Recent Advances, Challenges, and Functional Applications of Protein Chemical Modification in the Food Industry
Abstract
1. Introduction
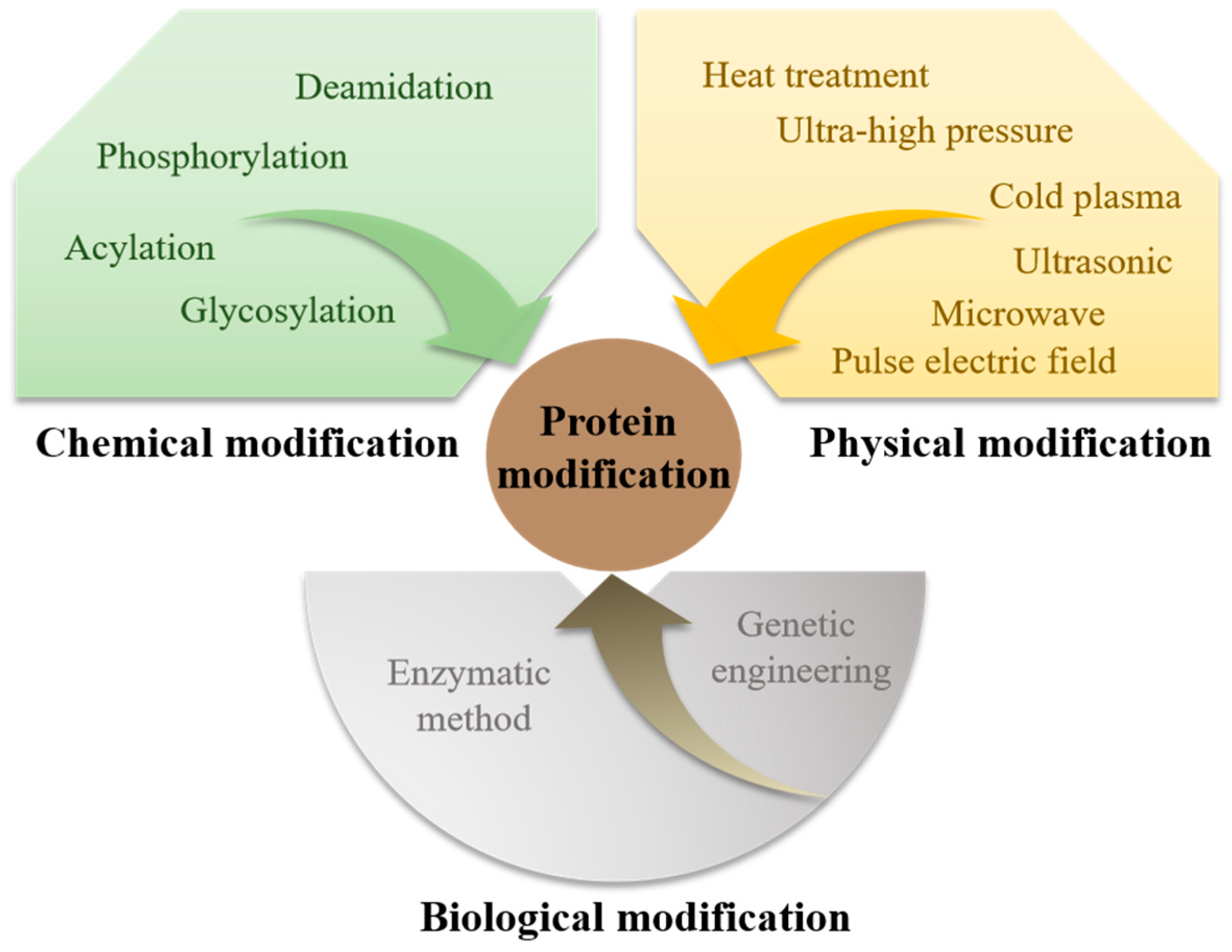
2. Types and Methods of Protein Chemical Modification
2.1. Deamidation
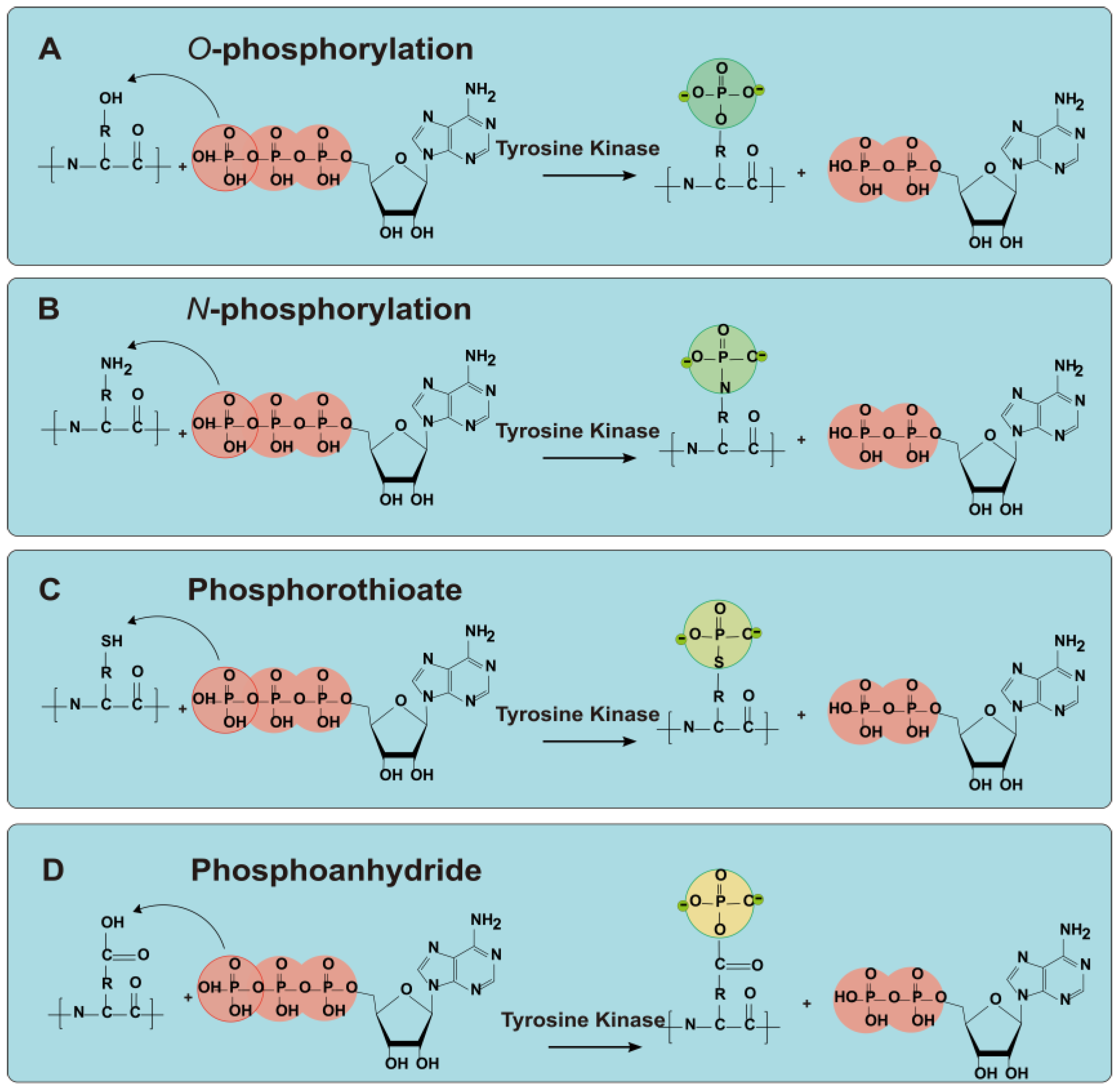
2.2. Phosphorylation
2.3. Glycosylation
2.4. Acylation
2.5. Other Chemical Modifications
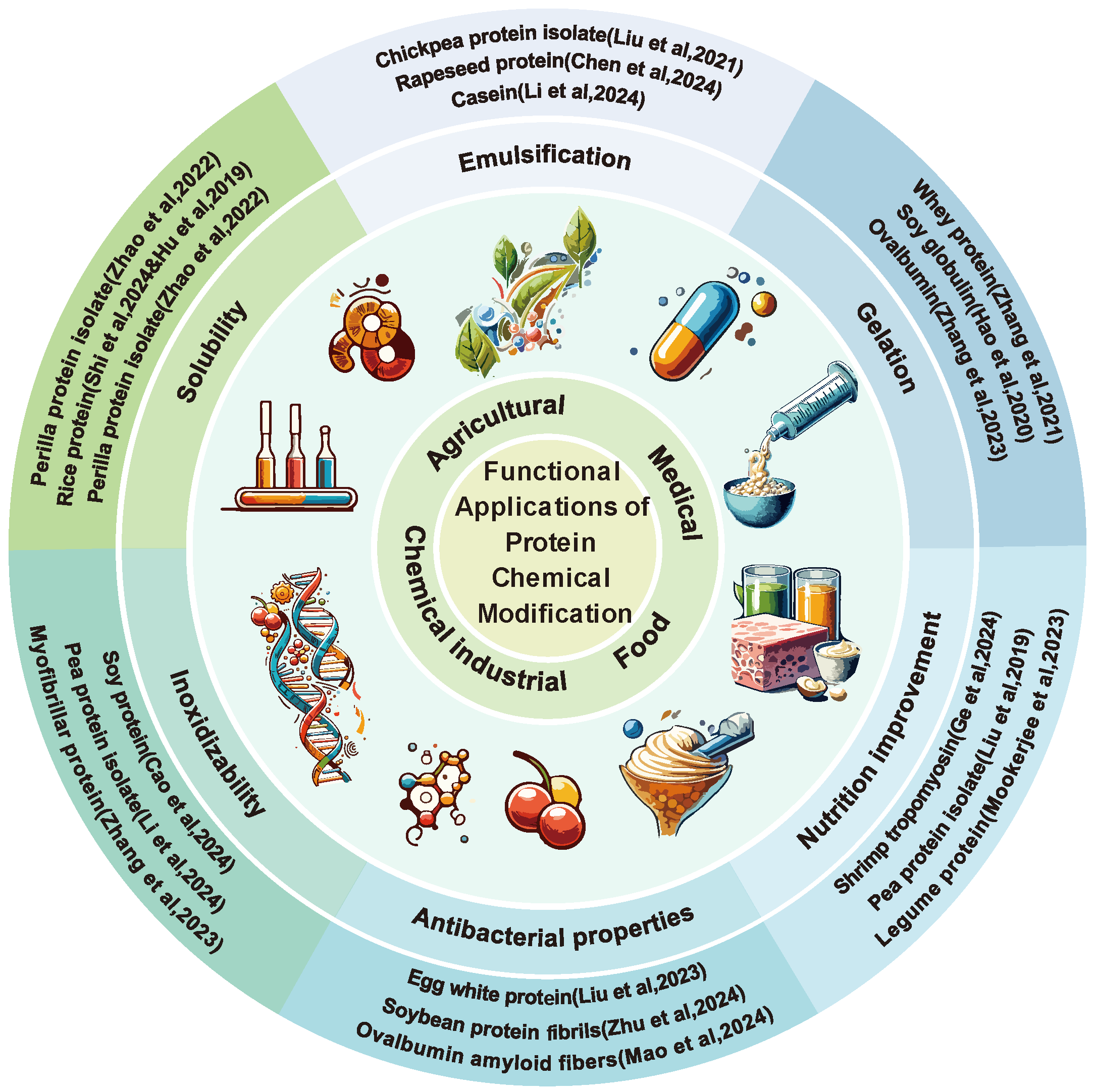
3. The Improvement of Protein Functional Properties Through Chemical Modification
3.1. The Improvement of Protein Solubility Through Chemical Modification
3.2. The Improvement of Protein Emulsification Through Chemical Modification
3.3. The Improvement of Protein Gelation Through Chemical Modification
3.4. The Improvement of Protein Inoxidizability Through Chemical Modification
3.5. The Improvement of Protein Antibacterial Properties Through Chemical Modification
3.6. The Improvement of Protein Nutrition Through Chemical Modification
4. The Effects of Chemical Modification Combined with Other Modification Methods on the Functional Properties of Proteins
4.1. Chemical Modification Combined with Physical Modification
4.2. Chemical Modification Combined with Biological Modification
5. Artificial Intelligence Empowers the Chemical Modification of Proteins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Y.L. 5—Muscle proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 127–148. [Google Scholar]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- King, J.; Leong, S.Y.; Alpos, M.; Johnson, C.; McLeod, S.; Peng, M.; Sutton, K.; Oey, I. Role of food processing and incorporating legumes in food products to increase protein intake and enhance satiety. Trends Food Sci. Technol. 2024, 147, 104466. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, P.; Li, N.; Li, J.; Chen, J.; Zhou, J. Production of 2-keto-L-gulonic acid by metabolically engineered Escherichia coli. Bioresour. Technol. 2020, 318, 124069. [Google Scholar] [CrossRef]
- Mefleh, M.; Omri, G.; Limongelli, R.; Minervini, F.; Santamaria, M.; Faccia, M. Enhancing nutritional and sensory properties of plant-based beverages: A study on chickpea and Kamut® flours fermentation using Lactococcus lactis. Front. Nutr. 2024, 11, 1269154. [Google Scholar] [CrossRef]
- Le Priol, L.; Dagmey, A.; Morandat, S.; Saleh, K.; El Kirat, K.; Nesterenko, A. Comparative study of plant protein extracts as wall materials for the improvement of the oxidative stability of sunflower oil by microencapsulation. Food Hydrocoll. 2019, 95, 105–115. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Islam, F.; Amer Ali, Y.; Imran, A.; Afzaal, M.; Zahra, S.M.; Fatima, M.; Saeed, F.; Usman, I.; Shehzadi, U.; Mehta, S.; et al. Vegetable proteins as encapsulating agents: Recent updates and future perspectives. Food Sci. Nutr. 2023, 11, 1705–1717. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2021, 39, 2428–2456. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef]
- Leroy, F.; Abraini, F.; Beal, T.; Dominguez-Salas, P.; Gregorini, P.; Manzano, P.; Rowntree, J.; van Vliet, S. Animal board invited review: Animal source foods in healthy, sustainable, and ethical diets—An argument against drastic limitation of livestock in the food system. Animal 2022, 16, 100457. [Google Scholar] [CrossRef]
- Lin, X.; Ye, L.; He, K.; Zhang, T.; Sun, F.; Mei, T.; Wu, X. A new method to reduce allergenicity by improving the functional properties of soybean 7S protein through covalent modification with polyphenols. Food Chem. 2022, 373, 131589. [Google Scholar] [CrossRef] [PubMed]
- Pilolli, R.; Nitride, C.; Gillard, N.; Huet, A.C.; van Poucke, C.; de Loose, M.; Tranquet, O.; Larre, C.; Adel-Patient, K.; Bernard, H.; et al. Critical review on proteotypic peptide marker tracing for six allergenic ingredients in incurred foods by mass spectrometry. Food Res. Int. 2020, 128, 108747. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juarez, M.L.; Aalhus, J.L.; Shand, P.; Estevez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G. Chapter 9—The whey proteins in milk: Thermal denaturation, physical interactions, and effects on the functional properties of milk. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 325–384. [Google Scholar]
- Zha, F.; Rao, J.; Chen, B. Modification of pulse proteins for improved functionality and flavor profile: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3036–3060. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Structure, gelation mechanism of plant proteins versus dairy proteins and evolving modification strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Violleau, F.; Silvestre, F.; Durrieu, V. The effect of vegetable protein modifications on the microencapsulation process. Food Hydrocoll. 2014, 41, 95–102. [Google Scholar] [CrossRef]
