W.; He, X.; Tan, M. Artificial intelligence in food bioactive peptides screening: Recent advances and future prospects. Trends Food Sci. Technol. 2025, 156, 104845. [Google Scholar] [CrossRef]
- Torres, M.D.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Hou, J.H.; Yang, X.C.; Chen, C.M. Emerging trends and new developments in information science: A document co-citation analysis (2009–2016). Scientometrics 2018, 115, 869–892. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Cao, J.C.; Franco, O.L.; Lu, T.K.; de la Fuente-Nunez, C. Synthetic Biology and Computer-Based Frameworks for Antimicrobial Peptide Discovery. Acs Nano 2021, 15, 2143–2164. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Behzadipour, Y.; Haddad, M. Decoding the proteome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for cell-penetrating peptides involved in pathogenesis or applicable as drug delivery vectors. Infect. Genet. Evol. 2020, 85, 104474. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef]
- Pierre, J.F.; Peters, B.M.; La Torre, D.; Sidebottom, A.M.; Tao, Y.; Zhu, X.; Cham, C.M.; Wang, L.; Kambal, A.; Harris, K.G.; et al. Peptide YY: A Paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 2023, 381, 502–508. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.H.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Gong, L.M.; Zhao, H.M.; Liu, Y.H.; Wu, H.; Liu, C.; Chang, S.Y.; Chen, L.Q.; Jin, M.J.; Wang, Q.M.; Gao, Z.G.; et al. Research advances in peptide-drug conjugates. Acta Pharm. Sin. B 2023, 13, 3659–3677. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef]
- Mao, S.; Jiang, J.; Xiong, K.; Chen, Y.; Yao, Y.; Liu, L.; Liu, H.; Li, X. Enzyme Engineering: Performance Optimization, Novel Sources, and Applications in the Food Industry. Foods 2024, 13, 3846. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.L.; Nargotra, P.; Sun, P.P.; Chen, C.W.; Dong, C.D. A novel deep eutectic solvent-based green extraction and purification of DPP-IV inhibitory peptides from tilapia (Oreochromis niloticus) viscera hydrolysate. Food Biosci. 2024, 61, 104658. [Google Scholar] [CrossRef]
- Yang, X.X.; Wang, K.; Liu, Q.; Zhang, X.W. Discovery of monoamine oxidase A inhibitory peptides from hairtail (Trichiurus japonicus) using in vitro simulated gastrointestinal digestion and in silico studies. Bioorganic Chem. 2020, 101, 104032. [Google Scholar] [CrossRef]
- Asmild, M.; Hukom, V.; Nielsen, R.; Nielsen, M. Is economies of scale driving the development in shrimp farming from Penaeus monodon to Litopenaeus vannamei? The case of Indonesia. Aquaculture 2024, 579, 740178. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, J.M.J.; Anaya-Esparza, L.M.; Martínez-Montaño, E.; Montalvo-González, E.; García-Magaña, M.L. Bioactive peptides from by-products of shrimp processing: A review. Int. Food Res. J. 2024, 31, 530–550. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.S.; Marshall, M.; Raghavan, S.; Kristinsson, H. Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation. J. Sci. Food Agric. 2011, 91, 1920–1924. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Q.; Koyama, T.; Ishizaki, S. Preparation, Purification and Characterization of Antibacterial and ACE Inhibitory Peptides from Head Protein Hydrolysate of Kuruma Shrimp, Marsupenaeus japonicus. Molecules 2023, 28, 894. [Google Scholar] [CrossRef]
- Molchanov, V.; Yegorov, A.; Molchanov, M.; Timchenko, A.; Novikov, V.; Novojilov, N.; Timchenko, M. Novel Antimicrobial Peptide from the Hepatopancreas of the Red King Crab. Int. J. Mol. Sci. 2023, 24, 15607. [Google Scholar] [CrossRef]
