Effect of Space Allowance on Pig Performance, Carcass Traits and Meat Quality in Italian Heavy Pigs Reared Under Two Housing Systems
Abstract
1. Introduction
2. Materials and Methods
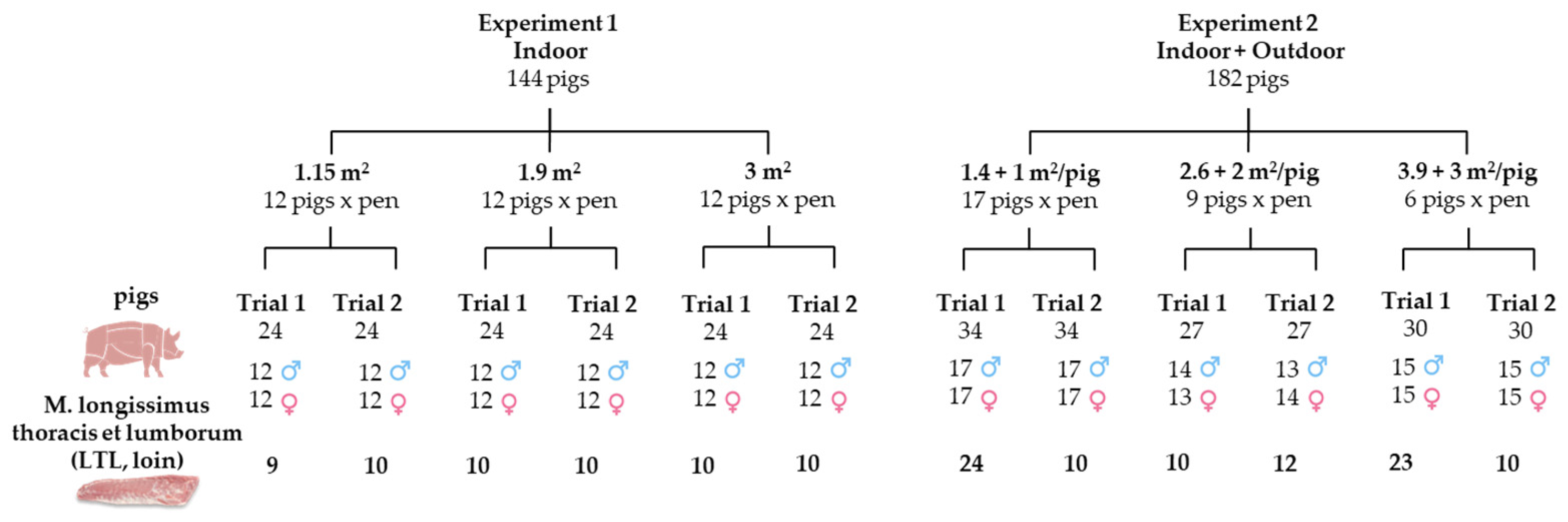
2.1. Animals
2.1.1. Pigs in Experiment 1
2.1.2. Pigs in Experiment 2
2.2. Housing and Feeding
2.3. Pig Welfare Observation Before Slaughter
2.4. Climate Conditions
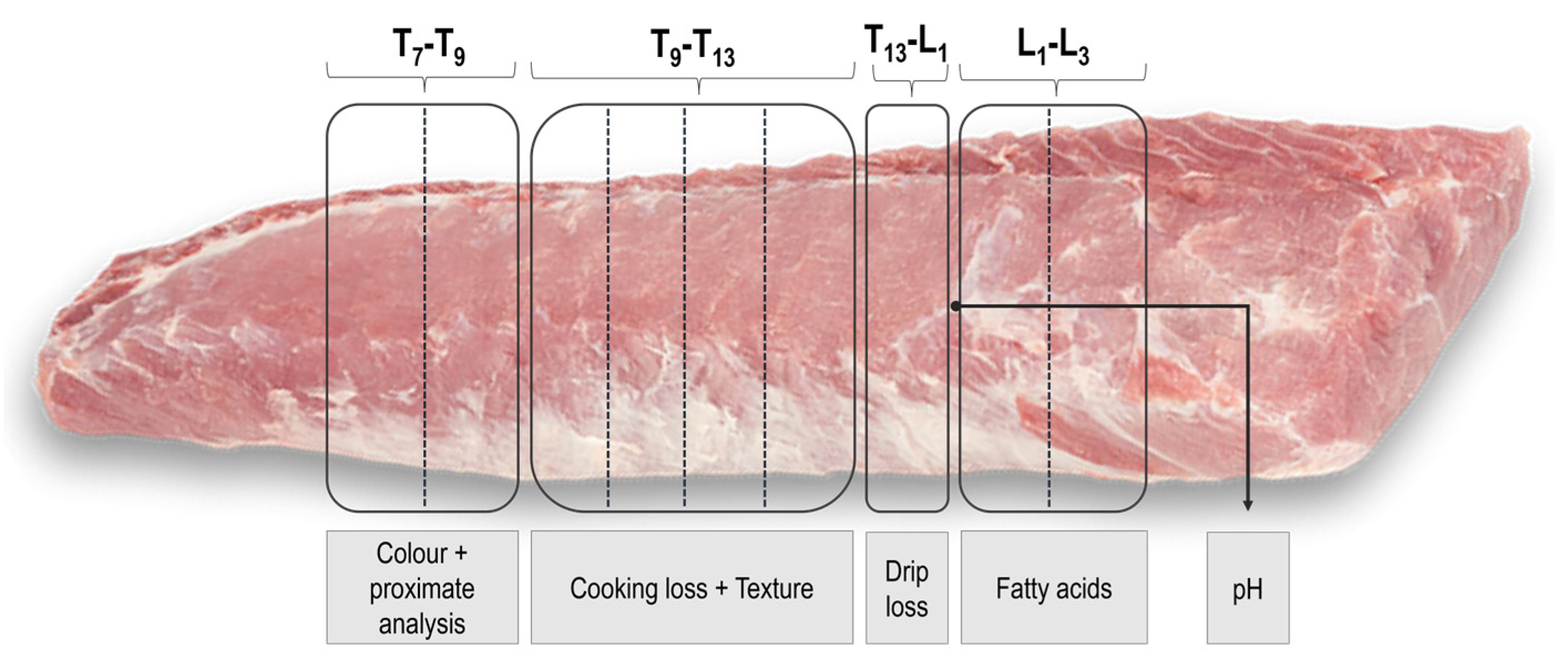
2.5. Carcass Measurement and Loin Sampling
2.6. Meat Quality Analyses
2.6.1. pH at 24 and 48 h
2.6.2. Color
2.6.3. Drip Loss
2.6.4. Proximate Composition
2.6.5. Texture Analysis (Slice Shear Force) and Cooking Loss
2.6.6. Fatty Acid Analyses
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Quality
3.2. Pig Welfare Observation Before Slaughter
3.3. Meat Quality Parameters
3.4. Effects of SA on Fatty Acid Composition in the Rearing Conditions of Experiments 1 and 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AW | Animal Welfare |
| SA | Space Allowance |
| FA | Fatty Acid |
| SFA | Saturated Fatty Acid |
| MUFA | Monounsaturated Fatty Acid |
| PUFA | Polyunsaturated Fatty Acid |
| ALA | Alpha-Linolenic Acid |
| EPA | Eicosapentaenoic Acid |
| DHA | Docosahexaenoic Acid |
| PDO | Protected Designation of Origin |
| BW | Body Weight |
| ADG | Average Daily Gain |
| FCR | Feed Conversion Ratio |
| SSF | Slice Shear Force |
| GLM | General Linear Model |
| EMM | Estimated Marginal Mean |
| SEM | Standard Error of Mean |
References
