Monascus Yellow Pigments Ameliorate Hyperuricemia via Dual Mechanisms: Xanthine Oxidase Inhibition and Uric Acid Transporter Modulation (ABCG2, URAT1, and GLUT9)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Preparation of Monascus Yellow Pigments
2.3. In Vitro XOD Inhibition Assay
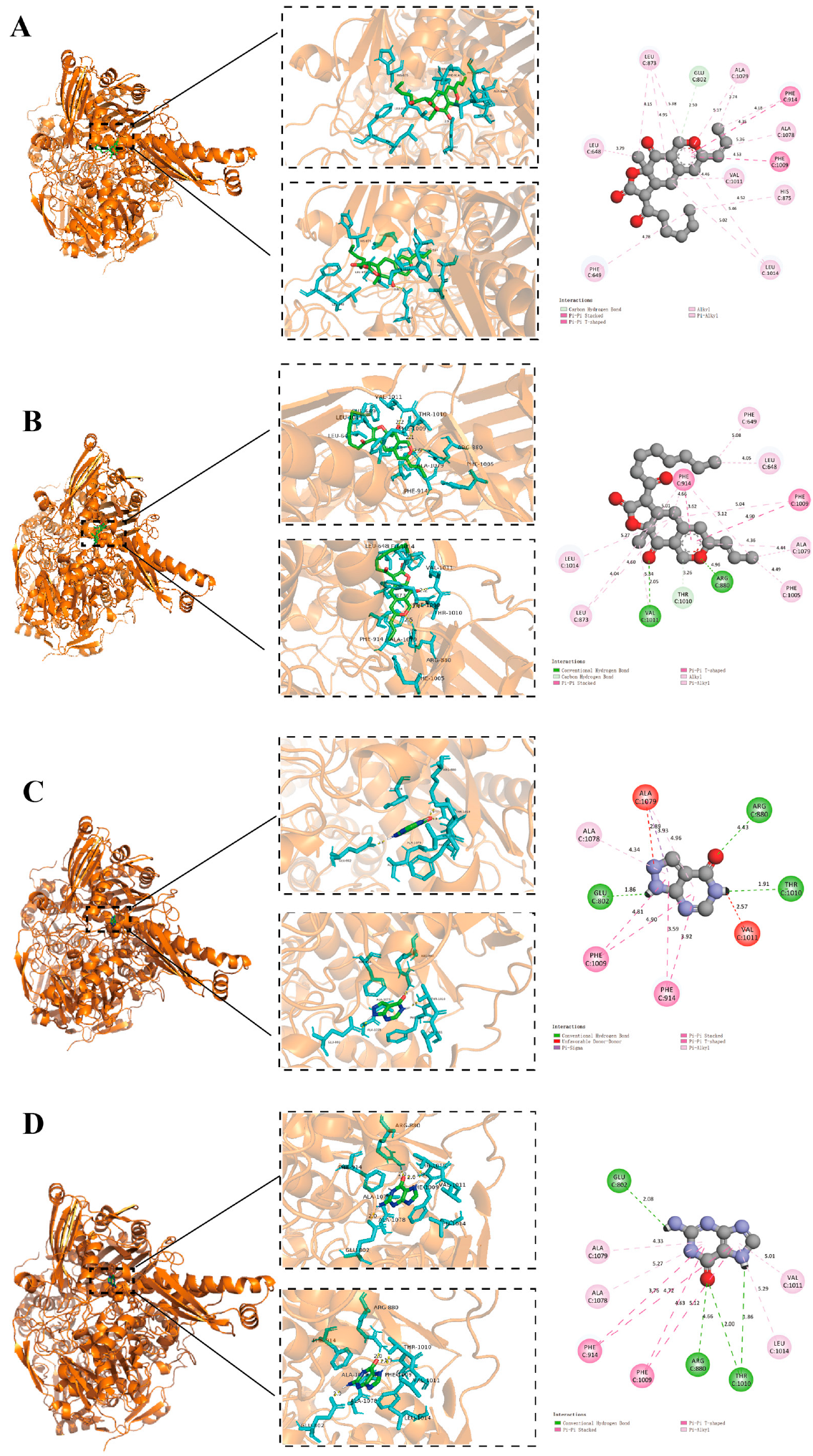
2.4. Molecular Docking