- Zheng, L.; San, Y.; Xing, Y.; Regenstein, J.M. Rice proteins: A review of their extraction, modification techniques and applications. Int. J. Biol. Macromol. 2024, 268, 131705. [Google Scholar] [CrossRef]
- Ni, X.; Chen, C.; Li, R.; Liu, Q.; Duan, C.; Wang, X.; Xu, M. Effects of ultrasonic treatment on the structure and functional characteristics of myofibrillar proteins from black soldier fly. Int. J. Biol. Macromol. 2024, 278, 135057. [Google Scholar] [CrossRef]
- Nakamura, S.; Kato, A. Multi-functional biopolymer prepared by covalent attachment of galactomannan to egg-white proteins through naturally occurring Maillard reaction. Nahrung/Food 2000, 44, 201–206. [Google Scholar] [CrossRef]
- Babiker, E.E. Effect of transglutaminase treatment on the functional properties of native and chymotrypsin-digested soy protein. Food Chem. 2000, 70, 139–145. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Colombo, A.; Rosell, C.M. Enzymatic modifications of pea protein and its application in protein–cassava and corn starch gels. Food Hydrocoll. 2012, 27, 185–190. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Liu, X.; Zhang, M.; Chu, P.; Zhu, B.; Liu, D.; Zhou, D. Achieving dual functions of texture modification and water retention of shrimp surimi products with the combination of epigallocatechin-3-gallate and gamma-cyclodextrin. Food Chem. 2023, 418, 136034. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-S.; Sun, Q.-Q.; Zhao, Y.-Y.; Zhong, X.-Y.; Mu, D.-D.; Jiang, S.-T.; Luo, S.-Z.; Zheng, Z. Transglutaminase-set colloidal properties of wheat gluten with ultrasound pretreatments. Ultrason. Sonochem. 2017, 39, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.A.; Lasse, M.; Cottam, J.; Healy, J.P.; Fayle, S.E.; Rasiah, I.; Brown, P.K.; BinYasir, S.M.; Sutton, K.H.; Larsen, N.G. Aspects of physical and chemical alterations to proteins during food processing—Some implications for nutrition. Br. J. Nutr. 2012, 108 (Suppl. S2), S288–S297. [Google Scholar] [CrossRef]
- Zeng, W.; Xue, J.; Geng, H.; Liu, X.; Yang, J.; Shen, W.; Yuan, Y.; Qiang, Y.; Zhu, Q. Research progress on chemical modifications of tyrosine residues in peptides and proteins. Biotechnol. Bioeng. 2024, 121, 799–822. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, X.; Ping, Q.; Cheng, J.; Sun, M.; Cao, F.; You, W.; Yuan, D. A novel lipoprotein-mimic nanocarrier composed of the modified protein and lipid for tumor cell targeting delivery. J. Control. Release 2010, 146, 299–308. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, H.; Li, X. Precision in protein chemical modification and total synthesis. Chem 2024, 10, 767–799. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, M.; Ren, J.; Zhao, H.; Cui, C.; Hu, X. Effect of acetic acid deamidation-induced modification on functional and nutritional properties and conformation of wheat gluten. J. Sci. Food Agric. 2009, 90, 409–417. [Google Scholar] [CrossRef]
- Shi, W.; Xie, H.; Ouyang, K.; Shi, Q.; Xiong, H.; Zhao, Q. Enhancing the solubility and emulsion properties of rice protein by deamidation of citric acid-based natural deep eutectic solvents. Food Res. Int. 2024, 175, 113762. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Wu, W.-K.; Hsiao, J.-T.; Hsieh, S.-C.; Sheu, F. Combination of chemical modifications improves rice protein solubility. J. Cereal Sci. 2024, 118, 103939. [Google Scholar] [CrossRef]
- Zhao, Q.; Hong, X.; Fan, L.; Liu, Y.; Li, J. Solubility and emulsifying properties of perilla protein isolate: Improvement by phosphorylation in the presence of sodium tripolyphosphate and sodium trimetaphosphate. Food Chem. 2022, 382, 132252. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.F.; Shih, F.F.; Marshall, W.E. Enzymic phosphorylation of soy protein isolate for improved functional properties. J. Agric. Food Chem. 1992, 40, 403–406. [Google Scholar] [CrossRef]
- Matsudomi, N.; Nakano, K.; Soma, A.; Ochi, A. Improvement of gel properties of dried egg white by modification with galactomannan through the Maillard reaction. J. Agric. Food Chem. 2002, 50, 4113–4118. [Google Scholar] [CrossRef]
- Hao, Z.-Z.; Peng, X.-Q.; Tang, C.-H. Edible pickering high internal phase emulsions stabilized by soy glycinin: Improvement of emulsification performance and pickering stabilization by glycation with soy polysaccharide. Food Hydrocoll. 2020, 103, 105672. [Google Scholar] [CrossRef]
- Chobert, J.-M.; Gaudin, J.-C.; Dalgalarrondo, M.; Haertlé, T. Impact of Maillard type glycation on properties of beta-lactoglobulin. Biotechnol. Adv. 2006, 24, 629–632. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- Lang, Y.; Huang, L.; Han, D.; Li, C.; Bian, P.; Xie, P.; Yang, X. Octenyl succinylation of myofibrillar protein: Structural, physicochemical and emulsifying properties. LWT 2024, 201, 116279. [Google Scholar] [CrossRef]
