Revisiting Spectrophotometric Methods in the FoodOmics Era: The Influence of Phytochemicals in the Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant and Fruit Samples and Extract Preparation
2.3. Physico-Chemical Characterisation of Samples
2.4. Quantification of Soluble Sugars by Spectrophotometric Methods
2.5. Quantification of Soluble Sugars by Gas Chromatography Coupled with a Flame Ionisation Detector (GC-FID)
2.6. Statistical Analysis
3. Results
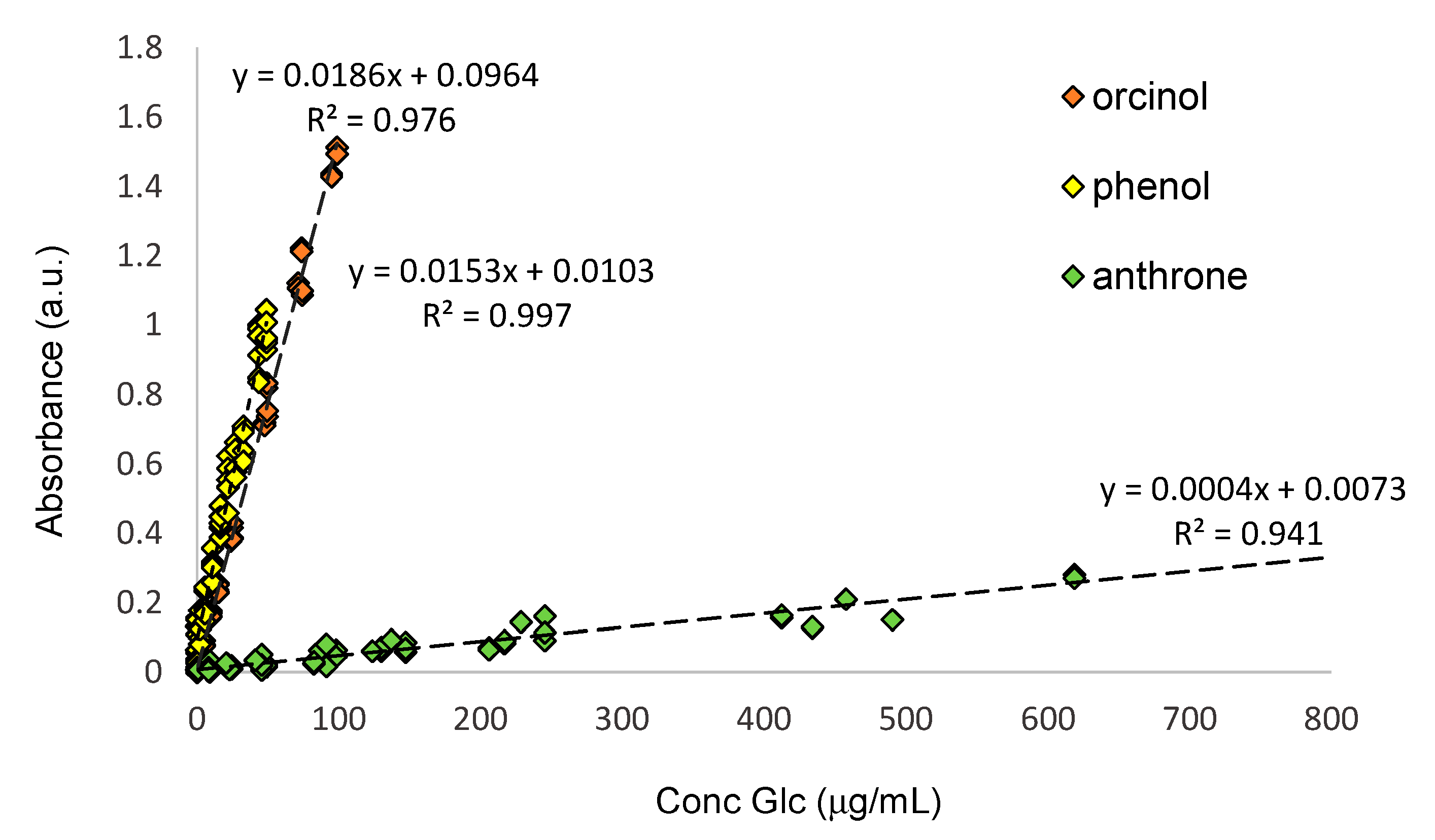
3.1. Performance of Spectrophotometric Methods Using Standard Glucose Solution
3.2. Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts
3.2.1. Physico-Chemical Characteristics of Plant-Based Beverages, Drinks, and Aqueous Extracts
3.2.2. Evaluation of Spectrophotometric Protocols in the Quantification of Soluble Sugars
3.2.3. Quantification of Sugars by Gas Chromatography with Flame Ionisation Detector (GC-FID)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Heiss, C.; Sansone, R.; Karimi, H.; Krabbe, M.; Schuler, D.; Rodriguez-Mateos, A.; Kraemer, T.; Cortese-Krott, M.M.; Kuhnle, G.G.C.; Spencer, J.P.E.; et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age 2015, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Koponen, K.K.; Salosensaari, A.; Ruuskanen, M.O.; Havulinna, A.S.; Männistö, S.; Jousilahti, P.; Palmu, J.; Salido, R.; Sanders, K.; Brennan, C.; et al. Associations of healthy food choices with gut microbiota profiles. Am. J. Clin. Nutr. 2021, 114, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef]
- Vila-Real, C.; Costa, C.; Pimenta-Martins, A.; Mbugua, S.; Hagrétou, S.-L.; Katina, K.; Maina, N.H.; Pinto, E.; Gomes, A.M.P. Novel Fermented Plant-Based Functional Beverage: Biological Potential and Impact on the Human Gut Microbiota. Foods 2025, 14, 433. [Google Scholar] [CrossRef]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Wu, H.; Patterson, C.C.; Zhang, X.; Ghani, R.B.A.; Magliano, D.J.; Boyko, E.J.; Ogle, G.D.; Luk, A.O. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res. Clin. Pract. 2022, 185, 109785. [Google Scholar] [CrossRef]
- Vilela, A.; Cosme, F.; Inês, A. Wine and Non-Dairy Fermented Beverages: A Novel Source of Pro-and Prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef]
- Moors, E.H.M. Functional foods: Regulation and innovations in the EU. Innovation 2012, 25, 424–440. [Google Scholar] [CrossRef]
- Available online: https://single-market-economy.ec.europa.eu/sectors/agri-food-industrial-ecosystem_en (accessed on 8 August 2025).