- Mao, Z.J.; Jiang, H.; Mao, X.Z. Identification and Anti-Hyperuricemic Activity of Xanthine Oxidase Inhibitory Peptides from Pacific White Shrimp and Swimming Crab Based on Molecular Docking Screening. J. Agric. Food Chem. 2023, 71, 1620–1627. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Ulagesan, S.; Krishnan, S.; Nam, T.-J.; Choi, Y.-H. A Review of Bioactive Compounds in Oyster Shell and Tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, Y.; Jiang, B.; Chang, Y.; Xue, C. Identification and detection of protein-derived adulterants in oyster peptide powder through an untargeted and targeted proteomics workflow. Food Control 2023, 153, 109896. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Brennan, C.; Young, D.J.; Chang, K.; Wadewitz, P.; Zeng, Q.; Yuan, Y. Antioxidative activity of oyster protein hydrolysates Maillard reaction products. Food Sci. Nutr. 2020, 8, 3274–3286. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Zheng, H.-Z.; Wang, R.; Chen, H.; Xiao, J.-X.; Zheng, B.; Liu, S.-L.; Ding, Y.-T. Ameliorative Effects of Peptides Derived from Oyster (Crassostrea gigas) on Immunomodulatory Function and Gut Microbiota Structure in Cyclophosphamide-Treated Mice. Mar. Drugs 2021, 19, 456. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Bechaux, J.; Ferraro, V.; Sayd, T.; Chambon, C.; Le Page, J.F.; Drillet, Y.; Gatellier, P.; Santé-Lhoutellier, V. Workflow towards the generation of bioactive hydrolysates from porcine products by combining in silico and in vitro approaches. Food Res. Int. 2020, 132, 109123. [Google Scholar] [CrossRef]
- Chen, Q.; Ge, Y.X.; He, X.Y.; Li, S.S.; Fang, Z.F.; Li, C.; Chen, H. Virtual-screening of xanthine oxidase inhibitory peptides: Inhibition mechanisms and prediction of activity using machine-learning. Food Chem. 2024, 460, 140741. [Google Scholar] [CrossRef]
- Cui, P.B.; Shao, T.L.; Liu, W.L.; Li, M.Y.; Yu, M.X.; Zhao, W.X.; Song, Y.Z.; Ding, Y.T.; Liu, J.H. Advanced review on type II collagen and peptide: Preparation, functional activities and food industry application. Crit. Rev. Food Sci. Nutr. 2024, 64, 11302–11319. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xi, Q.H.; Kong, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef]
- Lu, S.S.; Pei, Z.S.; Lu, Q.H.; Li, Q.; He, Y.F.; Feng, A.G.; Liu, Z.Y.; Xue, C.F.; Liu, J.H.; Lin, X.D.; et al. Effect of a collagen peptide-fish oil high internal phase emulsion on the printability and gelation of 3D-printed surimi gel inks. Food Chem. 2024, 446, 138810. [Google Scholar] [CrossRef]
- Gu, Y.X.; Zhang, J.C.; Niu, Y.J.; Sun, B.G.; Liu, Z.Y.; Mao, X.Z.; Zhang, Y.Y. Virtual screening and characteristics of novel umami peptides from porcine type I collagen. Food Chem. 2024, 434, 137386. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, L.P.; Zhuang, Y.L.; Gu, Y.; Cheng, G.G.; Fan, X.J.; Ding, Y.Y.; Liu, H.T. Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review. Foods 2023, 12, 2703. [Google Scholar] [CrossRef]
- Ning, J.; Li, M.; Chen, W.; Zhao, H.; Chen, J.; Yang, M.; Cao, X.; Yue, X. Peptidomics as a tool to analyze endogenous peptides in milk and milk-related peptides. Food Biosci. 2022, 50, 102199. [Google Scholar] [CrossRef]
- Xiao, T.; Zeng, J.; Qiu, L.; Wang, R.; Li, N.; Deng, Z.; Zheng, L. Combining in silico and in vitro approaches to identify endogenous hypoglycemic peptides from human milk. Food Funct. 2022, 13, 2899–2912. [Google Scholar] [CrossRef]
- Zhang, H.; Abdallah, M.F.; Zhang, J.; Yu, Y.; Zhao, Q.; Tang, C.; Qin, Y.; Zhang, J. Comprehensive quantitation of multi-signature peptides originating from casein for the discrimination of milk from eight different animal species using LC-HRMS with stable isotope labeled peptides. Food Chem. 2022, 390, 133126. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Peptides Released from Foremilk and Hindmilk Proteins by Breast Milk Proteases Are Highly Similar. Front. Nutr. 2017, 4, 54. [Google Scholar] [CrossRef]