- Hamada, J.S. Deamidation of food proteins to improve functionality. Crit. Rev. Food Sci. Nutr. 1994, 34, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Zhou, P.; Fan, D. Beef tenderization without exacerbating the cooking loss: The way of enzymatic deamidation. Food Biosci. 2024, 58, 103806. [Google Scholar] [CrossRef]
- Hu, Y.; Du, L.; Sun, Y.; Zhou, C.; Pan, D. Recent developments in phosphorylation modification on food proteins: Structure characterization, site identification and function. Food Hydrocoll. 2023, 137, 108390. [Google Scholar] [CrossRef]
- Yu, L.; Yang, W.; Sun, J.; Zhang, C.; Bi, J.; Yang, Q. Preparation, characterisation and physicochemical properties of the phosphate modified peanut protein obtained from Arachin conarachin L. Food Chem. 2015, 170, 169–179. [Google Scholar] [CrossRef]
- Nayak, S.K.; Arora, S.; Sindhu, J.S.; Sangwan, R.B. Effect of chemical phosphorylation on solubility of buffalo milk proteins. Int. Dairy J. 2006, 16, 268–273. [Google Scholar] [CrossRef]
- Sanchez-Resendiz, A.; Rodriguez-Barrientos, S.; Rodriguez-Rodriguez, J.; Barba-Davila, B.; Serna-Saldivar, S.O.; Chuck-Hernandez, C. Phosphoesterification of soybean and peanut proteins with sodium trimetaphosphate (STMP): Changes in structure to improve functionality for food applications. Food Chem. 2018, 260, 299–305. [Google Scholar] [CrossRef]
- Cao, R.; Wang, B.; Bai, T.; Zhu, Y.; Cheng, J.; Zhang, J. Structural and functional impacts of glycosylation-induced modifications in rabbit myofibrillar proteins. Int. J. Biol. Macromol. 2024, 283, 137583. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Cooking-Induced Protein Modifications in Meat. Compr. Rev. Food Sci. Food Saf. 2016, 16, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Jimenezcastano, L.; Lopezfandino, R.; Olano, A.; Villamiel, M. Study on β-lactoglobulin glycosylation with dextran: Effect on solubility and heat stability. Food Chem. 2005, 93, 689–695. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, H.; Zhao, M.; Cui, C.; Liu, L. Physicochemical properties of soy protein isolates-acacia gum conjugates. Czech J. Food Sci. 2011, 29, 129–136. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and emulsifying properties of whey protein isolate (WPI)-dextran conjugates produced in aqueous solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef]
- Alleyn, M.; Breitzig, M.; Lockey, R.; Kolliputi, N. The dawn of succinylation: A posttranslational modification. Am. J. Physiol. Cell Physiol. 2018, 314, C228–C232. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y. Acylation modification and/or guar gum conjugation enhanced functional properties of pea protein isolate. Food Hydrocoll. 2021, 117, 106686. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wang, Y.; Jiang, Y.; Min, E.H.; Rao, S.Q. Effects of dual succinylation and ultrasonication modification on the structural and functional properties of ovalbumin. Food Res. Int. 2023, 165, 112511. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar Singh, S.; Singha, P. A comprehensive review on the recent trends in extractions, pretreatments and modifications of plant-based proteins. Food Res. Int. 2024, 190, 114575. [Google Scholar] [CrossRef]
- Sani, M.A.; Mozafarpour, R.; Kia, A.G.; Khorasani, S.; Dara, A.; McClements, D.J. Grass pea protein as an emerging source of sustainable plant proteins: Structure, modification, functionality, and applications. Food Biosci. 2024, 62, 105092. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Comparative study of encapsulation of vitamins with native and modified soy protein. Food Hydrocoll. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein isolate. Food Chem. 2020, 325, 126921. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, J.; Zhang, X.; Xie, F.; Zhang, S.; Jiang, L.; Qi, B.; Li, Y. Effect of pH-shifting treatment on the structural and functional properties of soybean protein isolate and its interactions with (–)-epigallocatechin-3-gallate. Process Biochem. 2021, 101, 190–198. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Yuan, B.; Qi, B.; Li, Y. Fibrillation of soy protein isolate in the presence of metal ions: Structure and gelation behavior. Food Chem. 2024, 453, 139672. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, C.; Ma, Y.; Shen, L.; Gong, Q.; Hu, Z.; Wang, Z.; Liu, X.; Guo, Z.; Zhou, L. Study on the Structure, Function, and Interface Characteristics of Soybean Protein Isolate by Industrial Phosphorylation. Foods 2023, 12, 1108. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, L.; Sun, Y.; Xiong, H.; Ogra, Y. Improvement of the solubility and emulsifying properties of rice bran protein by phosphorylation with sodium trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Parandi, E.; Mousavi, M.; Assadpour, E.; Kiani, H.; Jafari, S.M. Sesame protein hydrolysate-gum Arabic Maillard conjugates for loading natural anthocyanins: Characterization, in vitro gastrointestinal digestion and storage stability. Food Hydrocoll. 2024, 148, 109490. [Google Scholar] [CrossRef]
