From Grass to Graph: NIRS Calibration for Fatty Acid Profiling in Grass-Raised Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Meat Samples
2.2. Analysis of Total FA Composition
2.3. NIRS and Chemometric Analysis
3. Results
3.1. Meat Fatty Acid Composition
3.2. NIRS Models
3.2.1. NIR Spectral Features
3.2.2. NIRS Calibration and Validation
3.2.3. External Validation of Fatty Acid Prediction
4. Discussion
4.1. NIRs Prediction
4.2. External Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Giaretta, E.; Mordenti, A.; Palmonari, A.; Brogna, N.; Canestrari, G.; Belloni, P.; Cavallini, D.; Mammi, L.; Cabbri, R.; Formigoni, A. NIRs calibration models for chemical composition and fatty acid families of raw and freeze-dried beef: A comparison. J. Food Compos. Anal. 2019, 83, 103257. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Aldai, N.; Dugan, M.E.R.; Kramer, J.K.G.; Martinez, A.C.; López-Campos, Ó.; Mantecón, Á.R.; Osoro, K. Length of concentrate finishing affects the fatty acid composition of grass-fed and genetically lean beef: An emphasis on trans-18:1 and conjugated linoleic acid profiles. Anim. Int. J. Anim. Biosci. 2011, 5, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Lamas, L.; Aldai, N.; Kramer, J.K.; Barron, L.J.R. Case study using commercial dairy sheep flocks: Comparison of the fat nutritional quality of milk produced in mountain and valley farms. LWT 2018, 89, 374–380. [Google Scholar] [CrossRef]
- Dilzer, A.M.; Park, Y. Implication of Conjugated Linoleic Acid (CLA) in Human Health. Crit. Rev. Food Sci. Nutr. 2012, 52, 488–513. [Google Scholar] [CrossRef]
- Morales, R.; Parga, J.; Subiabre, I.; Realini, C.E. Finishing strategies for steers based on pasture or silage plus grain and time on feed and their effects on beef quality. Cienc. E Investig. Agrar. 2015, 42, 5–18. [Google Scholar] [CrossRef]
- Toro-Mujica, P.; Vera-Infanzón, R. Evolving Dairy Cattle Systems in Chile: Structural Shifts and Adaptation Strategies. Animals 2024, 14, 2245. [Google Scholar] [CrossRef]
- Howes, N.L.; Bekhit, A.E.D.A.; Burritt, D.J.; Campbell, A.W. Opportunities and implications of pasture-based lamb fattening to enhance the long-chain fatty acid composition in meat. Compr. Rev. Food Sci. Food Saf. 2015, 14, 22–36. [Google Scholar] [CrossRef]
- Morales, R.; Folch, C.; Iraira, S.; Teuber, N.; Esteve-Garcia, E. Nutritional quality of beef produced in Chile from different production systems. Chil. J. Agric. Res. 2012, 72, 80–86. [Google Scholar] [CrossRef]
- Sales, F.; Bravo-Lamas, L.; Realini, C.E.; Lira, R.; Aldai, N.; Morales, R. Grain supplementation of calves as an alternative beef production system to pasture-finished steers in Chilean Patagonia: Meat quality and fatty acid composition. Transl. Anim. Sci. 2020, 4, 352–362. [Google Scholar] [CrossRef]
- Subiabre, I.; Rodríguez, R.A.; Aldai, N.; Allende, R.; Morales, R. Pasture type effects over beef quality: A comparison. Chil. J. Agric. Res. 2024, 84, 620–631. [Google Scholar] [CrossRef]
- Aldai, N.; Kramer, J.K.; Cruz-Hernandez, V.S.; Delmonte, P.; Mossoba, M.M.; Dugan, M.E.R. Appropiate extraction and methylation techniques for lipid analysis. In Fats and Fatty Acids in Poultry Nutrition and Health; Cherian, G., Poureslami, R., Eds.; Chapter 12; Context Products: London, UK, 2012; pp. 249–278. [Google Scholar]
- Kramer, J.K.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan, M.E. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Perez-Palacios, T.; Solomando, J.C.; Ruiz-Carrascal, J.; Antequera, T. Improvements in the methodology for fatty acids analysis in meat products: One-stage transmethylation and fast-GC method. Food Chem. 2022, 371, 130995. [Google Scholar] [CrossRef] [PubMed]