- Chatellier, V. Review: International Trade in Animal Products and the Place of the European Union: Main Trends over the Last 20 Years. Animal 2021, 15 (Suppl. S1), 100289. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2024–2033; OECD Publishing, FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2024; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Pugliese, C.; Madonia, G.; Chiofalo, V.; Margiotta, S.; Acciaioli, A.; Gandini, G. Comparison of the performances of Nero Siciliano pigs reared indoors and outdoors. 1. Growth and carcass. Meat Sci. 2003, 65, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Kapelański, W.; Bocian, M.; Pizzuto, R.; Kapelańska, J. Influence of rearing system, diet and gender on performance, carcass traits and meat quality of Polish Landrace pigs. Animal 2013, 7, 341–347. [Google Scholar] [CrossRef]
- Ammann, J.; Mack, G.; El Benni, N.; Jin, S.; Newell-Price, P.; Tindale, S.; Hunter, E.; Vicario-Modroño, V.; Gallardo-Cobos, R.; Sánchez-Zamora, P.; et al. Consumers across five European countries prioritise animal welfare above environmental sustainability when buying meat and dairy products. Food Qual. Prefer. 2024, 117, 105179. [Google Scholar] [CrossRef]
- Bozzo, G.; Barrasso, R.; Grimaldi, C.A.; Tantillo, G.; Roma, R. Consumer attitudes towards animal welfare and their willingness to pay. Vet. Ital. 2019, 55, 289–297. [Google Scholar] [CrossRef]
- Ferrari, P. EU CAP Network Focus Group ‘Alternative Solutions for Livestock Product Differentiation’ Starting Paper. 2024. Available online: https://eu-cap-network.ec.europa.eu/sites/default/files/2025-01/eu-cap-network-fg-livestock-starting-paper.pdf (accessed on 30 June 2025).