- Huang, S.; Feng, X.; Yue, W.; Madjirebaye, P.; Deng, X.; Fan, Y.; Chen, J.; Wu, X. Effects of chemical modifications on allergenicity and functional properties of silkworm pupae proteins. Food Chem. 2025, 477, 143635. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional properties and structural characteristics of phosphorylated pea protein isolate. Int. J. Food Sci. Technol. 2019, 55, 2002–2010. [Google Scholar] [CrossRef]
- He, W.; Yang, R.; Zhao, W. Effect of acid deamidation-alcalase hydrolysis induced modification on functional and bitter-masking properties of wheat gluten hydrolysates. Food Chem. 2019, 277, 655–663. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Gao, L.; Zhou, X.; Ma, P.; Wang, Q. Effect of different carbohydrates on the functional properties of black rice glutelin (BRG) modified by the maillard reaction. J. Cereal Sci. 2020, 93, 102979. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Wang, Z.; Lan, T.; Jiang, J.; Song, T.; Liu, J.; Zhang, H.; Lin, K. On the modification of plant proteins: Traditional methods and the Hofmeister effect. Food Chem. 2024, 451, 139530. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Ibarz, A. Modified mung bean protein: Optimization of microwave-assisted phosphorylation and its functional and structural characterizations. LWT 2021, 151, 112119. [Google Scholar] [CrossRef]
- Miedzianka, J.; Walkowiak, K.; Zielinska-Dawidziak, M.; Zambrowicz, A.; Wolny, S.; Kita, A. The Functional and Physicochemical Properties of Rice Protein Concentrate Subjected to Acetylation. Molecules 2023, 28, 770. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Zhou, X.; Deng, Q.; Dong, X.; Yang, C.; Huang, F. Astaxanthin-loaded emulsion gels stabilized by Maillard reaction products of whey protein and flaxseed gum: Physicochemical characterization and in vitro digestibility. Food Res. Int. 2021, 144, 110321. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, H.; Feng, T.; Bu, X.; Cui, C.; Yang, F.; Yu, C. Advancing soy protein isolate-ulvan film physicochemical properties and antioxidant activities through strategic high-pressure homogenization technique. Ind. Crops Prod. 2024, 215, 118704. [Google Scholar] [CrossRef]
- Li, W.; Han, S.; Huang, H.; McClements, D.J.; Chen, S.; Ma, C.; Liu, X.; Liu, F. Fabrication, characterization, and application of pea protein isolate-polyphenol-iron complexes with antioxidant and antibacterial activity. Food Hydrocoll. 2024, 150, 109729. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Ruan, J.; Jiang, Z.; Gong, F.; Lei, W.; Wang, X.; Zhao, J.; Meng, Q.; Xu, M.; et al. Combination of millet pepper and garlic water extracts improves the antioxidant capability of myofibrillar protein under malondialdehyde-induced oxidative modification. LWT 2023, 174, 114472. [Google Scholar] [CrossRef]
- Mao, S.; Zeng, Y.; Ren, Y.; Ye, X.; Tian, J. EGCG induced the formation of protein nanofibrils hydrogels with enhanced anti-bacterial activity. Food Hydrocoll. 2024, 157, 110408. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Liu, S.; Miao, L.; Dong, H.; Tong, X.; Jiang, L. Complexes of soybean protein fibrils and chlorogenic acid: Interaction mechanism and antibacterial activity. Food Chem. 2024, 452, 139551. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Lv, J.; Wu, Y.; Guo, Y.; Sun, C.; Li, X. Biodegradable composite films based on egg white protein and tea polyphenol: Physicochemical, structural and antibacterial properties. Food Packag. Shelf Life 2023, 38, 101098. [Google Scholar] [CrossRef]
- Mookerjee, A.; Tanaka, T. Influence of enzymatic treatments on legume proteins for improved functional and nutritional properties: Expansion of legume protein utilization as food ingredients. Curr. Opin. Food Sci. 2023, 49, 100974. [Google Scholar] [CrossRef]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2006, 70, R54–R66. [Google Scholar] [CrossRef]
- Shepherd, R. Dairy glycoconjugate emulsifiers: Casein–maltodextrins. Food Hydrocoll. 2000, 14, 281–286. [Google Scholar] [CrossRef]
- Lam, R.S.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ru, Y.; Weng, H.; Zhang, Y.; Chen, J.; Xiao, A.; Xiao, Q. Agar-gelatin Maillard conjugates used for Pickering emulsion stabilization. Carbohydr. Polym. 2024, 340, 122293. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2011, 131, 901–906. [Google Scholar] [CrossRef]