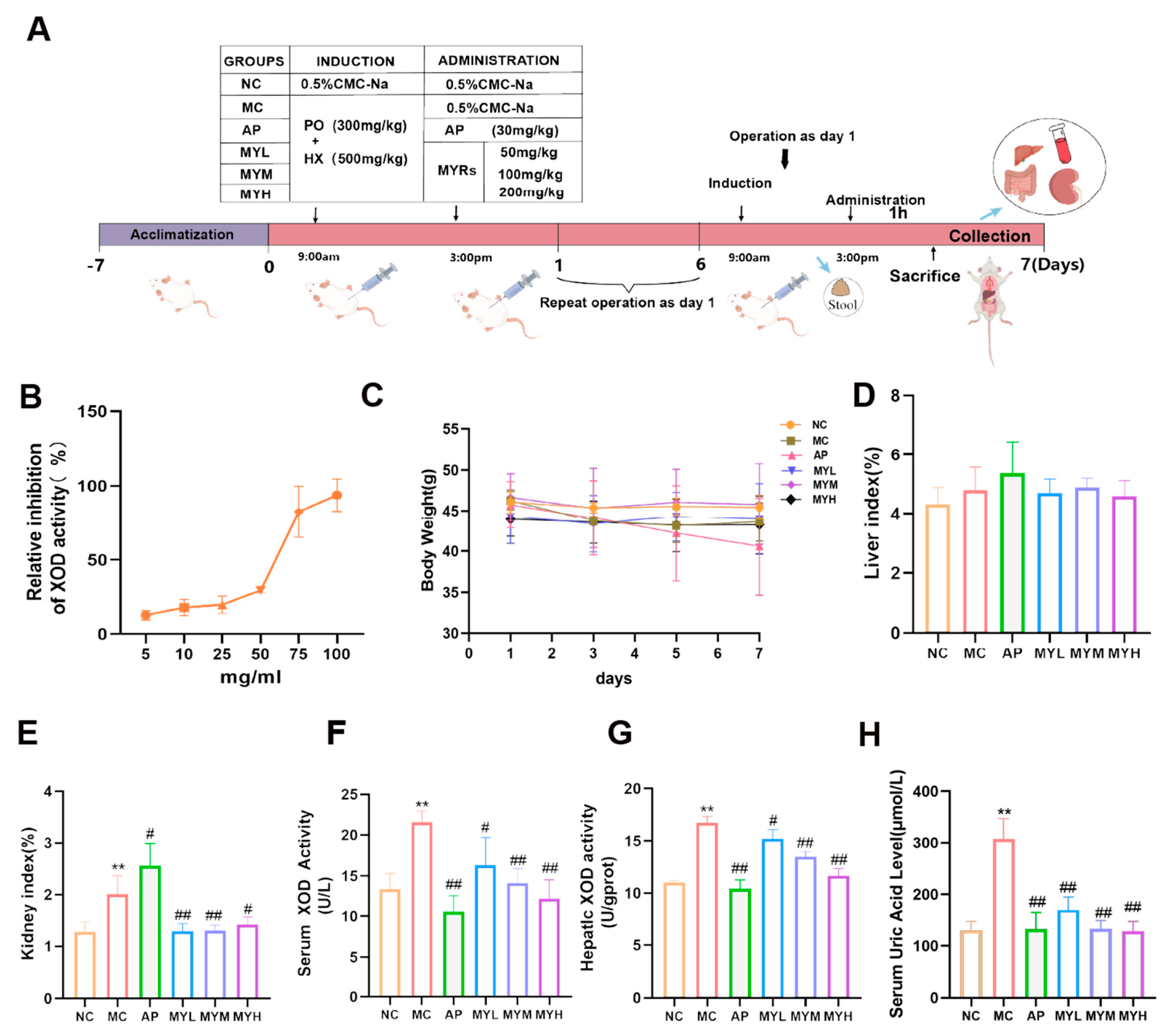
2.5. Establishment of Hyperuricemia Mouse Model and Treatment
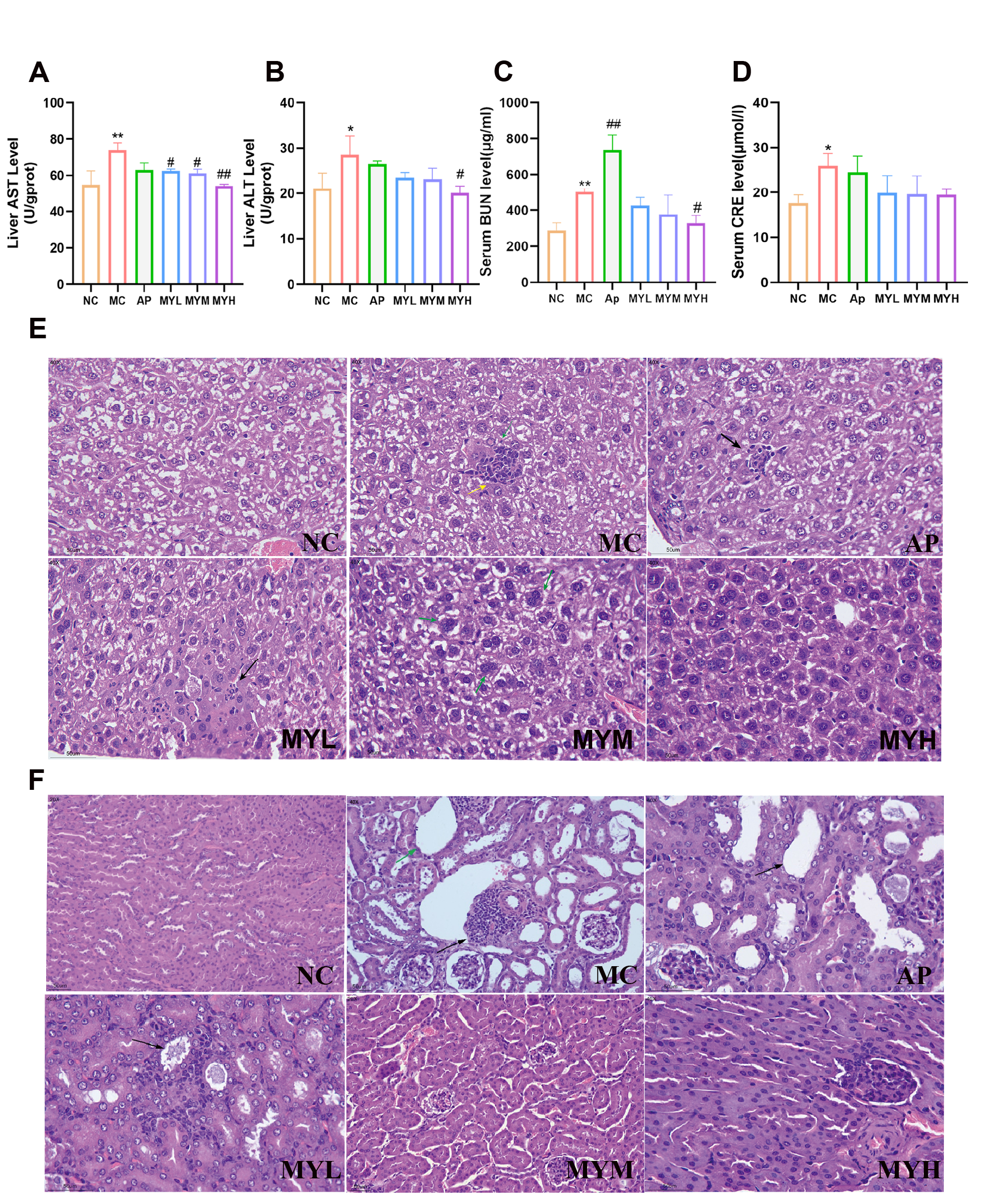
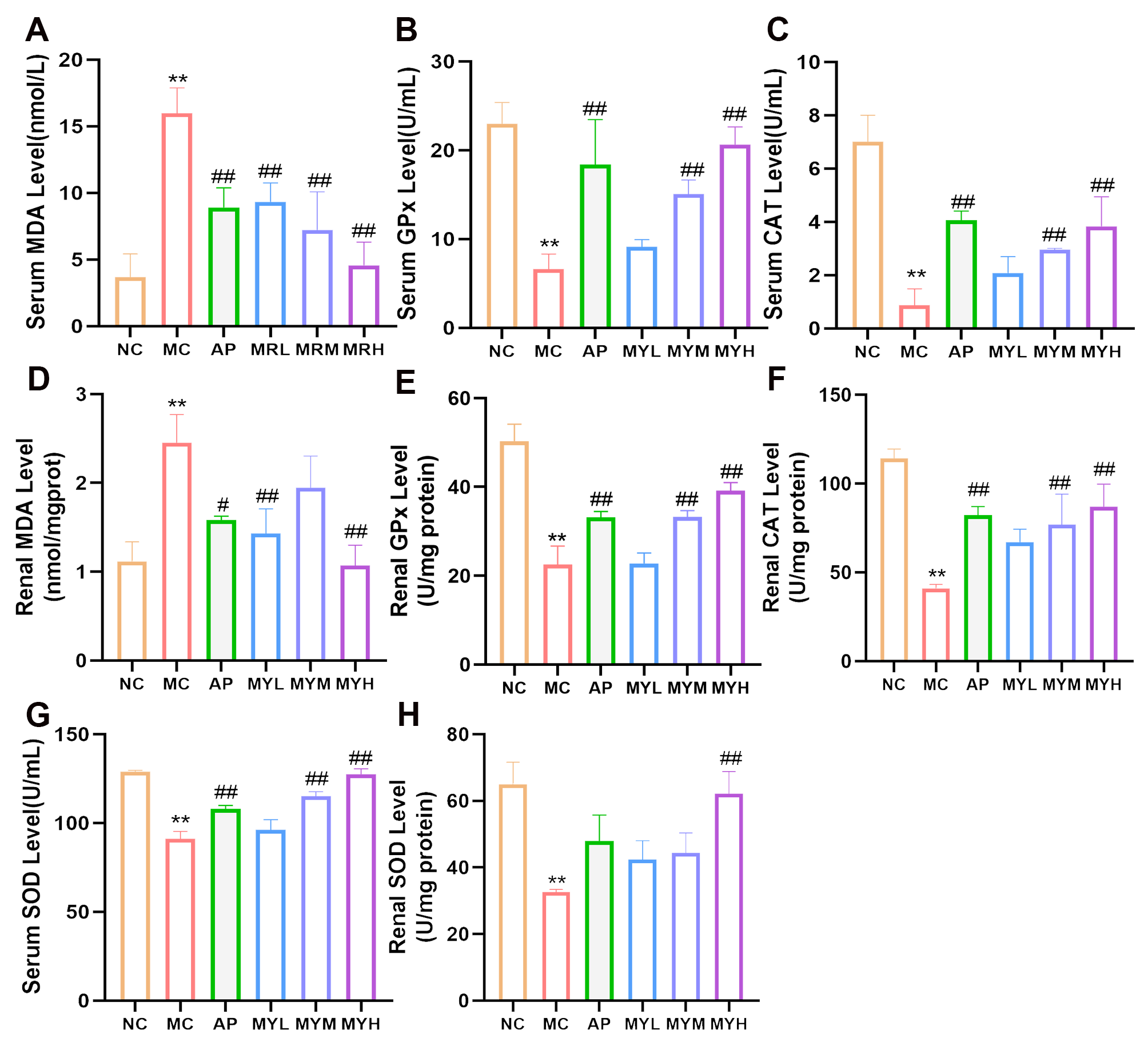