- Dugan, M.E.R.; Wood, J.D. Letter to the editor. Meat Sci. 2018, 143, 268. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Wang, X. Near-infrared spectroscopy for food quality evaluation. In Evaluation Technologies for Food Quality; Elsevier: Duxford, UK, 2019; pp. 105–118. [Google Scholar]
- Andueza, D.; Listrat, A.; Durand, D.; Normand, J.; Mourot, B.; Gruffat, D. Prediction of beef meat fatty acid composition by visible-near-infrared spectroscopy was improved by preliminary freeze-drying. Meat Sci. 2019, 158, 107910. [Google Scholar] [CrossRef]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Mourot, B.-P.; Gruffat, D.; Durand, D.; Chesneau, G.; Mairesse, G.; Andueza, D. Breeds and muscle types modulate performance of near-infrared reflectance spectroscopy to predict the fatty acid composition of bovine meat. Meat Sci. 2015, 99, 104–112. [Google Scholar] [CrossRef]
- Arias, R. Beef production and the beef evaluation system in Chile: Description, characterization, and quality. Anim. Front. 2024, 14, 21–28. [Google Scholar] [CrossRef]
- ODEPA. Encuesta Ganado Bovino Año 2019. [En Línea]; Oficina de Estudios y Políticas Agrarias (Odepa): Santiago, Chile, 2022. [Google Scholar]
- Delmonte, P.; Kia, A.-R.F.; Kramer, J.K.; Mossoba, M.M.; Sidisky, L.; Rader, J.I. Separation characteristics of fatty acid methyl esters using SLB-IL111, a new ionic liquid coated capillary gas chromatographic column. J. Chromatogr. A 2011, 1218, 545–554. [Google Scholar] [CrossRef]
- Alves, S.P.; Bessa, R.J. Comparison of two gas–liquid chromatograph columns for the analysis of fatty acids in ruminant meat. J. Chromatogr. A 2009, 1216, 5130–5139. [Google Scholar] [CrossRef]
- Alves, S.P.; Bessa, R.J. The trans-10, cis-15 18:2: A missing intermediate of trans-10 shifted rumen biohydrogenation pathway? Lipids 2014, 49, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, C.; Deng, Z.; Zhou, J.; Hill, A.R.; Yurawecz, M.P.; Delmonte, P.; Mossoba, M.M.; Dugan, M.E.; Kramer, J.K. Methods for analysis of conjugated linoleic acids and trans-18:1 isomers in dairy fats by using a combination of gas chromatography, silver-ion thin-layer chromatography/gas chromatography, and silver-ion liquid chromatography. J. AOAC Int. 2004, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, P.; Fardin-Kia, A.R.; Kramer, J.K.; Mossoba, M.M.; Sidisky, L.; Tyburczy, C.; Rader, J.I. Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J. Chromatogr. A 2012, 1233, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Wolff, R.L.; Precht, D.; Molkentin, J. Study of individual trans-and cis-16:1 isomers in cow, goat, and ewe cheese fats by gas-liquid chromatography with emphasis on the trans-Δ3 isomer. Lipids 2000, 35, 1027–1032. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Tyburczy, C.; Brenna, J.T.; Juárez, M.; de la Fuente, M.A. Characterization of cis-9 trans-11 trans-15 C18:3 in milk fat by GC and covalent adduct chemical ionization tandem MS. J. Lipid Res. 2009, 50, 2412–2420. [Google Scholar] [CrossRef]
- Rego, O.; Alves, S.; Antunes, L.; Rosa, H.; Alfaia, C.; Prates, J.; Cabrita, A.; Fonseca, A.; Bessa, R. Rumen biohydrogenation-derived fatty acids in milk fat from grazing dairy cows supplemented with rapeseed, sunflower, or linseed oils. J. Dairy Sci. 2009, 92, 4530–4540. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bravo-Lamas, L.; Kramer, J.K.; Aldai, N. Limitation of using silver ion solid-phase extraction for animal lipids with a low trans content. Eur. J. Lipid Sci. Technol. 2014, 116, 1621–1625. [Google Scholar] [CrossRef]
- Belaunzaran, X.; Bravo-Lamas, L.; Kramer, J.K.; Morales, R.; Aldai, N. Silver ion solid-phase extraction cartridges employing glass housings overcome the limitations observed in the GC analysis of animal lipids with low trans fatty acid content. Eur. J. Lipid Sci. Technol. 2017, 119, 1600124. [Google Scholar] [CrossRef]