- Li, W.; Xi, Y.; Wang, J.; Zhang, Y.; Li, H.; Liu, X. Food-derived protein hydrolysates and peptides: Anxiolytic and antidepressant activities, characteristics, and mechanisms. Food Sci. Hum. Wellness 2024, 13, 1168–1185. [Google Scholar] [CrossRef]
- Dela Pena, I.J.I.; Hong, E.; de la Pena, J.B.; Kim, H.J.; Botanas, C.J.; Hong, Y.S.; Hwang, Y.S.; Moon, B.S.; Cheong, J.H. Milk Collected at Night Induces Sedative and Anxiolytic-Like Effects and Augments Pentobarbital-Induced Sleeping Behavior in Mice. J. Med. Food 2015, 18, 1255–1261. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef]
- Mann, K.; Mann, M. In-depth analysis of the chicken egg white proteome using an LTQ Orbitrap Velos. Proteome Sci. 2011, 9, 7. [Google Scholar] [CrossRef]
- Tenovuo, J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: Efficacy and safety. Oral Dis. 2002, 8, 23–29. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Rupa, P.; Kovacs-Nolan, J.; Turner, P.V.; Matsui, T.; Mine, Y. Oral Administration of Hen Egg White Ovotransferrin Attenuates the Development of Colitis Induced by Dextran Sodium Sulfate in Mice. J. Agric. Food Chem. 2015, 63, 1532–1539. [Google Scholar] [CrossRef]
- Zhou, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.S.; Du, H.Y.; Wu, J.P.; Tu, Y.G. Antioxidant Stress and Anti-Inflammatory Activities of Egg White Proteins and Their Derived Peptides: A Review. J. Agric. Food Chem. 2022, 70, 5–20. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. MilkAMP: A comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol. 2014, 94, 181–193. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-based proteins and their multifaceted industrial applications. LWT Food Sci. Technol. 2022, 154, 112620. [Google Scholar] [CrossRef]
- Cavaliere, C.; Montone, A.M.I.; Aita, S.E.; Capparelli, R.; Cerrato, A.; Cuomo, P.; Laganà, A.; Montone, C.M.; Piovesana, S.; Capriotti, A.L. Production and Characterization of Medium-Sized and Short Antioxidant Peptides from Soy Flour-Simulated Gastrointestinal Hydrolysate. Antioxidants 2021, 10, 734. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Y.; Zeng, Q.; Xiong, J.; Gan, Y.; Jiang, K.; Xie, N. Plant protein-derived anti-breast cancer peptides: Sources, therapeutic approaches, mechanisms, and nanoparticle design. Front. Pharmacol. 2025, 15, 1468977. [Google Scholar] [CrossRef]
- Duan, X.J.; Dong, Y.F.; Zhang, M.; Li, Z.H.; Bu, G.H.; Chen, F.S. Identification and molecular interactions of novel ACE inhibitory peptides from rapeseed protein. Food Chem. 2023, 422, 136085. [Google Scholar] [CrossRef]
- Das, S.; Behera, M.; Das, S.R.; Behera, K.C.; Singh, L. Green Seaweeds as a Potential Source of Biomolecules and Bioactive Peptides: Recent Progress and Applications—A Review. Chem. Biodivers. 2024, 22, e202401695. [Google Scholar] [CrossRef]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Lo, S.-C.; Li, X.; Henzl, M.T.; Beamer, L.J.; Hannink, M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006, 25, 3605–3617. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef]
- Han, J.B.; Shi, X.W.; Xu, J.J.; Lin, W.T.; Chen, Y.H.; Han, B.J.; Wang, Y.; Xu, J.J. DL-3-n-butylphthalide prevents oxidative stress and atherosclerosis by targeting Keap-1 and inhibiting Keap-1/Nrf-2 interaction. Eur. J. Pharm. Sci. 2022, 172, 106164. [Google Scholar] [CrossRef]
- Xin, X.-Y.; Ruan, C.-H.; Liu, Y.-H.; Jin, H.-N.; Park, S.-K.; Hur, S.-J.; Li, X.-Z.; Choi, S.-H. Identification of novel antioxidant and anti-inflammatory peptides from bovine hemoglobin by computer simulation of enzymolysis, molecular docking and molecular dynamics. Curr. Res. Food Sci. 2024, 9, 100931. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Liang, X.J.; Bao, X.F.; Xiao, W.; Chen, G.L. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Rivero-Pino, F.; la Rosa, T.G.D.; Villanueva, A.; la Paz, S.M.D. Identification, characterization, and molecular docking of immunomodulatory oligopeptides from bioavailable hempseed protein hydrolysates. Food Res. Int. 2024, 176, 113712. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Huang, J.H.; Huang, N. Structure, Function, and Pharmaceutical Ligands of 5-Hydroxytryptamine 2B Receptor. Pharmaceuticals 2021, 14, 76. [Google Scholar] [CrossRef]