- Vermeir, S.; Nicolaï, B.M.; Jans, K.; Maes, G.; Lammertyn, J. High-throughput microplate enzymatic assays for fast sugar and acid quantification in apple and tomato. J. Agric. Food Chem. 2007, 55, 3240–3248. [Google Scholar] [CrossRef]
- Chen, Y.; Vaidyanathan, S. Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Le Parc, A.; Lee, H.; Chen, K.; Barile, D. Rapid Quantification of Functional Carbohydrates in Food Products. Food Nutr. Sci. 2014, 5, 71–78. [Google Scholar] [CrossRef][Green Version]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef]
- Campo-Martínez, J.F.; González-Castro, M.J.; Enseñat-Berea, M.L.; Fernández-Paz, J. Validation of an Automated Enzymatic Method for the Determination of Fermentable Sugars in Wines. Food Anal. Methods 2022, 15, 1851–1858. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, J. Estimation of monosaccharides by the orcinol-sulphuric acid reaction. Biochem. J. 1955, 60, 200–205. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Kondo, M.; Mulianda, R.; Matamura, M.; Shibata, T.; Mishima, T.; Jayanegara, A.; Isono, N. Validation of a phenol-sulfuric acid method in a microplate format for the quantification of soluble sugars in ruminant feeds. Anim. Sci. J. 2021, 92, e13530. [Google Scholar] [CrossRef]
- Pereira, S.G.; Teixeira-Guedes, C.; Souza-Matos, G.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Teixeira, J.A.; Pereira, R.N.; Rocha, C.M. Influence of ohmic heating in the composition of extracts from Gracilaria vermiculophylla. Algal Res. 2021, 58, 102360. [Google Scholar] [CrossRef]
- Chen, W.; Gao, L.; Song, L.; Sommerfeld, M.; Hu, Q. An improved phenol-sulfuric acid method for the quantitative measurement of total carbohydrates in algal biomass. Algal Res. 2023, 70, 102986. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Waldron, K.W.; Selvendran, R.R. Isolation and characterization of cell wall polymers from the heavily lignified tissues of olive pulp (Olea europaea L.) seed hull. Carbohydr. Polym. 1995, 27, 285–294. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef]
- Veloso, M.I.; Coelho, E.; Trabulo, O.; Coimbra, M.A. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Appl. Sci. 2022, 12, 9463. [Google Scholar] [CrossRef]
- Phenol Safety Data Sheet. 2025. Available online: https://www.sigmaaldrich.com/PT/en/sds/sial/p5566?srsltid=AfmBOooQV34mXh2cSK481L6-PFirdHim39IxR29XrBr6yCnHbEySCIi- (accessed on 23 July 2025).