- Velarde, A.; Fàbrega, E.; Blanco-Penedo, I.; Dalmau, A. Animal welfare towards sustainability in pork meat production. Meat Sci. 2015, 109, 13–17. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion of the Panel on Animal Health and Welfare on a request from the Commission on Animal health and welfare in fattening pigs in relation to housing and husbandry. EFSA J. 2007, 564, 1–14. [Google Scholar] [CrossRef]
- European Commission. Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs (Codified version). Off. J. Eur. Union 2009, L47, 5–13. [Google Scholar]
- European Commission. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, L150, 1–92. [Google Scholar]
- Commission Regulation (EC) 889/2008 of 5 September 2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Available online: http://data.europa.eu/eli/reg/2008/889/oj (accessed on 30 June 2025).
- Spoolder, H.A.M.; Edwards, S.A.; Corning, S. Legislative methods for specifying stocking density and consequences for the welfare of finishing pigs. Livest. Prod. Sci. 2000, 64, 167–173. [Google Scholar] [CrossRef]
- Liorančas, V.; Bakutis, B.; Januŝkevičiene, G. Influence of rearing space on the behavior, performance, carcass and meat quality of pigs. Med. Weter. 2006, 62, 274–277. [Google Scholar]
- Nannoni, E.; Martelli, G.; Rubini, G.; Sardi, L. Effects of increased space allowance on animal welfare, meat and ham quality of heavy pigs slaughtered at 160Kg. PLoS ONE 2019, 14, e0212417. [Google Scholar] [CrossRef] [PubMed]
- Averós, X.; Aparicio, M.A.; Ferrari, P.; Guy, J.H.; Hubbard, C.; Schmid, O.; Ilieski, V.; Spoolder, H.A.M. The effect of steps to promote higher levels of farm animal welfare across the EU. societal versus animal scientists’ perceptions of animal welfare. Animals 2013, 3, 786–807. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Zhang, S.; Xie, J.; Shan, T. The Effect of Rearing Conditions on Carcass Traits, Meat Quality and the Compositions of Fatty Acid and Amino Acid of LTL in Heigai Pigs. Animals 2022, 12, 14. [Google Scholar] [CrossRef]
- Pugliese, C.; Bozzi, R.; Campodoni, G.; Acciaioli, A.; Franci, O.; Gandini, G. Performance of Cinta Senese pigs reared outdoors and indoors.: 1. Meat and subcutaneous fat characteristics. Meat Sci. 2005, 69, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.P.; Camara, L.; Morales, J.I.; Berrocoso, J.D.; Lopez Bote, C.J.; Mateos, G.G. Effect of gender, housing density and the interaction on growth performance and carcass and meat quality of pigs slaughtered at 110 kg body weight. Span. J. Agric. Res. 2013, 11, 89–99. [Google Scholar] [CrossRef]
- Lebret, B. Effects of feeding and rearing systems on growth, carcass composition and meat quality in pigs. Animals 2008, 2, 1548–1558. [Google Scholar] [CrossRef]
- Patton, B.S.; Huff-Lonergan, E.; Honeyman, M.S.; Kerr, B.J.; Lonergan, S.M. Effects of space allocation within a deep-bedded finishing system on pig growth performance, fatty acid composition and pork quality. Animals 2008, 2, 471–478. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Składanowska-Baryza, J.; Rodriguez-Estevez, V.; Sanz-Fernandez, S.; Diaz-Gaona, C.; Ferrari, P.; Pedersen, L.J.; Couto, M.Y.R.; et al. Husbandry practices associated with extensification in European pig production and their effects on pork quality. Meat Sci. 2023, 206, 109339. [Google Scholar] [CrossRef]
- Toscano, A.; Giannuzzi, D.; Malgwi, I.H.; Halas, D.; Carnier, P.; Gallo, L.; Schiavon, S. Impact of innovative rearing strategies for the Italian heavy pigs: Technological traits and chemical composition of dry–cured hams. Meat Sci. 2023, 204, 109266. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on the use of animal-based measures to assess welfare in pigs. EFSA J. 2012, 10, 2512. [Google Scholar] [CrossRef]
- The SusPigSys Team. Condensed Protocol from Era-Net SusAn Project “Sustainable Pig Production Systems” (SusPigSys). A Starting Point for Connecting Data Bases for Integrated Sustainability Assessment. Deliverable 5.1 2020v. Available online: https://www.researchgate.net/publication/348466379_Condensed_protocol_from_Era-Net_SusAn_project_Sustainablepig_production_systems_SusPigSys (accessed on 21 June 2025).