- Göthert, M.; Bönisch, H.; Malinowska, B.; Schlicker, E. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part II. Some contributions of Manfred Göthert. Pharmacol. Rep. 2020, 72, 271–284. [Google Scholar] [CrossRef]
- Tanti, A.; Belzung, C. Open questions in current models of antidepressant action. Br. J. Pharmacol. 2010, 159, 1187–1200. [Google Scholar] [CrossRef]
- Mizushige, T.; Uchida, T.; Ohinata, K. Dipeptide tyrosyl-leucine exhibits antidepressant-like activity in mice. Sci. Rep. 2020, 10, 2257. [Google Scholar] [CrossRef]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme–inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Li, Z.K.; Zhang, W.H.; Abubaker, M.A.; Shu, Q.; Liu, Y.F. In silico identification and experimental validation of two types of angiotensin-converting enzyme (ACE) and xanthine oxidase (XO) milk inhibitory peptides. Food Chem. 2025, 464, 141864. [Google Scholar] [CrossRef]
- Yu, Z.P.; Fu, L.; Zhang, Q.; Zhao, W.Z. In silico identification and molecular mechanism of novel egg white-derived tyrosinase inhibitory peptides. Food Biosci. 2024, 57, 103567. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, X.; Mao, Z.; Zhang, J.; Sun, J.; Mao, X. Exploration, sequence optimization and mechanism analysis of novel xanthine oxidase inhibitory peptide from Ostrea rivularis Gould. Food Chem. 2023, 404, 134537. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Shin, T.H.; Lee, G. Machine intelligence in peptide therapeutics: A next-generation tool for rapid disease screening. Med. Res. Rev. 2020, 40, 1276–1314. [Google Scholar] [CrossRef]
- Zhou, X.R.; Liu, G.X.; Cao, S.Y.; Lv, J. Deep Learning for Antimicrobial Peptides: Computational Models and Databases. J. Chem. Inf. Model. 2025, 65, 1708–1717. [Google Scholar] [CrossRef]
- Fu, H.H.; Comer, J.; Cai, W.S.; Chipot, C. Sonoporation at Small and Large Length Scales: Effect of Cavitation Bubble Collapse on Membranes. J. Phys. Chem. Lett. 2015, 6, 413–418. [Google Scholar] [CrossRef]
- Shamloo, A.; Pedram, M.Z.; Heidari, H.; Alasty, A. Computing the blood brain barrier (BBB) diffusion coefficient: A molecular dynamics approach. J. Magn. Magn. Mater. 2016, 410, 187–197. [Google Scholar] [CrossRef]
- Stevens, J.A.; Grünewald, F.; van Tilburg, P.A.M.; König, M.; Gilbert, B.R.; Brier, T.A.; Thornburg, Z.R.; Luthey-Schulten, Z.; Marrink, S.J. Molecular dynamics simulation of an entire cell. Front. Chem. 2023, 11, 1106495. [Google Scholar] [CrossRef]
- Dorey, A.; Howorka, S. Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics. Nat. Chem. 2024, 16, 314–334. [Google Scholar] [CrossRef]
- Bian, S.Q.; Wang, Z.K.; Gong, J.S.; Su, C.; Li, H.; Xu, Z.H.; Shi, J.S. Protein Engineering of Substrate Specificity toward Nitrilases: Strategies and Challenges. J. Agric. Food Chem. 2025, 73, 1775–1789. [Google Scholar] [CrossRef]
- Alder, B.J.; Wainwright, T.E. Phase Transition for a Hard Sphere System. J. Chem. Phys. 1957, 27, 1208–1209. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Xie, J.; Cheng, M.; Sun, L.; Zhang, S.; Xin, J.; Zhang, N. A review on application of molecular simulation technology in food molecules interaction. Curr. Res. Food Sci. 2022, 5, 1873–1881. [Google Scholar] [CrossRef]
- Ma, R.; Wong, S.W.; Ge, L.; Shaw, C.; Siu, S.W.; Kwok, H.F. In vitro and MD simulation study to explore physicochemical parameters for antibacterial peptide to become potent anticancer peptide. Mol. Ther. Oncolytics 2020, 16, 7–19. [Google Scholar] [CrossRef]