- Berzaghi, P.; Riovanto, R. Near infrared spectroscopy in animal science production: Principles and applications. Ital. J. Anim. Sci. 2009, 8 (Suppl. S3), 39–62. [Google Scholar] [CrossRef]
- Conzen, J.P. Multivariate Calibration: A Practical Guide for Developing Methods in the Quantitative Analytical Chemistry, 3rd English Edition ed.; Optik Bruker, Ed.; Optik Bruker: Ettlingen, Germany, 2014; p. 127. [Google Scholar]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Fearn, T. Assessing calibrations: SEP, RPD, RER and R2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Williams, P. The RPD statistic: A tutorial note. NIR News 2014, 25, 22–26. [Google Scholar] [CrossRef]
- Duckett, S.; Neel, J.; Lewis, R.M.; Fontenot, J.; Clapham, W. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef]
- Ripoll García, G.; Failla, S.; Panea Doblado, B.; Hocquette, J.F.; Dunner, S.; Olleta Castañer, J.L.; Chistensen, M.; Ertbjerg, P.; Richardson, I.; Conto, M. Near-Infrared Reflectance Spectroscopy for Predicting the Phospholipid Fraction and the Total Fatty Acid Composition of Freeze-Dried Beef. Sensors 2021, 21, 4230. [Google Scholar] [CrossRef]
- Prieto, N.; Dugan, M.; Juárez, M.; López-Campos, Ó.; Zijlstra, R.; Aalhus, J. Using portable near-infrared spectroscopy to predict pig subcutaneous fat composition and iodine value. Can. J. Anim. Sci. 2017, 98, 221–229. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.; Li, J.; Wang, Z.; Zhang, L. Determination of fatty acids in broiler breast meat by near-infrared reflectance spectroscopy. Meat Sci. 2012, 90, 658–664. [Google Scholar] [CrossRef]
- Gonzalez-Martın, I.; González-Pérez, C.; Alvarez-Garcıa, N.; González-Cabrera, J. On-line determination of fatty acid composition in intramuscular fat of Iberian pork loin by NIRs with a remote reflectance fibre optic probe. Meat Sci. 2005, 69, 243–248. [Google Scholar] [CrossRef]
- Pla, M.; Hernández, P.; Ariño, B.; Ramírez, J.; Díaz, I. Prediction of fatty acid content in rabbit meat and discrimination between conventional and organic production systems by NIRS methodology. Food Chem. 2007, 100, 165–170. [Google Scholar] [CrossRef]
- Lobos-Ortega, I.; Pizarro-Aránguiz, N.; Urrutia, N.; Silva-Lemus, M.; Pavez-Andrades, P.; Subiabre-Riveros, I.; Torres-Püschel, D. Determination of nutritional health indexes of fresh bovine milk using near infrared spectroscopy. Grasas Y Aceites 2022, 73, e458. [Google Scholar] [CrossRef]
- Zomeño, C.; Juste, V.; Hernández, P. Application of NIRS for predicting fatty acids in intramuscular fat of rabbit. Meat Sci. 2012, 91, 155–159. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Revilla, I.; Hernández-Ramos, P.; Vivar-Quintana, A.M. Prediction of the fatty acid profiles of iberian pig products by near infrared spectroscopy: A comparison between multiple regression tools and artificial neural Networks. Food Bioprocess Technol. 2025, 18, 737–755. [Google Scholar] [CrossRef]
- Prieto, N.; Dugan, M.; Lopez-Campos, O.; Aalhus, J.; Uttaro, B. At line prediction of PUFA and biohydrogenation intermediates in perirenal and subcutaneous fat from cattle fed sunflower or flaxseed by near infrared spectroscopy. Meat Sci. 2013, 94, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Dugan, M.; López-Campos, O.; McAllister, T.; Aalhus, J.; Uttaro, B. Near infrared reflectance spectroscopy predicts the content of polyunsaturated fatty acids and biohydrogenation products in the subcutaneous fat of beef cows. Meat Sci. 2012, 90, 43–51. [Google Scholar] [CrossRef]
- Guy, F.; Prache, S.; Thomas, A.; Bauchart, D.; Andueza, D. Prediction of lamb meat fatty acid composition using near-infrared reflectance spectroscopy (NIRS). Food Chem. 2011, 127, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
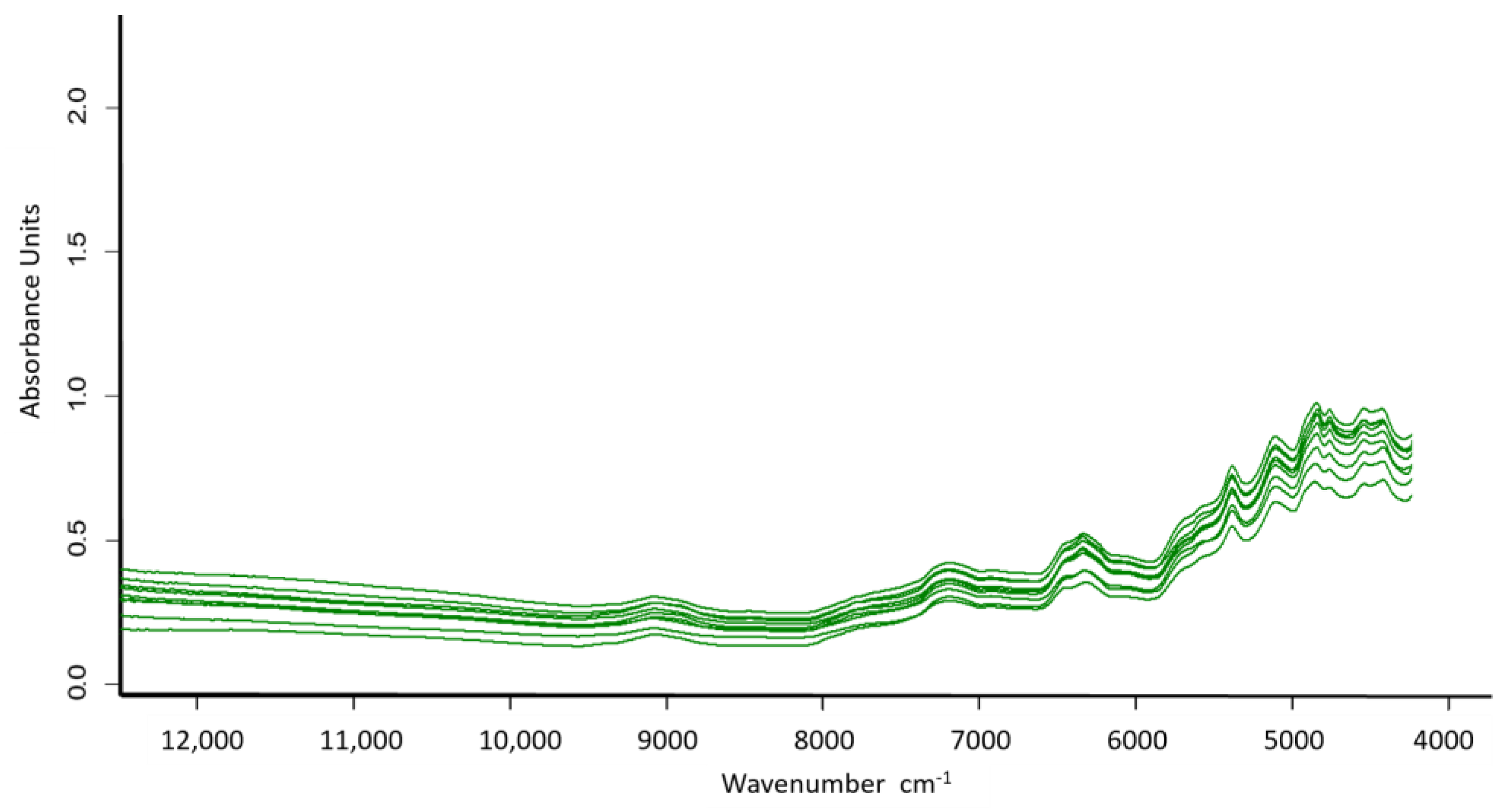