- Tan, M.; Xu, J.; Gao, H.; Yu, Z.; Liang, J.; Mu, D.; Li, X.; Zhong, X.; Luo, S.; Zhao, Y.; et al. Effects of combined high hydrostatic pressure and pH-shifting pretreatment on the structure and emulsifying properties of soy protein isolates. J. Food Eng. 2021, 306, 110622. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, Y.; Tan, Z.; Zhou, D.; Li, D. Characterization of a novel chickpea protein-pullulan hydrogels that efficiently load and release sodium salts: Microstructures, molecular dynamics, and rheological properties. Food Res. Int. 2025, 204, 115951. [Google Scholar] [CrossRef] [PubMed]
- Speroni, F.; Jung, S.; de Lamballerie, M. Effects of calcium and pressure treatment on thermal gelation of soybean protein. J. Food Sci. 2010, 75, E30–E38. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, X.; Ma, B.; Chen, Y.; Batool, Z.; Fu, X.; Jin, Y. Modification methods and applications of egg protein gel properties: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef]
- Xu, M.; Li, R.; Li, M.; Duan, C.; Wei, Y.; Ni, X.; Wang, X.; Fu, S.; Yu, R. Tenebrio molitor powder enhances gelation properties of Penaeus vannamei myofibrillar protein: Mechanistic insights into structural optimization and digestibility. Food Chem. 2025, 490, 145065. [Google Scholar] [CrossRef]
- Liang, P.J.; Chen, S.M.; Fang, X.; Wu, J.F. Recent advance in modification strategies and applications of soy protein gel properties. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13276. [Google Scholar] [CrossRef]
- Spotti, M.J.; Loyeau, P.A.; Marangón, A.; Noir, H.; Rubiolo, A.C.; Carrara, C.R. Influence of Maillard reaction extent on acid induced gels of whey proteins and dextrans. Food Hydrocoll. 2019, 91, 224–231. [Google Scholar] [CrossRef]
- Guan, H.; Feng, C.; Ren, M.; Xu, X.; Liu, D.; Diao, X. Emulsifying property, antioxidant activity, and bitterness of soybean protein isolate hydrolysate obtained by Corolase PP under high hydrostatic pressure. Food Sci. Hum. Wellness 2024, 13, 1271–1278. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; He, J.; Tang, W.; Liu, J. Innovative multistep modifications of β-Lactoglobulin for enhanced emulsifying and antioxidant activities. Food Hydrocoll. 2024, 148, 109465. [Google Scholar] [CrossRef]
- Zhang, W.; Boateng, I.D.; Xu, J.; Zhang, Y. Proteins from Legumes, Cereals, and Pseudo-Cereals: Composition, Modification, Bioactivities, and Applications. Foods 2024, 13, 1974. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Ding, Q.; Wang, D.; Yuan, T.; Song, G.; Seong, H.; Gong, J. Formation and functional improvement of α-casein, β-lactoglobulin, and hyaluronic acid conjugates via the Maillard reaction: Comparison with different mass ratios. Food Chem. 2025, 475, 143322. [Google Scholar] [CrossRef] [PubMed]
- Briceño-Islas, G.; Mojica, L.; Urías-Silvas, J.E. Functional chia (Salvia hispanica L.) co-product protein hydrolysate: An analysis of biochemical, antidiabetic, antioxidant potential and physicochemical properties. Food Chem. 2024, 460, 140406. [Google Scholar] [CrossRef]
- Yan, X.; Gao, Y.; Liu, S.; Zhang, G.; Zhao, J.; Cheng, D.; Zeng, Z.; Gong, X.; Yu, P.; Gong, D. Covalent modification by phenolic extract improves the structural properties and antioxidant activities of the protein isolate from Cinnamomum camphora seed kernel. Food Chem. 2021, 352, 129377. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.-Q.; Zhang, R.-Y.; Gao, X.-R.; Wu, L.; Zhang, Y.; Wang, Z.-R.; Gao, L.; Yang, Z.-Q. Formation mechanism, environmental stability and antibacterial activity of succinylated ovalbumin/ε-polylysine nanogel loaded with thymol: The synergistic roles of succinylation modification and ε-polylysine. Ind. Crops Prod. 2024, 220, 119192. [Google Scholar] [CrossRef]
- Keppler, J.K.; Martin, D.; Garamus, V.M.; Berton-Carabin, C.; Nipoti, E.; Coenye, T.; Schwarz, K. Functionality of whey proteins covalently modified by allyl isothiocyanate. Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocoll. 2017, 65, 130–143. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.; Du, Z.; Zhao, G.; Zang, J. Construction of protein hydrogels with antibacterial activity by interaction of β-lactoglobulin amyloid fibrils with epigallocatechin-3-gallate. Food Biosci. 2024, 58, 103632. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, X.; Chai, Y.; Li, X.; Chen, L.; Feng, X. Superabsorbent whey protein isolates/chitosan-based antibacterial aerogels: Preparation, characterization and application in chicken meat preservation. Int. J. Biol. Macromol. 2024, 259, 128961. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.-G.; Cheng, J.-H.; Sun, D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.; Shi, C.; Gu, J.; Shao, Y.; Chen, Q.; Li, Y.; Mezzenga, R. Amyloid-Polyphenol Hybrid Nanofilaments Mitigate Colitis and Regulate Gut Microbial Dysbiosis. ACS Nano 2020, 14, 2760–2776. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Liang, Y.; Wu, W. Potential implications of oxidative modification on dietary protein nutritional value: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 714–751. [Google Scholar] [CrossRef]