- Li, T.; Ren, X.; Luo, X.; Wang, Z.; Li, Z.; Luo, X.; Shen, J.; Li, Y.; Yuan, D.; Nussinov, R.; et al. A Foundation Model Identifies Broad-Spectrum Antimicrobial Peptides against Drug-Resistant Bacterial Infection. Nat. Commun. 2024, 15, 7538. [Google Scholar] [CrossRef]
- Hao, Y.J.; Xing, L.J.; Wang, Z.X.; Cai, J.M.; Toldrá, F.; Zhang, W.A. Study on the anti-inflammatory activity of the porcine bone collagen peptides prepared by ultrasound-assisted enzymatic hydrolysis. Ultrason. Sonochem. 2023, 101, 106697. [Google Scholar] [CrossRef]
- Gu, Z.F.; Hao, Y.D.; Wang, T.Y.; Cai, P.L.; Zhang, Y.; Deng, K.J.; Lin, H.; Lv, H. Prediction of blood-brain barrier penetrating peptides based on data augmentation with Augur. BMC Biol. 2024, 22, 86. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Forster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Young, R.C.; Mitchell, R.C.; Brown, T.H.; Ganellin, C.R.; Griffiths, R.; Jones, M.; Rana, K.K.; Saunders, D.; Smith, I.R.; Sore, N.E. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. J. Med. Chem. 1988, 31, 656–671. [Google Scholar] [CrossRef]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A Method to Predict Blood-Brain Barrier Permeability of Drug-Like Compounds Using Molecular Dynamics Simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef]
- Mosalaganti, S.; Obarska-Kosinska, A.; Siggel, M.; Taniguchi, R.; Turoňová, B.; Zimmerli, C.E.; Buczak, K.; Schmidt, F.H.; Margiotta, E.; Mackmull, M.-T.; et al. AI-based structure prediction empowers integrative structural analysis of human nuclear pores. Science 2022, 376, eabm9506. [Google Scholar] [CrossRef]
- Yu, I.; Mori, T.; Ando, T.; Harada, R.; Jung, J.; Sugita, Y.; Feig, M. Biomolecular interactions modulate macromolecular structure and dynamics in atomistic model of a bacterial cytoplasm. eLife 2016, 5, e19274. [Google Scholar] [CrossRef]
- Roohani, Y.H.; Hua, T.J.; Tung, P.Y.; Bounds, L.R.; Yu, F.B.; Dobin, A.; Teyssier, N.; Adduri, A.; Woodrow, A.; Plosky, B.S.; et al. Virtual Cell Challenge Toward a Turing test for the virtual cell. Cell 2025, 188, 3370–3374. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J.P. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, Z.J.; Aweya, J.J.; Huang, S.Y.; Deng, S.G.; Shi, L.F.; Zheng, M.J.; Zhang, Y.L.; Liu, G.M. Low-intensity ultrasound enhances the antimicrobial activity of neutral peptide TGH2 against Escherichia coli. Ultrason. Sonochem. 2021, 77, 105676. [Google Scholar] [CrossRef]
- Mori, Y.; Okumura, H. Molecular dynamics of the structural changes of helical peptides induced by pressure. Proteins-Struct. Funct. Bioinform. 2014, 82, 2970–2981. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhu, Z.H.; Pan, F.; Zhou, Q.H.; Zhao, L.; Zhao, L. Enhancing walnut protein isolate functionality with ultrasound treatment: An integrated experimental and molecular dynamics simulation study. Food Hydrocoll. 2025, 163, 111135. [Google Scholar] [CrossRef]
- Foguel, D.; Suarez, M.C.; Ferrão-Gonzales, A.D.; Porto, T.C.R.; Palmieri, L.; Einsiedler, C.M.; Andrade, L.R.; Lashuel, H.A.; Lansbury, P.T.; Kelly, J.W.; et al. Dissociation of amyloid fibrils of α-synuclein and transthyretin by pressure reveals their reversible nature and the formation of water-excluded cavities. Proc. Natl. Acad. Sci. USA 2003, 100, 9831–9836. [Google Scholar] [CrossRef]
- Yagi, H.; Hasegawa, K.; Yoshimura, Y.; Goto, Y. Acceleration of the depolymerization of amyloid β fibrils by ultrasonication. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 2480–2485. [Google Scholar] [CrossRef]
- Koshiyama, K.; Kodama, T.; Yano, T.; Fujikawa, S. Molecular dynamics simulation of structural changes of lipid bilayers induced by shock waves: Effects of incident angles. Biochim. Biophys. Acta 2008, 1778, 1423–1428. [Google Scholar] [CrossRef]