- Welfare Quality®. Welfare Quality® Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- ILMeteo.it. Archivio Meteo. iLMeteo Srl. Available online: https://www.ilmeteo.it/portale/archivio-meteo (accessed on 29 June 2025).
- ASPA. Metodologie Relative alla Macellazione Degli Animali di Interesse Zootecnico ed alla Valutazione e Dissezione Della Loro Carcassa; ISMEA: Roma, Italy, 1991; pp. 66–70. [Google Scholar]
- Commission Implementing Decision of 24 January 2014 Authorising Methods for Grading Pig Carcases in Italy (Notified Under Document C(2014) 279) (2014/38/EU). Available online: http://data.europa.eu/eli/dec_impl/2014/38/oj (accessed on 30 June 2025).
- Rasmussen, A.J.; Andersson, M. New method for determination of drip loss in pork muscles. In Proceedings of the 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, 1–6 September 1996; Volume 42. [Google Scholar]
- AOAC. Official methods 950.46 moisture, 981.10 crude protein and 991.36 fat (crude) contents in meat and meat products. In Official Methods of Analysis of the AOAC International, Cunniff, P., Ed.; 16th ed.; AOAC International: Gaithersburg, MD, USA, 1999; Volume 2, pp. 1–15. [Google Scholar]
- Pinna, A.; Loffi, C.; Ferrari, P.; Bertolini, A.; Virgili, R. Conversion from Warner-Bratzler Shear Force (WBSF) to Slice Shear Force (SSF) to classify pork loin tenderness. In Proceedings of the 71th International Congress of Meat Science and Technology (ICOMST), Girona, Spain, 3–8 August 2025. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanlye, G.H. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–508. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids (ISO 12966-2:2017). Available online: https://store.uni.com/en-iso-12966-2-2017 (accessed on 30 June 2025).
- Jang, J.C.; Jin, X.H.; Hong, J.S.; Kim, Y.Y. Effects of different space allowances on growth performance, blood profile and pork quality in a grow-to-finish production system. Asian-Australas. J. Anim. Sci. 2017, 30, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Temple, D.; Courboulay, V.; Velarde, A.; Dalmau, A.; Manteca, X. The welfare of growing pigs in five different production systems in France and Spain: Assessment of health. Anim. Welf. 2012, 21, 257–271. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion of the Panel on Animal Health and Welfare on a request from Commission on the risks associated with tail biting in pigs and possible means to reduce the need for tail-docking considering the different housing and husbandry systems. EFSA J. 2007, 611, 1–13. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef]
- Goldberg, A.L.; Jablecki, C.; Li, J.B. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Effects of use and disuse on amino acid transport and protein turnover in muscle. Ann. N. Y. Acad. Sci. 1974, 228, 190–201. [Google Scholar] [CrossRef]
- Sarri, L.; Balcells, J.; Reza Seradj, A.; de la Fuente, J. Protein turnover in pigs: A review of interacting factors. J. Anim. Physiol. Anim. Nutr. 2024, 108, 451–469. (accessed on 30 June 2025). [Google Scholar] [CrossRef]