- Orcinol Safety Data Sheet. 2021. Available online: https://www.fishersci.com/store/msds?partNumber=AC416581000&countryCode=US&language=en (accessed on 23 July 2025).
- Mateus, N.; Proença, S.; Ribeiro, P.; Machado, J.M.; De Freitas, V. Grape and wine polyphenolic composition of red Vitis vinifera varieties concerning vineyard altitude. Cienc. Tecnol. Aliment. 2001, 3, 102–110. [Google Scholar] [CrossRef]
- Cordeiro, T.; Fernandes, I.; Pinho, O.; Calhau, C.; Mateus, N.; Faria, A. Anthocyanin content in raspberry and elderberry: The impact of cooking and recipe composition. Int. J. Gastron. Food Sci. 2021, 24, 100316. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Fan, F.Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.H.; Liang, Y.R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fu, Y.Q.; Granato, D.; Yu, P.; Yin, J.-F.; Zeng, L.; Xu, Y.-Q. Study on the color effects of (-)-epigallocatechin-3-gallate under different pH and temperatures in a model beverage system. Food Control 2022, 139, 109112. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Schmidtke, L.M.; Reis, A.; Domingues, M.R.M.; Delgadillo, I.; Debus, B.; Kirsanov, D.; Legin, A. Measurements of the effects of wine maceration with oak chips using an electronic tongue. Food Chem. 2017, 229, 20–27. [Google Scholar] [CrossRef]
- MacIerzyński, J.; Sójka, M.; Kosmala, M.; Karlińska, E. Transformation of Oligomeric Ellagitannins, Typical for Rubus and Fragaria Genus, during Strong Acid Hydrolysis. J. Agric. Food Chem. 2020, 68, 8212–8222. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Bastos, R.; Calvão, J.; Neto, F.; Coelho, E.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A.; Passos, C.P. Microwave hydrodiffusion and gravity as a sustainable alternative approach for an efficient apple pomace drying. Bioresour. Technol. 2021, 333, 125207. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Petronilho, S.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Carbohydrates as targeting compounds to produce infusions resembling espresso coffee brews using quality by design approach. Food Chem. 2021, 344, 128613. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J. Appl. Phycol. 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- Silventoinen-Veijalainen, P.; Sneck, A.M.; Nordlund, E.; Rosa-Sibakov, N. Influence of oat flour characteristics on the physicochemical properties of oat-based milk substitutes. Food Hydrocoll. 2024, 147, 109402. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Cheang, S.E.; Jiang, J.; Suarez, C.; Weng, C.-Y.; Couture, G.; Bacalzo, N.P.; Phillips, K.M.; Fukagawa, N.K.; Lebrilla, C.B. Combined alcohol soluble carbohydrate determination (CASCADE) of Food. ACS Food Sci. Technol. 2024, 4, 554–560. [Google Scholar] [CrossRef]

| Samples | pH | CIELab Colour | CIELab Parameters | °Brix | Sugar # (g/L) | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| Fermented drinks | Coffee (expresso) | 5.30 ± 0.01 | 24.35 ± 0.02 | 1.59 ± 0.01 | 1.11 ± 0.04 | 2.6 | 26.4 | |
| Red wine | 3.49 ± 0.01 | 24.47 ± 0.01 | 4.36 ± 0.03 | 1.60 ± 0.01 | 4.6 | 47.0 | ||
| Beer | 4.05 ± 0.01 | 54.78 ± 0.01 | 2.03 ± 0.02 | 24.74 ± 0.02 | 3.8 | 38.7 | ||