- Sagui, C.; Darden, T.A. Molecular Dynamics Simulations of Biomolecules: Long-Range Electrostatic Effects. Annu. Rev. Biophys. 1999, 28, 155–179. [Google Scholar] [CrossRef]
- Qu, W.; Xie, Y.; Shen, Y.; Han, J.; You, M.; Zhu, T. Simulation on the effects of various factors on the motion of ultrasonic cavitation bubble. Math. Model. Eng. Probl. 2017, 4, 173–178. [Google Scholar] [CrossRef]
- Khalaf, M.H.; Mansoori, G.A.; Yong, C.W. Magnetic treatment of petroleum and its relation with asphaltene aggregation onset (an atomistic investigation). J. Pet. Sci. Eng. 2019, 176, 926–933. [Google Scholar] [CrossRef]
- Lappala, A. The next revolution in computational simulations: Harnessing AI and quantum computing in molecular dynamics. Curr. Opin. Struct. Biol. 2024, 89, 102919. [Google Scholar] [CrossRef]
- Cui, T.; Tang, C.; Zhou, D.; Li, Y.; Gong, X.; Ouyang, W.; Su, M.; Zhang, S. Online test-time adaptation for better generalization of interatomic potentials to out-of-distribution data. Nat. Commun. 2025, 16, 1891. [Google Scholar] [CrossRef]
- Wang, T.; He, X.; Li, M.; Li, Y.; Bi, R.; Wang, Y.; Cheng, C.; Shen, X.; Meng, J.; Zhang, H.; et al. Ab initio characterization of protein molecular dynamics with AI2BMD. Nature 2024, 635, 1019–1027. [Google Scholar] [CrossRef]
- Zeng, W.F.; Zhou, X.X.; Willems, S.; Ammar, C.; Wahle, M.; Bludau, I.; Voytik, E.; Strauss, M.T.; Mann, M. AlphaPeptDeep: A modular deep learning framework to predict peptide properties for proteomics. Nat. Commun. 2022, 13, 7238. [Google Scholar] [CrossRef] [PubMed]
- Luttens, A.; Cabeza de Vaca, I.; Sparring, L.; Brea, J.; Martínez, A.L.; Kahlous, N.A.; Radchenko, D.S.; Moroz, Y.S.; Loza, M.I.; Norinder, U.; et al. Rapid traversal of vast chemical space using machine learning-guided docking screens. Nat. Comput. Sci. 2025, 5, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, M.; Vassilev-Galindo, V.; Poltavsky, I.; Martín Pendás, Á.; Tkatchenko, A. Explainable chemical artificial intelligence from accurate machine learning of real-space chemical descriptors. Nat. Commun. 2024, 15, 4345. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kortemme, T. Recent advances in de novo protein design: Principles, methods, and applications. J. Biol. Chem. 2021, 296, 100558. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Albanese, K.I.; Barbe, S.; Tagami, S.; Woolfson, D.N.; Schiex, T. Computational protein design. Nat. Rev. Methods Primers 2025, 5, 13. [Google Scholar] [CrossRef]
- Khakzad, H.; Igashov, I.; Schneuing, A.; Goverde, C.; Bronstein, M.; Correia, B. A new age in protein design empowered by deep learning. Cell Syst. 2023, 14, 925–939. [Google Scholar] [CrossRef]
- Wang, B.; Lin, P.; Zhong, Y.; Tan, X.; Shen, Y.; Huang, Y.; Jin, K.; Zhang, Y.; Zhan, Y.; Shen, D.; et al. Explainable deep learning and virtual evolution identifies antimicrobial peptides with activity against multidrug-resistant human pathogens. Nat. Microbiol. 2025, 10, 332–347. [Google Scholar] [CrossRef]
- Yan, K.; Lv, H.W.; Guo, Y.C.; Peng, W.; Liu, B. sAMPpred-GAT: Prediction of antimicrobial peptide by graph attention network and predicted peptide structure. Bioinformatics 2023, 39, btac715. [Google Scholar] [CrossRef]
- Liu, H.; Song, Z.; Zhang, Y.; Wu, B.; Chen, D.; Zhou, Z.; Zhang, H.; Li, S.; Feng, X.; Huang, J.; et al. De novo design of self-assembling peptides with antimicrobial activity guided by deep learning. Nat. Mater. 2025, 24, 1295–1306. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Xiao, S.; Zhou, G.C.; Chen, X.; Wang, B.; Wang, J.H. Bioactive peptides in dry-cured ham: A comprehensive review of preparation methods, metabolic stability, safety, health benefits, and regulatory frameworks. Food Res. Int. 2024, 186, 114367. [Google Scholar] [CrossRef] [PubMed]