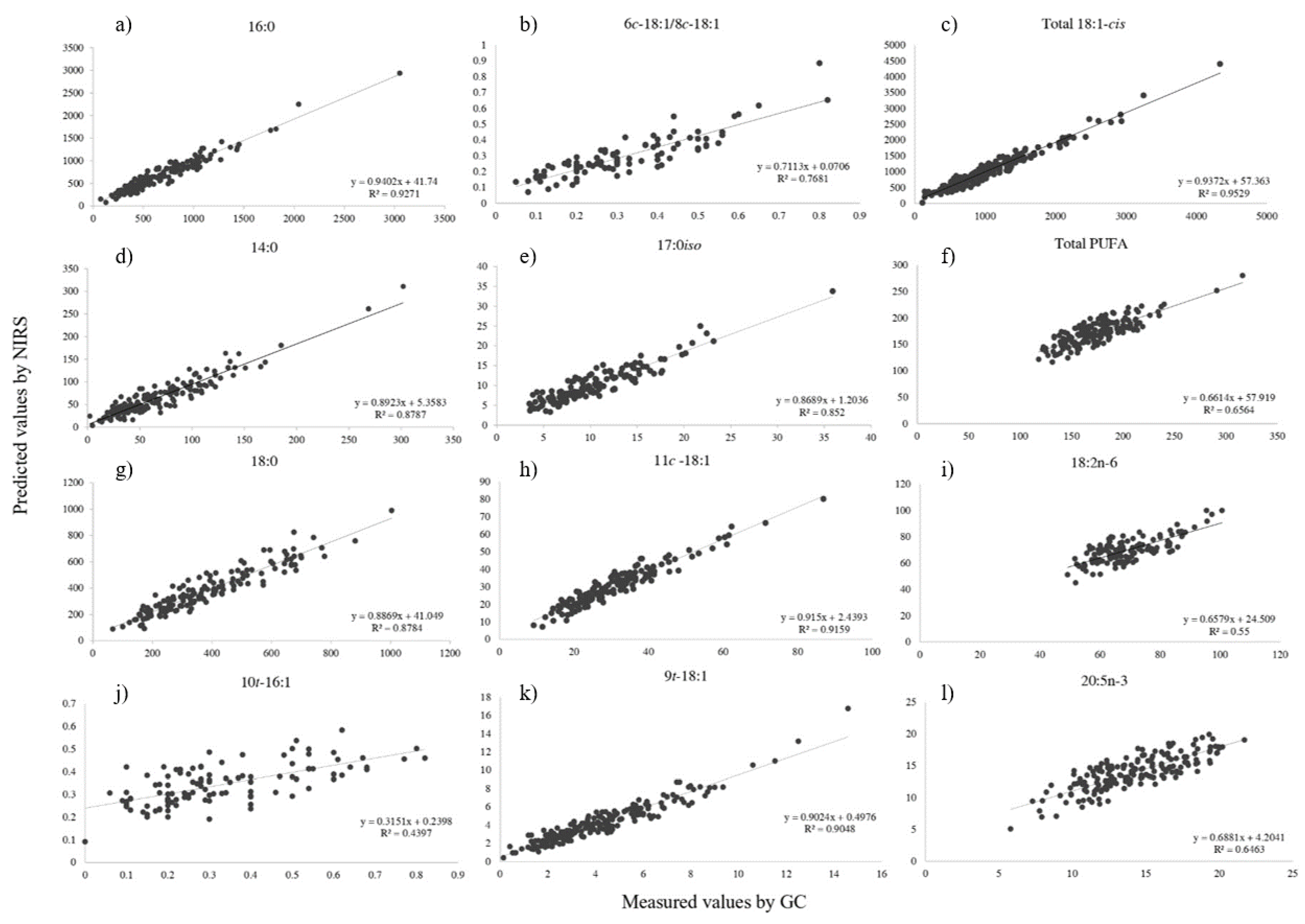
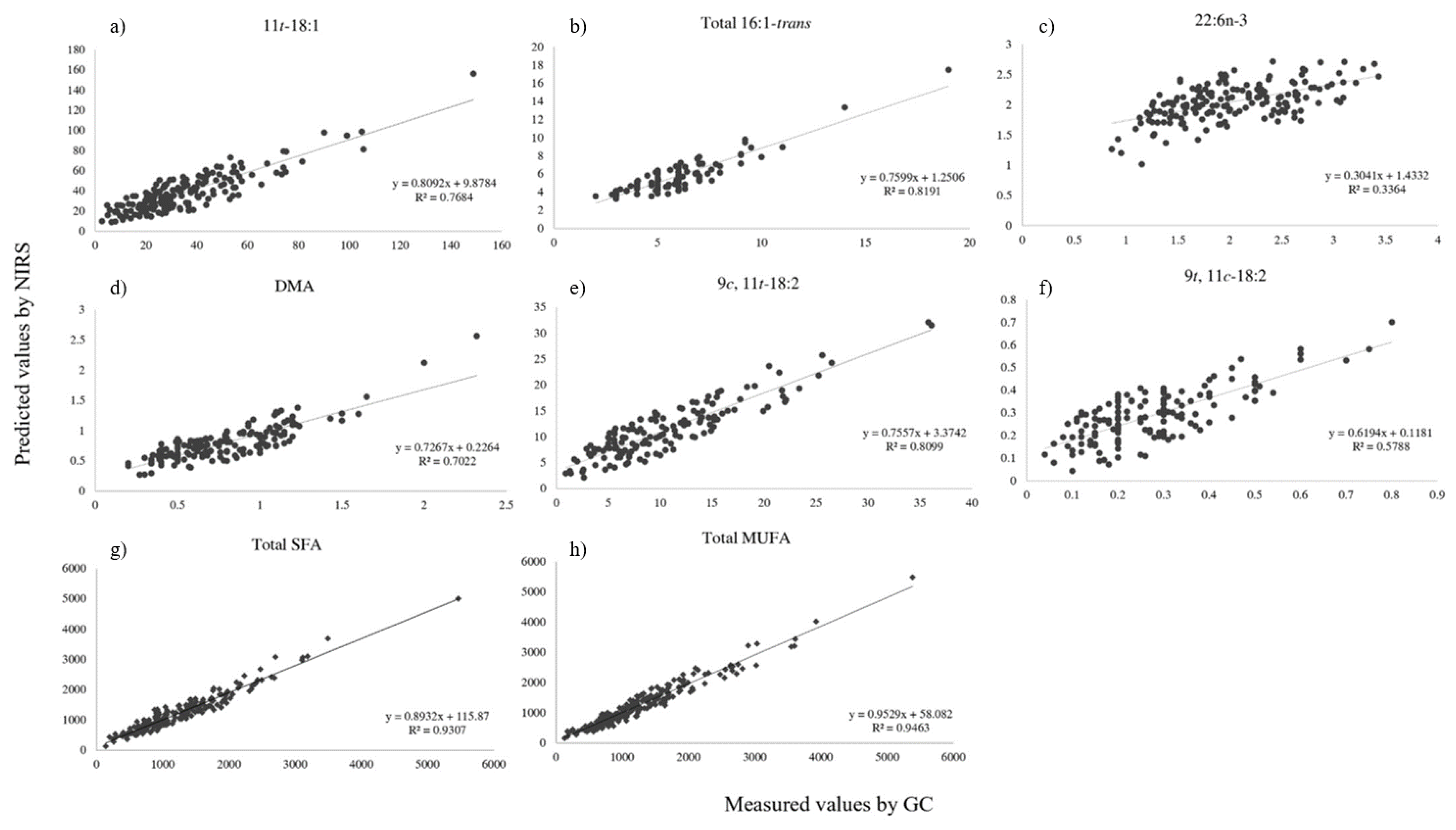
| Calibration (n = 272) | Validation (n = 271) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid | Mean ± SD | Min | Max | CV | Mean ± SD | Min | Max | CV |
| (mg · 100 g−1 Meat) | (%) | (mg · 100 g−1 Meat) | (%) | |||||
| Σ FAME | 2695.1 ± 1493.9 | 550.3 | 12,351.9 | 0.55 | 2825.9 ± 1516.2 | 523.7 | 11,427.3 | 0.54 |
| Σ SFA | 1196.3 ± 691.5 | 154.0 | 4746.0 | 0.58 | 1278.8 ± 755.5 | 140.0 | 5462.0 | 0.59 |
| 10:0 | 1.3 ± 0.9 | 0.1 | 7.2 | 0.68 | 1.5 ± 1.2 | 0.1 | 9.3 | 0.79 |
| 12:0 | 1.7 ± 1.2 | 0.2 | 9.9 | 0.70 | 1.8 ± 1.3 | 0.2 | 8.0 | 0.73 |
| 14:0 | 63.9 ± 46.8 | 2.2 | 375.3 | 0.73 | 69.2 ± 50.8 | 1.8 | 301.9 | 0.73 |
| 15:0 | 10.3 ± 6.6 | 1.3 | 46.4 | 0.65 | 11.1 ± 7.7 | 1.1 | 61.0 | 0.69 |
| 16:0 | 660.7 ± 397.4 | 74.3 | 3014.0 | 0.60 | 706.0 ± 423.3 | 75.6 | 3051.9 | 0.60 |
| 17:0 | 23.7 ± 13.9 | 2.5 | 79.3 | 0.59 | 25.6 ± 16.1 | 2.5 | 116.8 | 0.63 |
| 18:0 | 384.3 ± 213.0 | 66.7 | 1343.1 | 0.55 | 410.0 ± 241.3 | 51.4 | 1636.0 | 0.59 |
| 19:0 | 1.6 ± 1.0 | 0.1 | 6.3 | 0.65 | 1.7 ± 1.1 | 0.1 | 6.4 | 0.67 |
| 20:0 | 2.3 ± 1.2 | 0.5 | 7.4 | 0.55 | 2.6 ± 1.7 | 0.5 | 10.9 | 0.67 |
| 22:0 | 0.9 ± 0.5 | 0.0 | 4.7 | 0.64 | 0.9 ± 0.6 | 0.0 | 4.8 | 0.68 |
| Σ BCFA | ||||||||
| 15:0iso | 4.9 ± 3.4 | 0.2 | 20.7 | 0.69 | 5.2 ± 4.0 | 0.3 | 36.2 | 0.78 |
| 17:0iso | 10.7 ± 6.6 | 2.0 | 50.2 | 0.61 | 11.3 ± 6.9 | 2.0 | 60.7 | 0.61 |
| 18:0iso | 2.8 ± 2.1 | 0.3 | 17.8 | 0.74 | 3.0 ± 2.2 | 0.3 | 17.1 | 0.74 |
| 15:0ai | 5.0 ± 3.4 | 0.7 | 26.6 | 0.68 | 5.3 ± 3.8 | 0.5 | 29.3 | 0.72 |
| 17:0ai/13t-16:1 | 15.7 ± 10.4 | 1.7 | 77.7 | 0.66 | 16.7 ± 10.9 | 1.6 | 83.5 | 0.65 |
| Σ MUFA | 1198.9 ± 779.0 | 132.0 | 6954.0 | 0.65 | 1248.9 ± 732.7 | 128.0 | 5379.0 | 0.59 |
| Σ 16:1-cis | 95.8 ± 77.4 | 8.0 | 802.0 | 0.81 | 99.1 ± 65.7 | 8.0 | 442.0 | 0.66 |
| Σ 18:1-cis | 977.8 ± 634.3 | 109.0 | 5589.0 | 0.65 | 1019.4 ± 593.7 | 109.0 | 4333.0 | 0.58 |
| 9c-14:1 | 15.2 ± 16.6 | 0.4 | 169.8 | 1.09 | 15.8 ± 13.0 | 0.2 | 81.1 | 0.82 |
| 7c-16:1 | 5.9 ± 3.9 | 1.0 | 39.9 | 0.66 | 6.1 ± 3.5 | 1.2 | 27.4 | 0.57 |
| 9c-16:1 | 85.6 ± 69.8 | 6.6 | 722.0 | 0.82 | 88.7 ± 59.9 | 6.2 | 394.6 | 0.68 |
| 11c-16:1 | 3.1 ± 3.5 | 0.0 | 37.5 | 1.14 | 3.1 ± 2.4 | 0.2 | 14.0 | 0.78 |
| 13c-16:1 | 1.2 ± 1.3 | 0.0 | 13.7 | 1.11 | 1.2 ± 1.0 | 0.0 | 5.9 | 0.82 |
| 9c-17:1 | 17.1 ± 11.1 | 2.3 | 107.7 | 0.65 | 17.8 ± 10.5 | 2.5 | 81.7 | 0.59 |
| 6c-18:1/8c-18:1 | 0.4 ± 0.4 | 0.0 | 3.4 | 1.06 | 0.4 ± 0.4 | 0.0 | 4.5 | 1.03 |
| 9c-18:1/10c-18:1 | 932.9 ± 604.5 | 98.9 | 5218.7 | 0.65 | 973.3 ± 569.2 | 101.8 | 4150.9 | 0.58 |
| 11c-18:1 | 32.1 ± 22.7 | 8.1 | 284.0 | 0.71 | 32.8 ± 17.5 | 6.1 | 126.8 | 0.53 |
| 12c-18:1 | 2.3 ± 1.6 | 0.3 | 13.6 | 0.68 | 2.5 ± 2.0 | 0.4 | 18.3 | 0.78 |
| 13c-18:1 | 6.7 ± 6.5 | 0.2 | 67.0 | 0.98 | 6.8 ± 4.7 | 0.3 | 28.9 | 0.69 |
| 15c-18:1 | 3.3 ± 2.2 | 0.1 | 12.6 | 0.67 | 3.4 ± 2.6 | 0.3 | 17.1 | 0.76 |
| 10c-20:1 | 1.6 ± 1.4 | 0.1 | 12.6 | 0.86 | 1.7 ± 1.2 | 0.1 | 7.8 | 0.73 |
| 11c-20:1 | 2.9 ± 2.8 | 0.4 | 35.0 | 0.97 | 3.0 ± 2.4 | 0.2 | 23.4 | 0.79 |
| Σ 16:1-trans | 6.3 ± 3.5 | 2.0 | 36.0 | 0.56 | 6.4 ± 3.5 | 1.8 | 26.0 | 0.55 |
| Σ 18:1-trans | 81.6 ± 52.6 | 9.0 | 297.0 | 0.64 | 84.6 ± 60.5 | 5.0 | 398.0 | 0.72 |
| 6t-16:1/8t-16:1 | 0.3 ± 0.3 | 0.0 | 1.9 | 0.84 | 0.4 ± 0.3 | 0.0 | 3.1 | 0.94 |
| 9t-16:1 | 3.1 ± 1.7 | 0.6 | 10.7 | 0.55 | 3.0 ± 1.7 | 0.3 | 10.8 | 0.56 |
| 10t-16:1 | 0.4 ± 0.4 | 0.0 | 5.1 | 1.11 | 0.5 ± 0.6 | 0.0 | 7.1 | 1.27 |
| 11t-16:1/12t-16:1 | 1.7 ± 1.3 | 0.0 | 13.5 | 0.77 | 1.7 ± 1.2 | 0.2 | 7.8 | 0.67 |
| 14t-16:1/12c-16:1 | 0.8 ± 0.9 | 0.0 | 11.9 | 1.12 | 0.8 ± 0.6 | 0.1 | 4.4 | 0.75 |
| 6t-18:1/8t-18:1 | 3.2 ± 2.1 | 0.5 | 16.0 | 0.65 | 3.4 ± 2.5 | 0.2 | 17.7 | 0.74 |
| 9t-18:1 | 4.5 ± 2.9 | 0.4 | 20.3 | 0.65 | 4.7 ± 3.2 | 0.1 | 22.6 | 0.68 |