| Whisky | 3.96 ± 0.01 | 52.11 ± 0.01 | 4.99 ± 0.01 | 32.5 ± 0.04 | 7.8 | 80.6 | ||
| Irish afternoon tea | 4.77 ± 0.02 | 42.75 ± 0.08 | 16.83 ± 0.01 | 33.62 ± 0.02 | 0.8 | 8.1 | ||
| Fresh drinks | Ginger tea | 7.49 ± 0.04 | 57.89 ± 0.01 | −2.36 ± 0.01 | 25.54 ± 0.01 | 0.2 | 2.0 | |
| Elderberry juice | 4.06 ± 0.01 | 24.01 ± 0.03 | 1.85 ± 0.00 | 0.75 ± 0.00 | 5.2 | 53.2 | ||
| Orange juice | 3.92 ± 0.02 | 61.58 ± 0.02 | −1.50 ± 0.00 | 8.63 ± 0.03 | 6.4 | 65.8 | ||
| Oat milk | 7.09 ± 0.01 | 56.98 ± 0.01 | −0.05 ± 0.01 | 10.51 ± 0.01 | 0.6 | 6.0 | ||
| Algae extract | 6.23 ± 0.02 | 48.49 ± 0.01 | 6.26 ± 0.01 | 26.23 ± 0.03 | 0.6 | 6.0 | ||
| mint leaves extract | 6.76 ± 0.01 | 37.74 ±0.01 | 5.44 ± 0.02 | 17.59 ± 0.03 | 0.5 | 5.0 | ||
| Spinach leaves extract | 5.96 ± 0.01 | 48.89 ± 0.01 | 2.68 ± 0.01 | 23.56 ± 0.03 | 0.8 | 8.1 | ||
| Sugar Quantification (mg/mL) | |||||
|---|---|---|---|---|---|
| Samples | Refractometer * | Spectrophotometric Assays | Chromatography Method | ||
| Phenol | Orcinol | ||||
| Fermented Drinks | Coffee (espresso) | 26.4 | 0.30 ± 0.03 a | 0.63 ± 0.02 a | 0.205 ± 0.003 |
| Red wine | 47.0 | 0.82 ± 0.07 b | 0.42 ± 0.04 b | 0.217 ± 0.002 | |
| Beer | 38.7 | 8.27 ± 0.35 b | 2.79 ± 0.17 b | 2.26 ± 0.09 | |
| Whisky | 80.6 | 0.121 ± 0.004 | 0.034 ± 0.003 | 0.034 ± 0.004 | |
| Irish tea | 8.1 | - (♦) | 0.118 ± 0.002 | 0.06 ± 0.02 | |
| Fresh drinks | Ginger tea | 2.0 | 0.072 ± 0.008 | 0.062 ± 0.005 | 0.029 ± 0.07 |
| Elderberry juice | 53.2 | 18.7 ± 1.37 b | 4.57 ± 0.28 b | 1.88 ± 0.02 | |
| Orange juice | 65.8 | 35.2 ± 1.74 b | 8.12 ± 0.38 b | 6.85 ± 0.19 | |
| Oat milk | 6.0 | 0.19 ± 0.02 | 0.25 ± 0.01 | 0.062 ± 0.003 | |
| Algae ext. | 6.0 | 0.23 ± 0.02 | 0.24 ± 0.02 | 0.142 ± 0.004 | |
| Mint Leaves ext. | 5.0 | 0.018 ± 0.005 | 0.022 ± 0.002 | 0.009 ± 0.003 | |
| Spinach leaves ext. | 8.1 | 0.019 ± 0.002 | 0.039 ± 0.003 | 0.004 ± 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.; Passos, C.P.; Brandão, E.; Teixeira, N.; Alves, T.; Mateus, N.; de Freitas, V. Revisiting Spectrophotometric Methods in the FoodOmics Era: The Influence of Phytochemicals in the Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts. Foods 2025, 14, 2889. https://doi.org/10.3390/foods14162889
Reis A, Passos CP, Brandão E, Teixeira N, Alves T, Mateus N, de Freitas V. Revisiting Spectrophotometric Methods in the FoodOmics Era: The Influence of Phytochemicals in the Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts. Foods. 2025; 14(16):2889. https://doi.org/10.3390/foods14162889
Chicago/Turabian StyleReis, Ana, Cláudia P. Passos, Elsa Brandão, Natércia Teixeira, Tiago Alves, Nuno Mateus, and Victor de Freitas. 2025. "Revisiting Spectrophotometric Methods in the FoodOmics Era: The Influence of Phytochemicals in the Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts" Foods 14, no. 16: 2889. https://doi.org/10.3390/foods14162889
APA StyleReis, A., Passos, C. P., Brandão, E., Teixeira, N., Alves, T., Mateus, N., & de Freitas, V. (2025). Revisiting Spectrophotometric Methods in the FoodOmics Era: The Influence of Phytochemicals in the Quantification of Soluble Sugars in Plant-Based Beverages, Drinks, and Extracts. Foods, 14(16), 2889. https://doi.org/10.3390/foods14162889