- Bernhofer, M.; Dallago, C.; Karl, T.; Satagopam, V.; Heinzinger, M.; Littmann, M.; Olenyi, T.; Qiu, J.J.; Schütze, K.; Yachdav, G.; et al. PredictProtein—Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021, 49, W535–W540. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Keller, R.C.A. New user-friendly approach to obtain an Eisenberg plot and its use as a practical tool in protein sequence analysis. Int. J. Mol. Sci. 2011, 12, 5577–5591. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Wang, S.; Yin, Z.; An, T.; Zhang, J.; Liu, Y. Research progress on fermentation-produced plant-derived bioactive peptides. Front. Pharmacol. 2024, 15, 1438947. [Google Scholar] [CrossRef]
- Fricker, L.D. Limitations of Mass Spectrometry-Based Peptidomic Approaches. J. Am. Soc. Mass Spectrom. 2015, 26, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.L.; Habeshian, S.; Li, B.; David, J.-A.G.L.; Nielsen, A.L.; Ji, X.; Il Khwildy, K.; Duany Benitez, M.M.; Phothirath, P.; Heinis, C. De novo development of small cyclic peptides that are orally bioavailable. Nat. Chem. Biol. 2024, 20, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Chawathe, A.; Ahire, V.; Luthra, K.; Patil, B.; Garkhal, K.; Sharma, N. Analytical and drug delivery strategies for short peptides: From manufacturing to market. Anal. Biochem. 2025, 696, 115699. [Google Scholar] [CrossRef]
- Guo, T.; Steen, J.A.; Mann, M. Mass-spectrometry-based proteomics: From single cells to clinical applications. Nature 2025, 638, 901–911. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Zhou, C.S.; Wahia, H.; Yao, D.Y.; Song, L.L.; Otu, P.; Zhang, K.; Niu, Y.W.; Hua, C.H. Preparation of umami peptides from chicken breast by batch coupled enzymatic hydrolysis and membrane separation mode and the taste mechanism of identified umami peptides. Food Chem. 2024, 456, 139963. [Google Scholar] [CrossRef] [PubMed]
- Boiko, D.A.; MacKnight, R.; Kline, B.; Gomes, G. Autonomous chemical research with large language models. Nature 2023, 624, 570–578. [Google Scholar] [CrossRef]
- Vincenzi, M.; Mercurio, F.A.; Leone, M. Virtual Screening of Peptide Libraries: The Search for Peptide-Based Therapeutics Using Computational Tools. Int. J. Mol. Sci. 2024, 25, 1798. [Google Scholar] [CrossRef]
- Mercer, D.K.; Torres, M.D.T.; Duay, S.S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell. Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]


| Receptor Name | PDB ID | Method and Resolution | Small Molecular Ligands |
|---|---|---|---|
| Keap 1 | 5GIT | X-RAY DIFFRACTION 2.19 Å | XXT (C19 H26 O7) |
| 2FLU | X-RAY DIFFRACTION 1.50 Å | Nrf2 (16 amino acid residues) | |
| TLR4 | 2Z63 | X-RAY DIFFRACTION 2.00 Å | Two oligosaccharides |
| 3VQ2 | X-RAY DIFFRACTION 2.48 Å | One oligosaccharide, LP5 (C34 H66 N O12 P), LP4 (C34 H66 N O12 P), MYR (C14 H28 O2), NAG (C8 H15 N O6), DAO (C12 H24 O2) | |
| 5-HT | 7VOE (5-HT2A) | X-RAY DIFFRACTION 2.90 Å | 9SC (Subject of Investigation/LOI, C23 H27 Cl2 N3 O2), CLR (C27 H46 O), OLC (C21 H40 O4), 1PE (C10 H22 O6), MG (Mg) |
| 5TVN (5-HT2B) | X-RAY DIFFRACTION 2.90 Å | CLR (C27 H46 O), OLC (C21 H40 O4), 7LD (C20 H25 N3 O), PEG (C4 H10 O3), PO4 (O4 P) | |
| ACE | 1O8A | X-RAY DIFFRACTION 2.00 Å | NAG (C8 H15 N O6), NXA (C4 H7 N O4), ZN (Zn), ACT (C2 H3 O2), CL (Cl) |
| 6F9T | X-RAY DIFFRACTION 1.60 Å | Two oligosaccharides, D0Z (C26 H40 N4 O9 S), PEG (C4 H10 O3), PGE (C6 H14 O4), IMD (C3 H5 N2), ZN (Zn), EDO (C2 H6 O2), BO3 (B H3 O3), CL (Cl) | |
| Tyrosinase | 2Y9X | X-RAY DIFFRACTION 2.78 Å | HO (Ho), 0TR (C7 H6 O2), CU (Cu) |
| 1WX5 | X-RAY DIFFRACTION 2.02 Å | CL (Cl), NA (Na) | |
| XO | 1FIQ | X-RAY DIFFRACTION 2.50 Å | FAD (C27 H33 N9 O15 P2), MTE (C10 H14 N5 O6 P S2), FES (Fe2 S2), MOS (H Mo O2 S), TEI (C16 H16 N2 O3 S) |
| 1N5X | X-RAY DIFFRACTION 2.80 Å | FAD (C27 H33 N9 O15 P2), MTE (C10 H14 N5 O6 P S2), FES (Fe2 S2), MOS (H Mo O2 S), PM6 (C5 H4 N4 S) | |