| 10t-18:1 | 4.5 ± 3.7 | 0.3 | 26.8 | 0.84 | 4.9 ± 5.0 | 0.2 | 36.8 | 1.02 |
| 11t-18:1 | 45.7 ± 34.8 | 5.4 | 200.9 | 0.76 | 46.1 ± 37.9 | 2.6 | 216.6 | 0.82 |
| 12t-18:1 | 5.8 ± 3.6 | 0.8 | 28.4 | 0.63 | 6.2 ± 4.1 | 0.6 | 27.5 | 0.67 |
| 13t-18:1/14t-18:1 | 10.4 ± 6.7 | 0.9 | 40.7 | 0.64 | 11.2 ± 8.2 | 0.9 | 66.1 | 0.73 |
| 15t-18:1 | 2.1 ± 2.0 | 0.1 | 10.6 | 0.96 | 2.3 ± 2.6 | 0.1 | 22.8 | 1.12 |
| 16t-18:1/c,t-18:2 | 5.5 ± 3.7 | 0.4 | 20.5 | 0.68 | 5.9 ± 4.6 | 0.2 | 33.1 | 0.79 |
| Σ PUFA | 176.4 ± 37.1 | 81.0 | 356.0 | 0.21 | 175.3 ± 37.5 | 80.6 | 316.0 | 0.21 |
| Σ n-6 | 108.8 ± 29.6 | 50.0 | 245.2 | 0.27 | 108.3 ± 28.8 | 59.0 | 203.0 | 0.27 |
| 18:2n-6 | 72.7 ± 22.4 | 30.8 | 180.9 | 0.31 | 72.3 ± 22.2 | 33.0 | 153.9 | 0.31 |
| 18:3n-6 | 0.7 ± 0.3 | 0.0 | 2.6 | 0.47 | 0.7 ± 0.3 | 0.0 | 2.0 | 0.48 |
| 20:2n-6 | 0.8 ± 0.4 | 0.1 | 2.1 | 0.46 | 0.8 ± 0.5 | 0.2 | 4.2 | 0.61 |
| 20:3n-6 | 6.8 ± 1.9 | 2.6 | 13.7 | 0.28 | 6.7 ± 1.8 | 2.9 | 13.2 | 0.26 |
| 20:4n-6 | 26.1 ± 7.4 | 8.3 | 54.4 | 0.28 | 25.9 ± 7.1 | 13.7 | 53.8 | 0.27 |
| 22:4n-6 | 1.9 ± 0.9 | 0.0 | 6.4 | 0.47 | 1.9 ± 0.9 | 0.0 | 5.0 | 0.47 |
| Σ n-3 | 67.5 ± 18.0 | 12.9 | 127.7 | 0.27 | 66.9 ± 18.2 | 15.2 | 128.3 | 0.27 |
| 18:3n-3 | 30.0 ± 12.0 | 3.5 | 83.1 | 0.40 | 29.5 ± 11.9 | 5.3 | 86.7 | 0.40 |
| 20:3n-3 | 0.8 ± 0.5 | 0.0 | 3.3 | 0.62 | 0.8 ± 0.4 | 0.0 | 3.5 | 0.57 |
| 20:4n-3 | 3.6 ± 1.4 | 0.0 | 8.6 | 0.40 | 3.5 ± 1.4 | 0.0 | 8.6 | 0.39 |
| 20:5n-3 (EPA) | 14.0 ± 4.4 | 2.5 | 29.4 | 0.32 | 14.2 ± 4.5 | 2.2 | 38.6 | 0.32 |
| 22:5n-3 (DPA) | 17.2 ± 3.5 | 6.2 | 26.3 | 0.21 | 17.1 ± 3.5 | 6.1 | 27.1 | 0.20 |
| 22:6n-3 (DHA) | 2.0 ± 1.0 | 0.0 | 5.3 | 0.49 | 1.9 ± 1.0 | 0.0 | 5.7 | 0.50 |
| Σ DMA | 79.9 ± 14.3 | 46.0 | 125.0 | 0.18 | 79.4 ± 14.1 | 39.0 | 144.0 | 0.18 |
| Σ CLA | 18.3 ± 14.5 | 2.4 | 102.6 | 0.79 | 18.2 ± 13.1 | 1.7 | 83.4 | 0.72 |
| Σ CLA-trans,trans | 1.9 ± 1.3 | 0.0 | 7.3 | 0.69 | 2.0 ± 1.5 | 0.0 | 10.1 | 0.76 |
| Σ CLA-cis,trans | 16.6 ± 13.6 | 2.2 | 96.7 | 0.82 | 16.4 ± 12.0 | 1.3 | 75.2 | 0.73 |
| 7t,9c-18:2 | 0.9 ± 0.8 | 0.1 | 5.3 | 0.82 | 0.9 ± 0.7 | 0.1 | 4.9 | 0.74 |
| 9c,11t-18:2 | 13.9 ± 11.8 | 1.6 | 82.9 | 0.85 | 13.6 ± 10.2 | 0.9 | 65.1 | 0.75 |
| 9t,11c-18:2 | 0.3 ± 0.3 | 0.0 | 2.2 | 0.92 | 0.3 ± 0.3 | 0.0 | 2.0 | 0.99 |
| 11c,13t-18:2/21:0 | 0.4 ± 0.3 | 0.0 | 2.2 | 0.78 | 0.4 ± 0.5 | 0.0 | 5.7 | 1.17 |
| 11t,13t-18:2 | 1.0 ± 0.7 | 0.1 | 4.3 | 0.73 | 1.1 ± 0.9 | 0.1 | 5.5 | 0.84 |
| 7t,9t-18:2/10t,12t-18:2 | 0.5 ± 0.4 | 0.0 | 2.5 | 0.77 | 0.5 ± 0.4 | 0.0 | 3.5 | 0.78 |
| 12t,14t-18:2 | 0.4 ± 0.4 | 0.0 | 3.5 | 1.02 | 0.4 ± 0.4 | 0.0 | 3.3 | 1.01 |
| Σ NC-dienes | ||||||||
| 9c,13t-18:2/8t,12c-18:2 | 5.2 ± 3.7 | 0.5 | 27.4 | 0.71 | 5.4 ± 3.8 | 0.4 | 25.9 | 0.71 |
| 8t,13c-18:2 | 2.8 ± 2.0 | 0.2 | 13.3 | 0.73 | 2.9 ± 2.0 | 0.2 | 15.6 | 0.71 |
| 9t,12c-18:2 | 0.7 ± 0.6 | 0.0 | 5.9 | 0.93 | 0.6 ± 0.5 | 0.0 | 3.8 | 0.81 |
| 11t,15c-18:2/10t,15c-18:2 | 6.8 ± 5.3 | 0.3 | 31.6 | 0.78 | 6.5 ± 5.3 | 0.0 | 35.1 | 0.82 |
| 9c,15c-18:2 | 2.4 ± 1.9 | 0.0 | 19.2 | 0.80 | 2.5 ± 1.7 | 0.0 | 12.2 | 0.67 |
| Σ NC-trienes | ||||||||
| 9c,11t,15c-18:3/20:3n-9 | 3.3 ± 1.4 | 0.4 | 9.1 | 0.42 | 3.3 ± 1.6 | 0.9 | 12.4 | 0.48 |
| 9c,11t,15t-18:3 | 0.8 ± 0.8 | 0.1 | 4.8 | 0.95 | 0.8 ± 0.6 | 0.0 | 3.4 | 0.84 |
| Mathematical Treatment | #PLS | Calibration | Validation | Frequency Ranges, cm−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEE | RPDc | R2p | RMSEP | RPDp | ||||
| Σ SFA | NSDP | 7 | 0.92 | 174.0 | 3.6 | 0.93 | 181.0 | 3.8 | 3594.9–12,489.5 |
| 10:0 | 1D+SNV | 2 | 0.80 | 0.3 | 2.3 | 0.79 | 0.4 | 2.2 | 7506–6796.3; 4852.3–4242.9 |
| 12:0 | 1D | 2 | 0.84 | 0.4 | 2.5 | 0.80 | 0.5 | 2.3 | 9403.7–5446.3; 4428–4242.9 |
| 14:0 | NSDP | 5 | 0.91 | 13.9 | 3.3 | 0.88 | 15.2 | 2.9 | 3594.9–12,489.5 |
| 15:0 | 1D | 4 | 0.84 | 2.7 | 2.5 | 0.83 | 1.8 | 2.4 | 9403.7–8447.2; 5454–4242.9 |