- Rennie, M.J.; Edwards, R.H.; Davies, C.T.; Krywawych, S.; Halliday, D.; Waterlow, J.C.; Millward, D.J. Protein and amino acid turnover during and after exercise. Biochem. Soc. Trans. 1980, 8, 499–501. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y.; Su, R.; Corazzin, M.; Li, J.; Huang, H.; Zhang, Y.; Yao, D.; Su, L.; Zhao, L.; et al. Effects of Physical Exercise on Muscle Metabolism and Meat Quality Characteristics of Mongolian Sheep. Food Sci. Nutr. 2022, 10, 1494–1509. [Google Scholar] [CrossRef]
- Andersson, A.; Sjödin, A.; Olsson, R.; Vessby, B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 1998, 274, E432–E438. [Google Scholar] [CrossRef]
- Gerlach, B.M. The Effects of Exercise on Beef Cattle Health, Performance, and Carcass Quality; and the Effects of Extended Aging, Blade Tenderization, and Degree of Doneness on Beef Aroma Volatile Formation. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2014; pp. 321–323. [Google Scholar]
- Quiles, J.L.; Huertas, J.R.; Mañas, M.; Battino, M.; Mataix, J. Physical exercise affects the lipid profile of mitochondrial membranes in rats fed with virgin olive oil or sunflower oil. Br. J. Nutr. 1999, 81, 21–24. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Rennie, M.J.; Hickson, R.C.; Conlee, R.K.; Hagberg, J.M. Physiological consequences of the biochemical adaptations to endurance exercise. Ann. N. Y. Acad. Sci. 1977, 301, 440–450. [Google Scholar] [CrossRef] [PubMed]
- McGivney, B.A.; McGettigan, P.A.; Browne, J.A.; Evans, A.C.O.; Fonseca, R.G.; Loftus, B.J.; Lohan, A.; MacHugh, D.E.; Murphy, B.A.; Katz, L.M.; et al. Characterization of the equine skeletal muscle transcriptome identifies novel functional responses to exercise training. BMC Genom. 2010, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Daza, A.; Rey, A.I.; Olivares, A.; Cordero, G.; Toldrà, F.; Lòpez-Bot, C.J. Physical activity-induced alterations on tissue lipid composition and lipid metabolism in fattening pigs. Meat Sci. 2009, 81, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.I.; Cava, R.; Mayoral, A.I.; Tejeda, J.F.; Morcuende, D.; Ruiz, J. Oxidative stability and fatty acid composition of pig muscles as affected by rearing system, crossbreading and metabolic type of muscle fibre. Meat Sci. 2001, 59, 39–47. [Google Scholar] [CrossRef]
- Mc Clelland, G.; Zwingelstein, G.; Taylor, C.R.; Weber, J.M. Effect of exercise on the plasma nonesterified fatty acid composition of dogs and goats: Species with different aerobic capacities and diets. Lipids 1995, 30, 147–153. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.A.J.; Wood, J.D. Fatty acid content and composition of english beef, lamb and pork at retail. Meat Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
| Space Allowance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1—Indoor | Experiment 2—Indoor + Outdoor | |||||||||
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |||||
| Pigs (n.) | 48 | 45 | 48 | 34 | 22 | 33 | ||||
| EEM | EEM | EEM | SEM | p | EEM | EEM | EEM | SEM | p | |
| Bwinitial (Kg) | 52.06 | 52.39 | 52.14 | 0.60 | 0.922 | 37.3 | 36.9 | 31.1 | N.A 1 | N.A 1 |
| Bwfinal (Kg) | 190.7 | 191.4 | 189.9 | 1.99 | 0.871 | 178.0 | 170.3 | 170.8 | 3.56 | 0.265 |
| ADG (Kg/d) | 0.749 | 0.754 | 0.752 | 0.009 | 0.945 | 0.836 | 0.799 | 0.832 | 0.022 | 0.506 |