| 3NVY | X-RAY DIFFRACTION 2.00 Å | FAD (C27 H33 N9 O15 P2), MTE (C10 H14 N5 O6 P S2), QUE (C15 H10 O7), FES (Fe2 S2), MOS (H Mo O2 S) | |
| Monoamine oxidase A | 2Z5X | X-RAY DIFFRACTION 2.20 Å | FAD (C27 H33 N9 O15 P2), DCX (C12 H27 O P), HRM (C13 H12 N2 O), GOL (C3 H8 O3) |
| 2BXR | X-RAY DIFFRACTION 3.00 Å | FAD (C27 H33 N9 O15 P2), MLG (C13 H15 Cl2 N O) | |
| Calcium-sensing receptor | 7DD7 | ELECTRON MICROSCOPY 3.20 Å | H43 (C24 H26 N2 O2), NAG (C8 H15 N O6), TRP (C11 H12 N2 O2), CA (Ca), CL (Cl) |
| Types | Category | Advantages | Disadvantages |
|---|---|---|---|
| Virtual enzymolysis | Enzyme | Obtain all the peptide sequences that can be theoretically generated. | Enzymatic hydrolysis conditions, such as temperature, pH, and ionic strength, were not fully considered. |
| Molecular dock | Rigid docking | Ultra-fast computation: ignores ligand-receptor conformational changes; high-throughput screening: handles > 100,000 compounds/day. | Low accuracy: fails to simulate induced-fit effects (binding pose error > 5 Å); limited applicability: only suitable for rigid binding pockets. |
| Semi-flexible docking | Balanced precision/efficiency: allows ligand flexibility; broad applicability: simulates small molecules/peptides binding to proteins. | Ignores receptor flexibility: critical residue motions; misses long-range interactions: cannot model electrostatic shielding in membrane proteins. | |
| Flexible docking | High-precision binding: full conformational sampling (RMSD ≤ 1.0 Å); captures allostery: simulates conformational shifts. | High computational cost: hours-to-days per task; inadequate sampling: prone to local minima; requires enhanced sampling. | |
| Molecular dynamic simulation | All-atom MD | Atomic resolution: resolves hydrogen bonds, water-mediated interactions; biophysical accuracy: simulates enzyme catalysis, ion channel gating. | Extreme computational demand; force field limitations. |
| Coarse-grained MD | 1000× faster: 4–8 atoms grouped per bead (e.g., Martini force field); simulates ms events; macroscale phenomena: captures membrane self-assembly, protein folding. | Loss of atomic detail: side-chain interactions (e.g., π-π stacking) unquantifiable; poor parameter transfer: system-specific re-parameterization needed. | |
| Deep learning/machine learning models | Various functional prediction models | Considerable accuracy, fast prediction rate and lower prediction cost. | A large number of high-quality databases are needed for training. |
| Virtual cell model | Multi-scale integration capability, visual analysis of complex cell relationships, assessment of controllability and traceability. | Data quality dependence, the contradiction between calculation accuracy and efficiency, and the complexity of verification experiments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Xu, Y.; Zhang, X.; Yin, F.; Cao, J.; Liu, Z.; Zhou, D.; Feng, A.; Li, C. Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides. Foods 2025, 14, 2890. https://doi.org/10.3390/foods14162890
Liu D, Xu Y, Zhang X, Yin F, Cao J, Liu Z, Zhou D, Feng A, Li C. Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides. Foods. 2025; 14(16):2890. https://doi.org/10.3390/foods14162890
Chicago/Turabian StyleLiu, Dongyin, Yuan Xu, Xin Zhang, Fawen Yin, Jun Cao, Zhongyuan Liu, Dayong Zhou, Aiguo Feng, and Chuan Li. 2025. "Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides" Foods 14, no. 16: 2890. https://doi.org/10.3390/foods14162890
APA StyleLiu, D., Xu, Y., Zhang, X., Yin, F., Cao, J., Liu, Z., Zhou, D., Feng, A., & Li, C. (2025). Organic Fusion of Molecular Simulation and Wet-Lab Validation: A Promising High-Throughput Strategy for Screening Bioactive Food Peptides. Foods, 14(16), 2890. https://doi.org/10.3390/foods14162890