| 16:0 | 1D | 3 | 0.92 | 109.0 | 3.5 | 0.93 | 104.0 | 3.7 | 7506–4242.9 |
| 17:0 | 1D | 8 | 0.88 | 4.3 | 3.0 | 0.86 | 4.0 | 2.7 | 9403.7–6094.3; 4605.4–4242.9 |
| 18:0 | 1D | 7 | 0.90 | 59.7 | 3.2 | 0.88 | 61.2 | 2.9 | 9403.7–544.3; 4605.4–4242.9 |
| 19:0 | 1D | 4 | 0.75 | 0.4 | 2.0 | 0.74 | 0.4 | 2.0 | 5778–5446.3 |
| 20:0 | SNV | 9 | 0.88 | 0.4 | 2.9 | 0.88 | 0.4 | 2.9 | 6120–4242.9 |
| 22:0 | 1D+SNV | 2 | 0.51 | 0.2 | 1.4 | 0.56 | 0.2 | 1.6 | 6102–4597.7 |
| Σ BCFA | |||||||||
| 15:0iso | 1D+SNV | 7 | 0.86 | 1.2 | 2.7 | 0.82 | 1.0 | 2.4 | 9403.7–5446.3; 4428–4242.9 |
| 17:0iso | NSDP | 5 | 0.89 | 2.2 | 3.0 | 0.85 | 1.8 | 2.6 | 3594.9–12,489.5 |
| 18:0iso | 1D | 4 | 0.87 | 0.5 | 2.8 | 0.88 | 0.5 | 3.0 | 9403.7–7498.3; 6102–4597.7 |
| 15:0ai | 1D+SNV | 3 | 0.81 | 1.3 | 2.3 | 0.80 | 1.0 | 2.4 | 6102–5446.3 |
| 17:0ai/13t-16:1 | 1D+SNV | 3 | 0.84 | 3.6 | 2.5 | 0.85 | 3.0 | 2.6 | 9403.7–8447.2; 5778–5446.3; 4605.4–4420.3 |
| Σ MUFA | 1D | 4 | 0.95 | 177.0 | 4.4 | 0.95 | 169.0 | 4.3 | 7506–5446.3; 4605.4–4242.9 |
| Σ 16:1-cis | 1D | 5 | 0.91 | 18.2 | 3.4 | 0.90 | 15.0 | 3.3 | 6102–4597.7 |
| Σ 18:1-cis | 1D | 4 | 0.95 | 141.0 | 4.6 | 0.95 | 130.0 | 4.6 | 6102–4597.7 |
| 9c-14:1 | 1D | 5 | 0.85 | 4.1 | 2.6 | 0.85 | 4.2 | 2.6 | 7506–4242.9 |
| 7c-16:1 | 1D+SNV | 3 | 0.83 | 1.2 | 2.4 | 0.84 | 0.9 | 2.6 | 7506–6094.3; 4605.4–4242.9 |
| 9c-16:1 | 1D | 4 | 0.86 | 17.4 | 2.7 | 0.83 | 19.7 | 0.3 | 6102–5770.3; 4428–4242.9 |
| 11c-16:1 | SNV | 6 | 0.84 | 0.5 | 2.5 | 0.80 | 0.5 | 2.3 | 5454–4242.9 |
| 13c-16:1 | 1D | 3 | 0.78 | 0.3 | 2.1 | 0.75 | 0.3 | 2.0 | 9403.7–7498.3; 6102–4242.9 |
| 6c-18:1/8c-18:1 | 1D | 3 | 0.78 | 0.1 | 2.1 | 0.75 | 0.1 | 2.1 | 7506–6796.3; 5454–4242.9 |
| 9c-17:1 | 1D+SNV | 2 | 0.84 | 2.8 | 2.5 | 0.91 | 2.0 | 3.4 | 6102–5446.3 |
| 9c-18:1/10c-18:1 | 1D | 3 | 0.95 | 145.0 | 4.3 | 0.96 | 107.0 | 4.8 | 6102–5168.6 |
| 11c-18:1 | 1D | 5 | 0.90 | 4.7 | 3.2 | 0.92 | 3.6 | 3.5 | 6102–5446.3; 4605.4–4242.9 |
| 12c-18:1 | SNV | 2 | 0.72 | 0.6 | 1.9 | 0.71 | 0.5 | 1.9 | 7506–4242.9 |
| 13c-18:1 | 1D | 5 | 0.88 | 1.6 | 2.9 | 0.86 | 1.1 | 2.7 | 7428.9–5446.3; 4428–4242.9 |
| 15c-18:1 | SNV | 5 | 0.85 | 0.7 | 2.6 | 0.84 | 0.6 | 2.6 | 9403.7–7498.3; 6102–4597.7 |
| 10c-20:1 | NSDP | 6 | 0.90 | 0.4 | 3.1 | 0.90 | 0.4 | 3.2 | 3594.9–1289.5 |
| 11c-20:1 | 1D | 2 | 0.88 | 0.6 | 2.9 | 0.87 | 0.7 | 2.8 | 6102–5770.3 |
| Σ 16:1-trans | 1D | 2 | 0.82 | 1.0 | 2.4 | 0.81 | 1.0 | 2.3 | 9403.7–7498.3; 5778–5446.3 |
| Σ 18:1-trans | 1D | 3 | 0.88 | 14.4 | 2.9 | 0.85 | 11.7 | 2.6 | 9403.7–7498.3; 4852.3–4242.9 |
| 6t-16:1/8t-16:1 | SNV | 3 | 0.71 | 0.1 | 1.9 | 0.71 | 0.1 | 1.9 | 9403.7–6094.3; 5454–4242.9 |
| 9t-16:1 | SNV | 1 | 0.05 | 0.9 | 1.0 | 0.87 | 0.7 | 2.8 | 6102–2446.3 |
| 10t-16:1 | SNV | 2 | 0.35 | 0.1 | 1.2 | 0.39 | 0.1 | 1.3 | 9403.7–8447.2; 5778–5446.3; 4428–4242.9 |
| 11t-16:1/12t-16:1 | 1D | 3 | 0.81 | 0.4 | 2.3 | 0.83 | 0.4 | 2.4 | 6102–4597.7 |
| 14t-16:1/12c-16:1 | SNV | 2 | 0.62 | 0.2 | 1.6 | 0.61 | 0.2 | 1.7 | 9403.7–7498.3; 4605.4–4242.9 |
| 6t-18:1/8t-18:1 | 1D | 4 | 0.83 | 0.8 | 2.4 | 0.87 | 0.7 | 2.8 | 9403.7–7498.3; 6102–4242.9 |
| 9t-18:1 | 1D | 3 | 0.90 | 0.9 | 3.2 | 0.90 | 0.7 | 3.2 | 9403.7–4242.9 |
| 10t-18:1 | 1D | 3 | 0.92 | 1.0 | 2.3 | 0.82 | 0.9 | 2.4 | 9403.7–6094.3; 5454–4242.9 |
| 11t-18:1 | 1D | 7 | 0.74 | 12.7 | 2.0 | 0.74 | 10.7 | 2.1 | 7506–6094.3 |
| 12t-18:1 | NSDP | 7 | 0.83 | 1.2 | 2.4 | 0.84 | 1.3 | 2.5 | 3594.9–12,489.5 |
| 13t-18:1/14t-18:1 | 1D+SNV | 5 | 0.81 | 2.0 | 2.3 | 0.82 | 1.8 | 2.4 | 5778–5446.3; 4605.4–4420.3 |
| 15t-18:1 | 1D | 3 | 0.78 | 0.6 | 2.1 | 0.78 | 0.4 | 2.1 | 5454–4242.9 |
| 16t-18:1/c,t-18:2 | SNV | 8 | 0.90 | 1.1 | 3.2 | 0.89 | 0.9 | 3.0 | 7506–5446.3; 4605.4–4242.9 |
| Σ PUFA | SNV | 5 | 0.69 | 17.9 | 1.8 | 0.65 | 1.7 | 1.1 | 9403.7–7498.3; 5454–4242.9 |