- Aziznia, S.; Askari, G.; Emamdjomeh, Z.; Salami, M. Effect of ultrasonic assisted grafting on the structural and functional properties of mung bean protein isolate conjugated with maltodextrin through maillard reaction. Int. J. Biol. Macromol. 2024, 254, 127616. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Li, L.; Chi, H.; Teng, F. High-pressure homogenization assisted pH-shifting modified soybean lipophilic protein interacting with chitosan hydrochloride: Double emulsion construction, physicochemical properties, stability, and in vitro digestion analysis. Food Hydrocoll. 2025, 160, 110834. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, C.; Bai, R.; Li, Z.; Zhang, L.; Chen, Y.; Ye, X.; Wang, S.; Jiang, H.; Ding, W. Modifying duck myofibrillar proteins using sodium bicarbonate under cold plasma treatment: Impact on the conformation, emulsification, and rheological properties. Food Hydrocoll. 2024, 150, 109682. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, D.; Chen, Z.; Li, M.; Jin, H. Effect of glucose glycosylation following limited enzymatic hydrolysis on functional and conformational properties of black bean protein isolate. Eur. Food Res. Technol. 2018, 244, 1111–1120. [Google Scholar] [CrossRef]
- Song, C.-L.; Zhao, X.-H. Structure and property modification of an oligochitosan-glycosylated and crosslinked soybean protein generated by microbial transglutaminase. Food Chem. 2014, 163, 114–119. [Google Scholar] [CrossRef]
- Fu, M.; Zhao, X.H. Modified properties of a glycated and cross-linked soy protein isolate by transglutaminase and an oligochitosan of 5 kDa. J. Sci. Food Agric. 2016, 97, 58–64. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, Q.; Yu, Z.; Wang, X.; Zhao, X.H. Effects of transglutaminase glycosylated soy protein isolate on its structure and interfacial properties. J. Sci. Food Agric. 2021, 101, 5097–5105. [Google Scholar] [CrossRef]
- Jiang, S.-J.; Zhao, X.-H. Transglutaminase-induced cross-linking and glucosamine conjugation of casein and some functional properties of the modified product. Int. Dairy J. 2011, 21, 198–205. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Fang, F.; Chen, D.; Federici, E.; Pan, S.; Jones, O.G. Limited hydrolysis and conjugation of zein with chitosan oligosaccharide by enzymatic reaction to improve functional properties. Food Chem. 2021, 348, 129035. [Google Scholar] [CrossRef]
- Liu, L.; Duan, J.; Zhang, X.; Su, K.; Duan, Y.; Cheng, W.; Zhang, X. Unlocking the functional potential of porcine hemoglobin: An enzymatic hydrolysis-phosphorylation synergistic modification to enhance minced pork quality. Food Biosci. 2025, 68, 106425. [Google Scholar] [CrossRef]
- Cizauskas, C.; DeBenedictis, E.; Kelly, P. How the past is shaping the future of life science: The influence of automation and AI on biology. New Biotechnol. 2025, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemi, M.; Ganbold, O.; Rotaru, K. Humans vs. large language models: Judgmental forecasting in an era of advanced AI. Int. J. Forecast. 2025, 41, 631–648. [Google Scholar] [CrossRef]
- Chia, B.S.; Seah, Y.F.S.; Wang, B.; Shen, K.; Srivastava, D.; Chew, W.L. Engineering a New Generation of Gene Editors: Integrating Synthetic Biology and AI Innovations. ACS Synth. Biol. 2025, 14, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Gnad, F.; Ren, S.; Choudhary, C.; Cox, J.; Mann, M. Predicting post-translational lysine acetylation using support vector machines. Bioinformatics 2010, 26, 1666–1668. [Google Scholar] [CrossRef]
- Holzinger, A.; Keiblinger, K.; Holub, P.; Zatloukal, K.; Muller, H. AI for life: Trends in artificial intelligence for biotechnology. New Biotechnol. 2023, 74, 16–24. [Google Scholar] [CrossRef]
- Fujita, S.; Terada, T. Enhanced prediction of protein functional identity through the integration of sequence and structural features. Comput. Struct. Biotechnol. J. 2024, 23, 4124–4130. [Google Scholar] [CrossRef]
- Meador, K.; Castells-Graells, R.; Aguirre, R.; Sawaya, M.R.; Arbing, M.A.; Sherman, T.; Senarathne, C.; Yeates, T.O. A suite of designed protein cages using machine learning and protein fragment-based protocols. Structure 2024, 32, 751–765.E11. [Google Scholar] [CrossRef]