| FCR | 3.786 | 3.790 | 3.783 | 0.045 | 0.995 | 3.621 | 3.743 | 3.602 | 0.093 | 0.547 |
| Space Allowance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1—Indoor | Experiment 2—Indoor + Outdoor | |||||||||
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |||||
| Pigs (n.) | 48 | 45 | 48 | 34 | 22 | 33 | ||||
| EEM | EEM | EEM | SEM | p | EEM | EEM | EEM | SEM | p | |
| Backfat thickness (mm) | 29.63 | 29.09 | 29.91 | 0.67 | 0.684 | 28.51 | 28.16 | 26.64 | 1.45 | 0.566 |
| Muscle thickness (mm) | 69.18 | 68.06 | 65.83 | 1.31 | 0.183 | 66.27 | 61.50 | 63.49 | 1.99 | 0.329 |
| Lean meat (%) | 52.35 | 52.56 | 52.13 | 0.31 | 0.625 | 52.73 | 53.27 | 53.58 | 0.69 | 0.637 |
| Space Allowance | ||||||
|---|---|---|---|---|---|---|
| Experiment 1—Indoor | Experiment 2—Indoor + Outdoor | |||||
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |
| Bedding in rest area (score 1 to 4) 1 | 4 | 4 | 4 | 2 | 2 | 2 |
| Dirtiness in rest area (score 1 to 3) 2 | 2 | 1.5 | 1 | 3 | 2 | 2 |
| Avoidance behavior (% of visible pigs) | 10 | 17 | 27 | 12 | 63 | 98 |
| Manipulation behavior (% of active pigs) | ||||||
| Wall | 0 | 2 | 0 | 2 | 4 | 2 |
| Floor | 17 | 26 | 16 | 7 | 4 | 7 |
| Pen fixture | 33 | 24 | 18 | 5 | 5 | 2 |
| Chain | 8 | 6 | 0 | 0 | 0 | 0 |
| Pig | 2 | 0 | 5 | 12 | 6 | 2 |
| Space Allowance | ||||||
|---|---|---|---|---|---|---|
| Experiment 1—Indoor | Experiment 2—Indoor + Outdoor | |||||
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |
| Short tail | 2 | 0 | 10 | 0 | 0 | 0 |
| Stump tail | 0 | 0 | 4 | 0 | 0 | 0 |
| Moderately dirty | 52 | 25 | 17 | 64 | 48 | 39 |
| Severely dirty | 19 | 0 | 0 | 30 | 15 | 15 |
| Mild body wounds | 0 | 4 | 0 | 7 | 0 | 0 |
| Severely lame | 2 | 0 | 0 | 4 | 5 | 0 |
| Hernia | 2 | 0 | 0 | 0 | 0 | 3 |
| Mortality rate | 0 | 8 | 0 | 0 | 6 | 2 |
| Space Allowance in Experiment 1—Indoor | |||||
|---|---|---|---|---|---|
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | |||
| Samples (n.) | 19 | 20 | 20 | ||
| EMM | EMM | EMM | SEM | p | |
| pH24h | 5.64 | 5.62 | 5.62 | 0.02 | 0.690 |
| pH48h | 5.64 | 5.64 | 5.63 | 0.02 | 0.755 |
| Drip loss | 1.58 | 1.51 | 2.21 | 0.22 | 0.413 |
| Thawing loss | 6.39 a | 5.14 b | 5.77 a,b | 0.24 | 0.002 |
| Lightness (L*) | 52.3 | 50.9 | 51.6 | 0.61 | 0.276 |
| Redness (a*) | 1.99 | 2.09 | 1.87 | 0.25 | 0.821 |
| Yellowness (b*) | 11.3 | 11.0 | 11.1 | 0.23 | 0.769 |
| Chroma | 11.6 | 11.2 | 11.4 | 0.26 | 0.455 |
| Hue angle (h°) | 80.9 | 80.6 | 81.8 | 1.14 | 0.746 |
| Moisture (%) | 73.3 | 73.0 | 73.3 | 0.15 | 0.367 |
| Protein (%) | 22.8 | 23.1 | 23.1 | 0.14 | 0.070 |
| Fat (%) | 3.31 | 2.94 | 2.83 | 0.22 | 0.270 |
| Cooking loss (%) | 18.6 | 18.2 | 19.5 | 0.66 | 0.386 |
| Slice Shear Force (N) | 111.9 a | 101.6 a,b | 95.1 b | 3.7 | 0.046 |
| Fracturability (N) | 22.7 a | 18.5 a,b | 16.0 b | 0.9 | 0.017 |
| Space Allowance in Experiment 2—Indoor + Outdoor | |||||
|---|---|---|---|---|---|
| 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |||
| Samples (n.) | 34 | 22 | 33 | ||
| EMM | EMM | EMM | SEM | p | |
| pH24h | 5.60 | 5.62 | 5.57 | 0.015 | 0.071 |
| pH48h | 5.57 | 5.61 | 5.58 | 0.014 | 0.158 |
| Drip loss | 1.78 | 1.42 | 1.73 | 0.215 | 0.511 |
| Thawing loss | 6.015 | 6.293 | 6.540 | 0.325 | 0.330 |