| Σ n-6 | NSDP | 4 | 0.43 | 15.1 | 1.3 | 0.44 | 10.3 | 1.3 | 3594.9–12,489.5 |
| 18:2n-6 | NSDP | 5 | 0.49 | 10.2 | 1.4 | 0.52 | 7.4 | 1.5 | 3594.9–12,489.5 |
| 18:3n-6 | COE | 4 | 0.46 | 0.1 | 1.4 | 0.44 | 0.2 | 1.3 | 3594.9–12,489.5 |
| 20:2n-6 | NSDP | 5 | 0.34 | 0.2 | 1.2 | 0.30 | 0.2 | 1.2 | 3594.9–12,489.5 |
| 20:3n-6 | NSDP | 2 | 0.16 | 1.2 | 1.1 | 0.18 | 0.9 | 1.1 | 3494.3–12,498.5 |
| 20:4n-6 | 1D | 2 | 0.42 | 3.9 | 1.3 | 0.44 | 2.5 | 1.4 | 9403.7–7498.3; 4605.4–4242.9 |
| 22:4n-6 | NSDP | 5 | 0.45 | 0.4 | 1.4 | 0.47 | 0.3 | 1.4 | 3594.9–12,489.5 |
| Σ n-3 | NSDP | 5 | 0.47 | 11.0 | 1.4 | 0.50 | 7.8 | 1.4 | 3594.3–12,489.5 |
| 18:3n-3 | NSDP | 4 | 0.22 | 7.6 | 1.1 | 0.41 | 5.2 | 1.3 | 3594.9–12,489.5 |
| 20:3n-3 | 1D+SNV | 8 | 0.69 | 0.2 | 1.8 | 0.66 | 0.2 | 1.7 | 9403.7–6094.3; 4605.4–4242.9 |
| 20:4n-3 | NSDP | 8 | 0.31 | 0.9 | 1.2 | 0.36 | 0.7 | 1.2 | 3594.9–12,489.5 |
| 20:5n-3 (EPA) | NSDP | 8 | 0.63 | 2.3 | 1.6 | 0.64 | 1.9 | 1.7 | 3594.9–12,489.5 |
| 22:5n-3 (DPA) | 1D+SNV | 5 | 0.40 | 1.6 | 1.3 | 0.38 | 1.4 | 1.3 | 8454.9–7498.3;6102–5446.3; 4605.4–4242.9 |
| 22:6n-3 (DHA) | NSDP | 4 | 0.33 | 0.5 | 1.2 | 0.33 | 0.5 | 1.2 | 3594.9–12,489.5 |
| Σ DMA | NSDP | 5 | 0.71 | 0.2 | 1.9 | 0.70 | 0.2 | 1.8 | 3594.9–12,489.5 |
| Σ CLA | 1D | 2 | 0.83 | 5.1 | 2.4 | 0.84 | 4.9 | 2.5 | 6102–5770.3 |
| Σ CLA-trans,trans | NSDP | 5 | 0.74 | 0.5 | 2.0 | 0.76 | 0.4 | 2.0 | 3594.9–12,489.5 |
| Σ CLA-cis,trans | NSDP | 5 | 0.81 | 5.0 | 2.3 | 0.80 | 4.2 | 2.3 | 3594.9–12,489.5 |
| 7t,9c-18:2 | NSDP | 6 | 0.77 | 0.2 | 2.1 | 0.75 | 0.2 | 2.1 | 3594.9–12,489.5 |
| 9c,11t-18:2 | NSDP | 6 | 0.78 | 3.9 | 2.1 | 0.79 | 2.9 | 2.3 | 3594.9–12,489.5 |
| 9t,11c-18:2 | NSDP | 1 | 0.55 | 0.1 | 1.5 | 0.57 | 0.1 | 1.5 | 3594.9–12,489.5 |
| 11c,13t-18:2/21:0 | NSDP | 5 | 0.65 | 0.1 | 1.7 | 0.64 | 0.1 | 1.8 | 3594.9–12,489.5 |
| 11t,13t-18:2 | NSDP | 5 | 0.74 | 0.3 | 2.0 | 0.76 | 0.2 | 2.1 | 3594.9–12,489.5 |
| 12t,14t-18:2 | NSDP | 5 | 0.67 | 0.1 | 1.8 | 0.69 | 0.1 | 1.8 | 3594.9–12,489.5 |
| 7t,9t/10t,12t-18:2 | NSDP | 5 | 0.74 | 0.2 | 2.0 | 0.73 | 0.1 | 1.9 | 3594.9–12,489.5 |
| Σ NC-dienes | |||||||||
| 9c,13t-18:2/8t,12c-18:2 | SNV | 4 | 0.87 | 1.2 | 2.8 | 0.87 | 0.1 | 2.9 | 6102–5770.3; 4605.4–4242.9 |
| 8t,13c-18:2 | NSDP | 5 | 0.83 | 0.7 | 2.4 | 0.84 | 0.6 | 2.5 | 3594.9–12,489.5 |
| 9t,12c-18:2 | NSDP | 5 | 0.73 | 0.2 | 1.9 | 0.72 | 0.2 | 1.9 | 3594.9–12,489.5 |
| 11t,15c-18:2/10t,15c-18:2 | SNV | 1 | 0.71 | 1.5 | 1.9 | 0.72 | 1.5 | 1.9 | 6102–5445.3; 4605.4–4242.9 |
| 9c,15c-18:2 | NSDP | 6 | 0.87 | 0.6 | 2.7 | 0.86 | 0.6 | 2.7 | 3594.9–12,489.5 |
| Σ NC-trienes | |||||||||
| 9c,11t,15c-18:3/20:3n-9 | NSDP | 5 | 0.54 | 0.7 | 1.5 | 0.55 | 0.7 | 1.5 | 3594.9–12,489.5 |
| 9c,11t,15t-18:3 | 1D | 1 | 0.77 | 0.2 | 2.1 | 0.77 | 0.2 | 2.1 | 6102–5770.3; 4428–4242.9 |
| Chemical Component | p-Value | Residual Mean | RMSE | R2 | Bias | Slope |
|---|---|---|---|---|---|---|
| Σ FAME | 0.62 | 136.61 | 273.00 | 0.01 | −137.00 | 0.98 |
| Σ SFA | 0.79 | −37.64 | 152.00 | 0.00 | 37.60 | 0.86 |
| 10:0 | 0.65 | 0.08 | 0.40 | 0.01 | −0.08 | 0.45 |
| 12:0 | 0.27 | 0.24 | 0.52 | 0.04 | −0.24 | 0.56 |
| 14:0 | 0.49 | −7.06 | 20.70 | 0.02 | 7.06 | 0.58 |
| 15:0 | 0.00 * | −11.92 | 730.00 | 0.45 | 701.00 | 0.01 |
| 16:0 | 0.50 | 51.68 | 763.00 | 0.02 | −740.00 | 14.57 |
| 17:0 | 0.00 * | 15.40 | 16.30 | 0.65 | −15.40 | 1.15 |
| 18:0 | 0.42 | 41.87 | 80.70 | 0.02 | −41.90 | 0.76 |
| 19:0 | 0.72 | −0.13 | 0.94 | 0.01 | 0.13 | 0.30 |
| 20:0 | 0.29 | 0.31 | 0.56 | 0.04 | −0.31 | 0.71 |
| 22:0 | 0.34 | −0.10 | 0.32 | 0.03 | 0.10 | 0.20 |
| 15:0iso | 0.02 * | 0.17 | 1.92 | 0.00 | −0.17 | 0.43 |
| 17:0iso | 0.69 | 0.46 | 2.71 | 0.01 | −0.46 | 0.48 |
| 18:0iso | 0.48 | 0.24 | 0.79 | 0.02 | −0.24 | 0.52 |
| 15:0ai | 0.58 | 0.48 | 2.40 | 0.01 | −0.48 | 0.30 |
| 17:0ai/13t-16:1 | 0.08 | 3.64 | 5.96 | 0.11 | −3.64 | 0.48 |