- Cheng, Y.; Bi, X.; Xu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Artificial intelligence technologies in bioprocess: Opportunities and challenges. Bioresour. Technol. 2023, 369, 128451. [Google Scholar] [CrossRef]
- Chou, K.-C. Pseudo Amino Acid Composition and its Applications in Bioinformatics, Proteomics and System Biology. Curr. Proteom. 2009, 6, 262–274. [Google Scholar] [CrossRef]
- Shen, M.; Zhan, Z.-H.; Chen, W.-N.; Gong, Y.-J.; Zhang, J.; Li, Y. Bi-Velocity Discrete Particle Swarm Optimization and Its Application to Multicast Routing Problem in Communication Networks. IEEE Trans. Ind. Electron. 2014, 61, 7141–7151. [Google Scholar] [CrossRef]
- Tomii, K.; Kanehisa, M. AAindex: A Database of Amino Acid Indices and Mutation Matrices. Genome Inform. 1995, 6, 142–143. [Google Scholar]
- Liu, Z.; Cao, J.; Gao, X.; Ma, Q.; Ren, J.; Xue, Y. GPS-CCD: A novel computational program for the prediction of calpain cleavage sites. PLoS ONE 2011, 6, e19001. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lyons, J.; Dehzangi, A.; Paliwal, K.K. A feature extraction technique using bi-gram probabilities of position specific scoring matrix for protein fold recognition. J. Theor. Biol. 2013, 320, 41–46. [Google Scholar] [CrossRef]
- Alasi, S.O.; Sanusi, M.S.; Sunmonu, M.O.; Odewole, M.M.; Adepoju, A.L. Exploring recent developments in novel technologies and AI integration for plant-based protein functionality: A review. J. Agric. Food Res. 2024, 15, 101036. [Google Scholar] [CrossRef]
- Esmaili, F.; Pourmirzaei, M.; Ramazi, S.; Shojaeilangari, S.; Yavari, E. A Review of Machine Learning and Algorithmic Methods for Protein Phosphorylation Site Prediction. Genom. Proteom. Bioinform. 2023, 21, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xue, Y.; Yao, X.; Xu, Y. CSS-Palm: Palmitoylation site prediction with a clustering and scoring strategy (CSS). Bioinformatics 2006, 22, 894–896. [Google Scholar] [CrossRef]
- Gou, Y.; Liu, D.; Chen, M.; Wei, Y.; Huang, X.; Han, C.; Feng, Z.; Zhang, C.; Lu, T.; Peng, D.; et al. GPS-SUMO 2.0: An updated online service for the prediction of SUMOylation sites and SUMO-interacting motifs. Nucleic Acids Res. 2024, 52, W238–W247. [Google Scholar] [CrossRef]
- Guo, Y.; Ning, W.; Jiang, P.; Lin, S.; Wang, C.; Tan, X.; Yao, L.; Peng, D.; Xue, Y. GPS-PBS: A Deep Learning Framework to Predict Phosphorylation Sites that Specifically Interact with Phosphoprotein-Binding Domains. Cells 2020, 9, 1266. [Google Scholar] [CrossRef]
- Ning, W.; Jiang, P.; Guo, Y.; Wang, C.; Tan, X.; Zhang, W.; Peng, D.; Xue, Y. GPS-Palm: A deep learning-based graphic presentation system for the prediction of S-palmitoylation sites in proteins. Brief. Bioinform. 2021, 22, 1836–1847. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, D.; Han, C.; Gou, Y.; Chen, M.; Huang, X.; Liu, D.; Zhao, M.; Xiao, L.; Xiao, Q.; et al. GPS-pPLM: A Language Model for Prediction of Prokaryotic Phosphorylation Sites. Cells 2024, 13, 1854. [Google Scholar] [CrossRef]
- Dipta, S.R.; Taherzadeh, G.; Ahmad, M.W.; Arafat, M.E.; Shatabda, S.; Dehzangi, A. SEMal: Accurate protein malonylation site predictor using structural and evolutionary information. Comput. Biol. Med. 2020, 125, 104022. [Google Scholar] [CrossRef]
- Li, Y.; Pu, F.; Feng, Y.; Ji, J.; Sun, H.; Wang, H. MRMD-palm: A novel method for the identification of palmitoylated protein. Chemom. Intell. Lab. Syst. 2021, 210, 104245. [Google Scholar] [CrossRef]
- Li, Y.X.; Shao, Y.H.; Deng, N.Y. Improved prediction of palmitoylation sites using PWMs and SVM. Protein Pept. Lett. 2011, 18, 186–193. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, R.; Kumar, M. PalmPred: An SVM based palmitoylation prediction method using sequence profile information. PLoS ONE 2014, 9, e89246. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zheng, Y.; Li, H.; Luo, X.; He, Z.; Cao, S.; Shi, Y.; Zhao, Q.; Xue, Y.; Zuo, Z.; et al. GPS-Lipid: A robust tool for the prediction of multiple lipid modification sites. Sci. Rep. 2016, 6, 28249. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Xu, H.; Jiang, P.; Cheng, H.; Deng, W.; Guo, Y.; Xue, Y. HybridSucc: A Hybrid-learning Architecture for General and Species-specific Succinylation Site Prediction. Genom. Proteom. Bioinform. 2020, 18, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.L.; Kao, H.J.; Huang, C.H.; Lee, T.Y. MDD-Palm: Identification of protein S-palmitoylation sites with substrate motifs based on maximal dependence decomposition. PLoS ONE 2017, 12, e0179529. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.J.; Wang, H.L.; Tan, H.; Zhang, Z.D.; Webb, G.I.; Song, J.N. Accurate in silico identification of species-specific acetylation sites by integrating protein sequence-derived and functional features. Sci. Rep. 2014, 4, 5765. [Google Scholar] [CrossRef]