| Lightness (L*) | 55.3 a | 57.2 b | 55.8 a,b | 0.574 | 0.037 |
| Redness (a*) | 2.34 a | 1.42 b | 1.95 a,b | 0.871 | 0.042 |
| Yellowness (b*) | 12.6 | 12.5 | 12.3 | 0.194 | 0.294 |
| Chroma | 13.0 | 12.7 | 12.6 | 0.224 | 0.267 |
| Hue angle (h°) | 80.2 b | 84.0 a | 81.6 a,b | 1.212 | 0.042 |
| Moisture (%) | 73.2 a | 72.8 a,b | 72.3 b | 0.235 | 0.001 |
| Protein (%) | 22.8 | 22.7 | 22.6 | 0.125 | 0.367 |
| Fat (%) | 3.54 | 3.92 | 4.26 | 0.218 | 0.073 |
| Cooking loss (%) | 20.6 | 20.6 | 20.7 | 0.654 | 0.994 |
| Slice Shear Force (N) | 82.4 | 73.6 | 79.2 | 2.871 | 0.094 |
| Fracturability (N) | 14.5 | 12.4 | 13.3 | 2.589 | 0.203 |
| Space Allowance in Experiment 1—Indoor | |||||
|---|---|---|---|---|---|
| 1.15 m2/pig | 1.9 m2/pig | 3 m2/pig | |||
| Samples (n.) | 19 | 20 | 20 | ||
| EMM | EMM | EMM | SEM | p | |
| FAs (% total FAs) | |||||
| C10:0 | 0.15 a,b | 0.16 a | 0.13 b | 0.004 | 0.001 |
| C12:0 | 0.11 a | 0.11 a | 0.09 b | 0.006 | 0.049 |
| C13:0 | 0.07 b | 0.13 a | 0.07 b | 0.02 | 0.013 |
| C14:0 | 1.47 | 1.46 | 1.38 | 0.04 | 0.142 |
| C14:1 cis-9 | 0.07 a,b | 0.09 a | 0.04 b | 0.01 | 0.001 |
| C15:0 | 0.06 | 0.07 | 0.14 | 0.06 | 0.238 |
| C15:1 cis-10 | 0.05 | 0.06 | 0.043 | 0.01 | 0.423 |
| C16:0 | 23.12 | 23.37 | 23.34 | 0.21 | 0.380 |
| C16:1 cis-9 | 3.86 | 3.79 | 3.87 | 0.11 | 0.838 |
| C17:0 | 0.18 | 0.19 | 0.16 | 0.01 | 0.076 |
| C17:1 cis-9 | 0.50 | 0.44 | 0.43 | 0.02 | 0.094 |
| C18:0 | 11.40 | 11.37 | 11.77 | 0.22 | 0.548 |
| C18:1 trans-9 | 0.230 a | 0.26 a,b | 0.22 b | 0.02 | 0.002 |
| C18:1 n-9 cis | 45.47 | 45.66 | 45.91 | 0.47 | 0.806 |
| C18:2 n-6 trans | 0.015 | 0.006 | 0.005 | 0.007 | 0.106 |
| C18:2 n-6 cis | 7.36 | 7.32 | 7.31 | 0.33 | 0.994 |
| C18:3 n-6 | 0.10 | 0.10 | 0.08 | 0.01 | 0.140 |
| C18:3 n-3 ALA | 0.76 a | 0.76 a | 0.65 b | 0.03 | 0.015 |
| C20:0 | 0.22 | 0.21 | 0.21 | 0.01 | 0.686 |
| C20:1 cis-9 | 0.38 a,b | 0.42 a | 0.33 b | 0.03 | 0.042 |
| C20:2 n-6 | 0.27 | 0.25 | 0.23 | 0.02 | 0.206 |
| C20:3 n-6 | 0.27 | 0.26 | 0.24 | 0.02 | 0.473 |
| C20:3 n-3 | 0.06 | 0.04 | 0.04 | 0.004 | 0.122 |
| C20:4 n-6 | 2.04 | 1.90 | 1.98 | 0.14 | 0.764 |
| C20:5 n-3 EPA | 0.06 | 0.06 | 0.05 | 0.004 | 0.347 |
| C22:0 | 0.12 a | 0.12 a | 0.09 b | 0.01 | 0.005 |
| C22:1 cis-9 | 0.02 a,b | 0.04 a | 0.01 b | 0.006 | 0.003 |
| C22:4 n-6 | 0.49 | 0.46 | 0.46 | 0.032 | 0.802 |
| C22:5 n-3 | 0.27 | 0.34 | 0.32 | 0.023 | 0.091 |
| C22:6 n-3 DHA | 0.12 a,b | 0.13 a | 0.09 b | 0.01 | 0.005 |
| C24:0 | 0.09 | 0.11 | 0.1 | 0.007 | 0.506 |
| C24:1 cis-9 | 0.07 | 0.06 | 0.06 | 0.005 | 0.829 |
| Σ SFAs | 37.40 | 37.47 | 37.63 | 0.36 | 0.894 |
| Σ MUFAs | 50.76 | 50.90 | 50.92 | 0.48 | 0.965 |
| Σ PUFAs | 11.84 | 11.60 | 11.45 | 0.56 | 0.880 |
| Σ n-6 | 10.54 | 10.30 | 10.30 | 0.52 | 0.935 |
| Σ n-3 | 1.26 a,b | 1.30 a | 1.15 b | 0.04 | 0.015 |
| n-6/n-3 | 8.35 a,b | 7.67 b | 8.98 a | 0.28 | 0.006 |
| Space Allowance in Experiment 2—Indoor + Outdoor | |||||
|---|---|---|---|---|---|
| 1.4 + 1 m2/pig | 2.6 + 2 m2/pig | 3.9 + 3 m2/pig | |||
| Samples (n.) | 34 | 22 | 33 | ||
| EMM | EMM | EMM | SEM | p | |
| FAs (% total FAs) | |||||
| C10:0 | 0.14 | 0.14 | 0.13 | 0.007 | 0.543 |
| C12:0 | 0.10 | 0.09 | 0.09 | 0.007 | 0.601 |
| C13:0 | 0.11 | 0.12 | 0.09 | 0.02 | 0.416 |