| Σ 16:1-cis | 0.63 | −5.40 | 19.80 | 0.01 | 5.40 | 0.75 |
| Σ 18:1-cis | 0.81 | 26.40 | 83.40 | 0.00 | −26.40 | 1.03 |
| 9c-14:1 | 0.36 | −2.62 | 7.17 | 0.03 | 2.62 | 0.44 |
| 7c-16:1 | 0.55 | 0.32 | 1.29 | 0.01 | −0.32 | 0.61 |
| 9c-16:1 | 0.91 | 1.21 | 19.50 | 0.00 | −1.21 | 0.60 |
| 11c-16:1 | 0.97 | 0.02 | 0.93 | 0.00 | −0.02 | 0.41 |
| 9c-17:1 | 0.38 | 1.30 | 3.07 | 0.03 | −1.30 | 0.68 |
| 9c-18:1/10c-18:1 | 0.98 | 2.14 | 75.30 | 0.00 | −2.14 | 1.05 |
| 11c-18:1 | 0.65 | −1.11 | 2.75 | 0.01 | 1.11 | 0.90 |
| 12c-18:1 | 0.55 | −0.24 | 1.29 | 0.01 | 0.24 | 0.16 |
| 13c:18:1 | 0.21 | −1.13 | 1.66 | 0.06 | 1.12 | 0.70 |
| 15c-18:1 | 0.76 | −0.19 | 1.99 | 0.00 | 0.19 | 0.17 |
| 11c-20:1 | 0.99 | −0.01 | 0.91 | 0.00 | 0.01 | 0.55 |
| Σ 16:1-trans | 0.00 * | 1.55 | 2.21 | 0.33 | −1.55 | −0.02 |
| Σ 18:1-trans | 0.40 | 8.91 | 24.50 | 0.03 | −1.55 | −0.02 |
| 9t-16:1 | 0.00 * | 1.02 | 1.25 | 0.55 | −1.02 | −0.15 |
| 11t-16:1/12t-16:1 | 0.04 * | 0.32 | 0.62 | 0.14 | −0.32 | 0.20 |
| 9t-18:1 | 0.68 | 0.25 | 0.92 | 0.01 | −0.25 | 0.72 |
| 10t-18:1 | 0.08 | −1.63 | 3.11 | 0.10 | 1.63 | 0.23 |
| 11t-18:1 | 0.12 | 8.34 | 16.80 | 0.09 | −8.33 | 0.34 |
| 12t-18:1 | 0.37 | −0.75 | 1.73 | 0.03 | 0.75 | 0.50 |
| 13t-18:1/14t-18:1 | 0.53 | 1.00 | 3.20 | 0.01 | −1.00 | 0.60 |
| 16t-18:1/cis,trans-18:2 | 0.71 | 0.31 | 1.87 | 0.01 | −0.31 | 0.60 |
| Σ PUFA | 0.08 | 13.43 | 30.20 | 0.10 | −13.40 | 0.03 |
| Σ n-6 | 0.97 | −0.27 | 23.90 | 0.00 | 0.27 | −0.01 |
| 18:2n-6 | 0.51 | −3.22 | 17.00 | 0.02 | 3.22 | 0.07 |
| 18:3n-6 | 0.01 * | −0.25 | 0.38 | 0.24 | 0.25 | 0.12 |
| 20:2n-6 | 0.15 | −0.29 | 0.74 | 0.07 | 0.29 | 0.05 |
| 20:3n-6 | 0.39 | −0.37 | 1.56 | 0.03 | 0.37 | 0.05 |
| 20:4n-6 | 0.45 | −1.11 | 4.72 | 0.02 | 1.11 | 0.16 |
| 22:4n-6 | 0.03 * | −0.58 | 1.04 | 0.15 | 0.58 | 0.10 |
| Σ n-3 | 0.00 * | 21.52 | 24.70 | 0.66 | −21.50 | −0.32 |
| 18:3n-3 | 0.00 * | 14.20 | 14.90 | 0.81 | −14.20 | 0.08 |
| 20:3n-3 | 0.16 | 0.13 | 0.35 | 0.07 | −0.13 | 0.05 |
| 20:4n-3 | 0.00 * | 1.15 | 1.34 | 0.59 | −1.15 | −0.03 |
| 20:5n-3 (EPA) | 0.16 | 1.22 | 3.09 | 0.07 | −1.22 | 0.18 |
| 22:5n-3 (DPA) | 0.74 | −0.20 | 2.37 | 0.00 | 0.20 | −0.11 |
| 22:6n-3 (DHA) | 0.05 * | −0.49 | 1.00 | 0.13 | 0.49 | 0.00 |
| Σ CLA | 0.00 * | 9.33 | 10.40 | 0.47 | −9.33 | 1.47 |
| Σ CLA-cis,trans | 0.00 * | 8.69 | 9.59 | 0.48 | −8.69 | 1.55 |
| 9c,13t-18:2/8t,12c-18:2 | 0.92 | 0.06 | 0.91 | 0.00 | −0.06 | 0.83 |
| 9c,15c-18:2 | 0.39 | 0.28 | 0.61 | 0.03 | −0.28 | 0.78 |
| 8t,13c-18:2 | 0.87 | −0.05 | 0.52 | 0.00 | 0.05 | 0.91 |
| 9t,12c-18:2 | 0.57 | 0.05 | 0.28 | 0.01 | −0.05 | 0.15 |
| 11t,15c-18:2/10t,15c-18:2 | 0.34 | 0.82 | 2.48 | 0.03 | −0.82 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobos-Ortega, I.; Silva, M.; Rodríguez-Pereira, R.; Saldaña, R.; Subiabre, I.; Rodríguez, M.; Morales, R. From Grass to Graph: NIRS Calibration for Fatty Acid Profiling in Grass-Raised Beef. Foods 2025, 14, 2767. https://doi.org/10.3390/foods14162767
Lobos-Ortega I, Silva M, Rodríguez-Pereira R, Saldaña R, Subiabre I, Rodríguez M, Morales R. From Grass to Graph: NIRS Calibration for Fatty Acid Profiling in Grass-Raised Beef. Foods. 2025; 14(16):2767. https://doi.org/10.3390/foods14162767
Chicago/Turabian StyleLobos-Ortega, Iris, Mariela Silva, Romina Rodríguez-Pereira, Rodolfo Saldaña, Ignacio Subiabre, Marion Rodríguez, and Rodrigo Morales. 2025. "From Grass to Graph: NIRS Calibration for Fatty Acid Profiling in Grass-Raised Beef" Foods 14, no. 16: 2767. https://doi.org/10.3390/foods14162767
APA StyleLobos-Ortega, I., Silva, M., Rodríguez-Pereira, R., Saldaña, R., Subiabre, I., Rodríguez, M., & Morales, R. (2025). From Grass to Graph: NIRS Calibration for Fatty Acid Profiling in Grass-Raised Beef. Foods, 14(16), 2767. https://doi.org/10.3390/foods14162767