- Biggar, K.K.; Charih, F.; Liu, H.; Ruiz-Blanco, Y.B.; Stalker, L.; Chopra, A.; Connolly, J.; Adhikary, H.; Frensemier, K.; Hoekstra, M.; et al. Proteome-wide Prediction of Lysine Methylation Leads to Identification of H2BK43 Methylation and Outlines the Potential Methyllysine Proteome. Cell Rep. 2020, 32, 107896. [Google Scholar] [CrossRef]
| Modification Method | Protein Type | Strategy | Critical Operational Step | Modified Effect | Bottlenecks | References |
|---|---|---|---|---|---|---|
| Deamidation | Wheat gluten; rice protein | Acetic acid (0.03–0.14 mol/L) and HCl (0.05–0.22 mol/L) were added separately to a 100 g/L wheat gluten suspension and heated at 121 °C. | Acid/enzyme concentration | Disrupts H-bonding; promotes backbone cleavage (Asn), enhancing the charge density and electrostatic repulsion of proteins and improving emulsification, emulsion stability, and solubility | Unstable modification efficiency | [34,35,36] |
| Phosphorylation | Perilla protein isolate; soy protein isolate | PPI was mixed with STPP and STMP, adjusted to pH 9, and agitated at 45 °C for 2 h. | Phosphorylating agent selection and dosage, pH regulation | Induces conformational shifts; alters electrostatic interactions, enhancing the electrostatic repulsion between protein molecules; results in improved solubility, emulsifying properties, and foaming ability | Reagent residue in final product, requiring additional purification steps | [37,38] |
| Glycosylation | Egg white protein; soybean globulin; casein | The mixture of GM and DEW, with a weight ratio of 1:4, was subjected to dry-heat treatment at 60 °C and 65% relative humidity. | Dry-heat duration/temperature | Adds hydrophilic glycans; significantly alters hydrophilicity; the mechanical strength and water retention ability of the gel were augmented, concurrently enhancing the gel’s transparency and thermal stability | Undesirable flavor compounds | [39,40,41] |
| Acylation | Oat protein isolate; myofibrillar proteins | Add succinic anhydride to an OPI aqueous suspension that has a pH of 8 and a concentration of 5%. | pH regulation and acylating agent dosage | Adds hydrophobic chains, significantly enhance the solubility and emulsifying properties of the protein | Unreacted acylating agents may remain, raising food safety concerns | [42,43] |
| Modification Method | Protein Type | Chemical Reagents | Functional Characteristics | References |
|---|---|---|---|---|
| Phosphorylation | Soybean protein isolate; buffalo milk proteins | Sodium tripolyphosphate and sodium hexametaphosphate | Structural changes, emulsibility, solubility | [48,66] |
| Acylation | Pea protein isolate; egg white proteins | Acetic anhydride and succinic anhydride | Oil-holding capacity, gelation, emulsibility | [29,58] |
| Phosphorylation | Rice bran protein | Sodium trimetaphosphate | Structural changes, solubility, emulsibility activity, solubility | [67] |
| Glycosylation | Sesame protein | Gum arabic | Solubility, thermal stability | [68] |
| Phosphorylation, succinylation, deamidation, and glycosylation | Silkworm pupae proteins | Sodium tripolyphosphate, succinic anhydride, and acetic acid | Water-holding capacity, foaming ability and foaming stability | [69] |
| Phosphorylation | Pea protein isolate | Sodium tripolyphosphate | Solubility, viscosity, emulsibility, foaming ability | [70] |
| Deamidation | Wheat gluten; beef myofibrillar proteins | Acetic acid, tartaric acid, and citric acid | Allergenicity, water-holding capacity, emulsibility, solubility | [45,71] |
| Glycosylation | Black rice glutelin; rabbit myofibrillar proteins | Arabinose, sodium alginate, maltodextrin, and lactose | Structural changes, solubility, emulsion stability | [50,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Zhang, Z.; Ran, W.; Bai, T.; Cheng, J.; Zhang, J. Recent Advances, Challenges, and Functional Applications of Protein Chemical Modification in the Food Industry. Foods 2025, 14, 2784. https://doi.org/10.3390/foods14162784
Zhao P, Zhang Z, Ran W, Bai T, Cheng J, Zhang J. Recent Advances, Challenges, and Functional Applications of Protein Chemical Modification in the Food Industry. Foods. 2025; 14(16):2784. https://doi.org/10.3390/foods14162784
Chicago/Turabian StyleZhao, Peiming, Zhiyan Zhang, Wei Ran, Ting Bai, Jie Cheng, and Jiamin Zhang. 2025. "Recent Advances, Challenges, and Functional Applications of Protein Chemical Modification in the Food Industry" Foods 14, no. 16: 2784. https://doi.org/10.3390/foods14162784
APA StyleZhao, P., Zhang, Z., Ran, W., Bai, T., Cheng, J., & Zhang, J. (2025). Recent Advances, Challenges, and Functional Applications of Protein Chemical Modification in the Food Industry. Foods, 14(16), 2784. https://doi.org/10.3390/foods14162784