| C14:0 | 1.35 b | 1.51 a | 1.39 a,b | 0.04 | 0.023 |
| C14:1 cis-9 | 0.10 | 0.10 | 0.07 | 0.02 | 0.321 |
| C15:0 | 0.07 | 0.07 | 0.13 | 0.04 | 0.269 |
| C15:1 cis-10 | 0.09 | 0.08 | 0.07 | 0.01 | 0.375 |
| C16:0 | 22.47 | 22.72 | 23.42 | 0.31 | 0.105 |
| C16:1 cis-9 | 3.59 | 3.43 | 3.59 | 0.09 | 0.353 |
| C17:0 | 0.22 a,b | 0.23 a | 0.19 b | 0.01 | 0.010 |
| C17:1 cis-9 | 0.52 a | 0.52 a | 0.45 b | 0.03 | 0.020 |
| C18:0 | 10.93 | 11.16 | 10.91 | 0.20 | 0.461 |
| C18:1 trans-9 | 0.27 | 0.27 | 0.26 | 0.02 | 0.968 |
| C18:1 n-9 cis | 45.39 a,b | 44.98 b | 46.11 a | 0.45 | 0.051 |
| C18:2 n-6 trans | 0.002 | 0.003 | 0.007 | 0.004 | 0.651 |
| C18:2 n-6 cis | 8.14 a | 8.27 a | 7.24 b | 0.32 | 0.008 |
| C18:3 n-6 | 0.09 | 0.10 | 0.08 | 0.007 | 0.076 |
| C18:3 n-3 ALA | 0.82 | 0.81 | 0.81 | 0.03 | 0.855 |
| C20:0 | 0.25 | 0.25 | 0.24 | 0.01 | 0.866 |
| C20:1 cis-9 | 0.58 | 0.61 | 0.55 | 0.03 | 0.294 |
| C20:2 n-6 | 0.29 a,b | 0.32 a | 0.27 b | 0.01 | 0.006 |
| C20:3 n-6 | 0.29 | 0.30 | 0.26 | 0.02 | 0.078 |
| C20:3 n-3 | 0.075 a,b | 0.083 a | 0.06 b | 0.005 | 0.042 |
| C20:4 n-6 | 2.13 a,b | 2.20 a | 1.82 b | 0.11 | 0.022 |
| C20:5 n-3 EPA | 0.11 a | 0.12 a | 0.09 b | 0.006 | 0.008 |
| C22:0 | 0.13 | 0.14 | 0.13 | 0.008 | 0.436 |
| C22:1 cis-9 | 0.02 | 0.02 | 0.02 | 0.006 | 0.591 |
| C22:4 n-6 | 0.49 | 0.49 | 0.44 | 0.031 | 0.292 |
| C22:5 n-3 | 0.47 a | 0.49 a | 0.39 b | 0.027 | 0.022 |
| C22:6 n-3 DHA | 0.15 a | 0.12 b | 0.12 b | 0.01 | 0.013 |
| C24:0 | 0.11 a | 0.11 a,b | 0.09 b | 0.007 | 0.017 |
| C24:1 cis-9 | 0.08 | 0.09 | 0.07 | 0.005 | 0.036 |
| Σ SFAs | 35.98 | 36.71 | 37.16 | 0.35 | 0.064 |
| Σ MUFAs | 50.95 a,b | 49.94 b | 51.23 a | 0.41 | 0.042 |
| Σ PUFAs | 13.05 a | 13.31 a | 11.61 b | 0.45 | 0.012 |
| Σ n-6 | 11.43 a | 11.68 a | 10.14 b | 0.41 | 0.013 |
| Σ n-3 | 1.62 a | 1.63 a | 1.47 b | 0.05 | 0.004 |
| n-6/n-3 | 7.05 | 7.17 | 6.89 | 0.18 | 0.217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, P.; Bertolini, A.; Garavaldi, A.; Faeti, V.; Bergamaschi, M.; Loffi, C.; Pinna, A.; Virgili, R. Effect of Space Allowance on Pig Performance, Carcass Traits and Meat Quality in Italian Heavy Pigs Reared Under Two Housing Systems. Foods 2025, 14, 2817. https://doi.org/10.3390/foods14162817
Ferrari P, Bertolini A, Garavaldi A, Faeti V, Bergamaschi M, Loffi C, Pinna A, Virgili R. Effect of Space Allowance on Pig Performance, Carcass Traits and Meat Quality in Italian Heavy Pigs Reared Under Two Housing Systems. Foods. 2025; 14(16):2817. https://doi.org/10.3390/foods14162817
Chicago/Turabian StyleFerrari, Paolo, Andrea Bertolini, Anna Garavaldi, Valerio Faeti, Monica Bergamaschi, Cecilia Loffi, Anna Pinna, and Roberta Virgili. 2025. "Effect of Space Allowance on Pig Performance, Carcass Traits and Meat Quality in Italian Heavy Pigs Reared Under Two Housing Systems" Foods 14, no. 16: 2817. https://doi.org/10.3390/foods14162817
APA StyleFerrari, P., Bertolini, A., Garavaldi, A., Faeti, V., Bergamaschi, M., Loffi, C., Pinna, A., & Virgili, R. (2025). Effect of Space Allowance on Pig Performance, Carcass Traits and Meat Quality in Italian Heavy Pigs Reared Under Two Housing Systems. Foods, 14(16), 2817. https://doi.org/10.3390/foods14162817