2.6. Biochemical Analyses
2.7. Histopathology
2.8. Cytokine Measurement
2.9. qRT-PCR
2.10. Western Blotting
2.11. Gut Microbiota Analysis
2.12. Statistical Analysis
3. Results
3.1. Effects of MYPs on In Vitro XOD Inhibition and General Parameters in HUA Mice
3.2. Molecular Docking Analysis
3.3. MYPs Preserve Liver and Renal Function
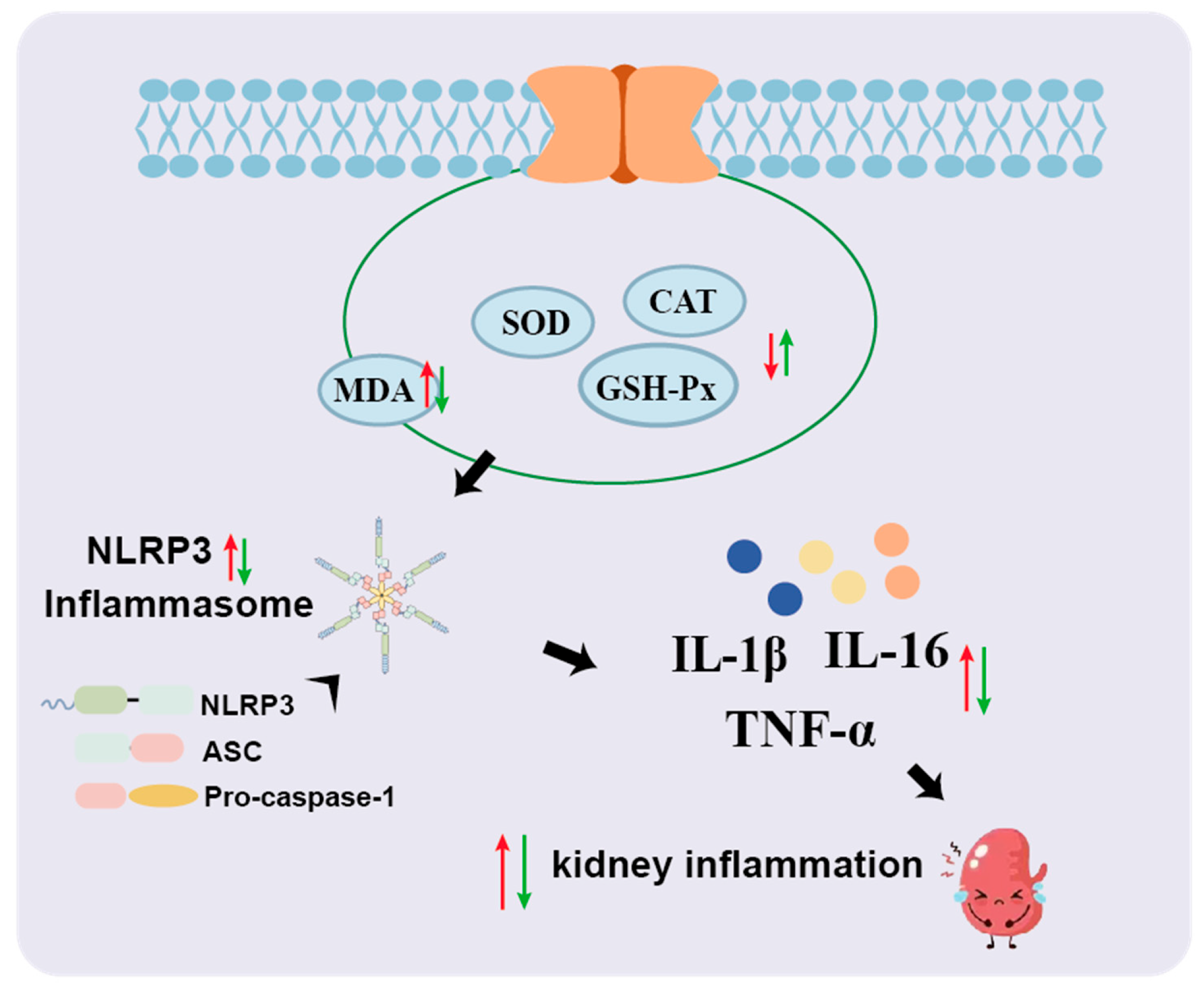
3.4. MYPs Alleviate Oxidative Stress in Hyperuricemic Mice
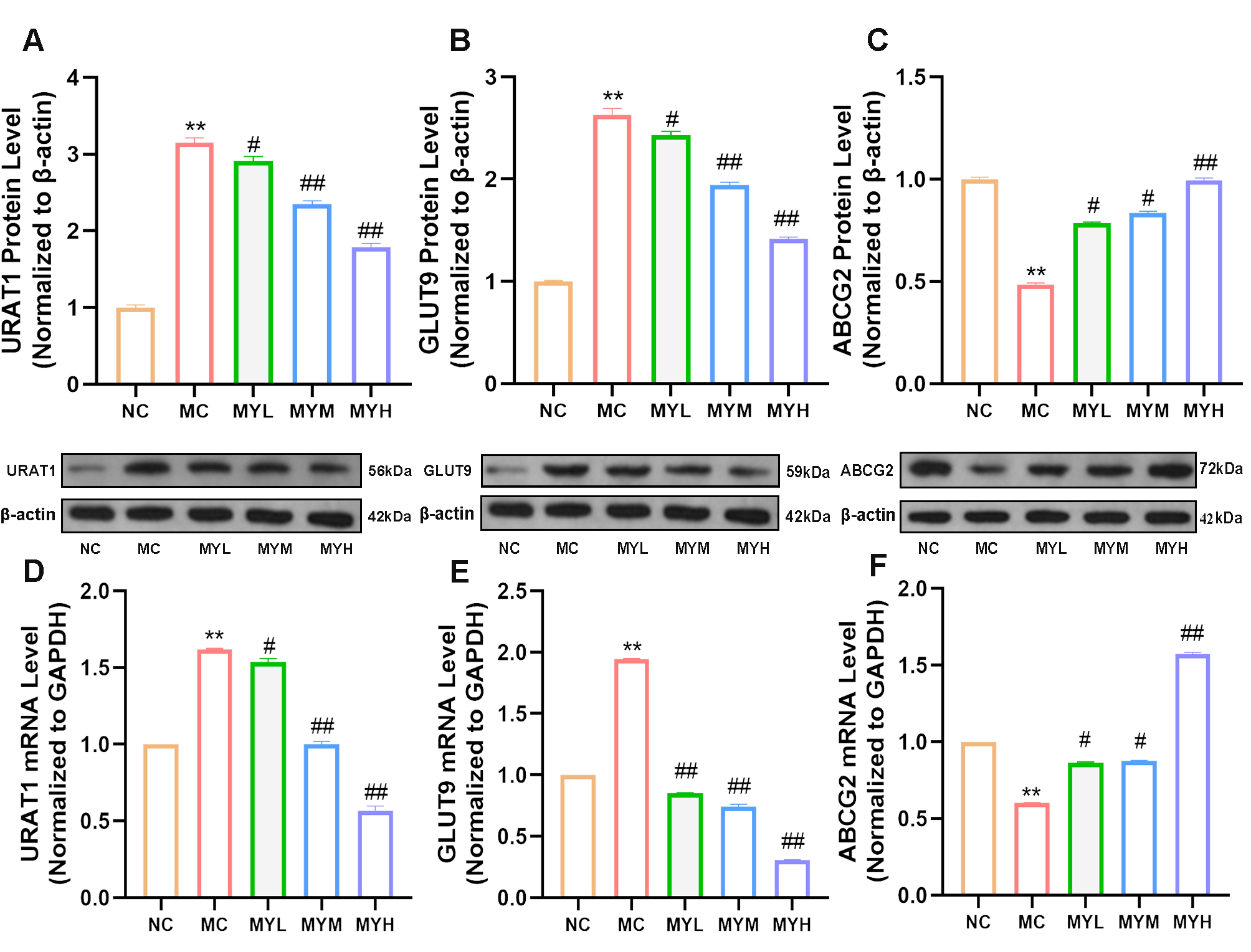
3.5. MYPs Restore Renal Uric Acid Transporter Expression
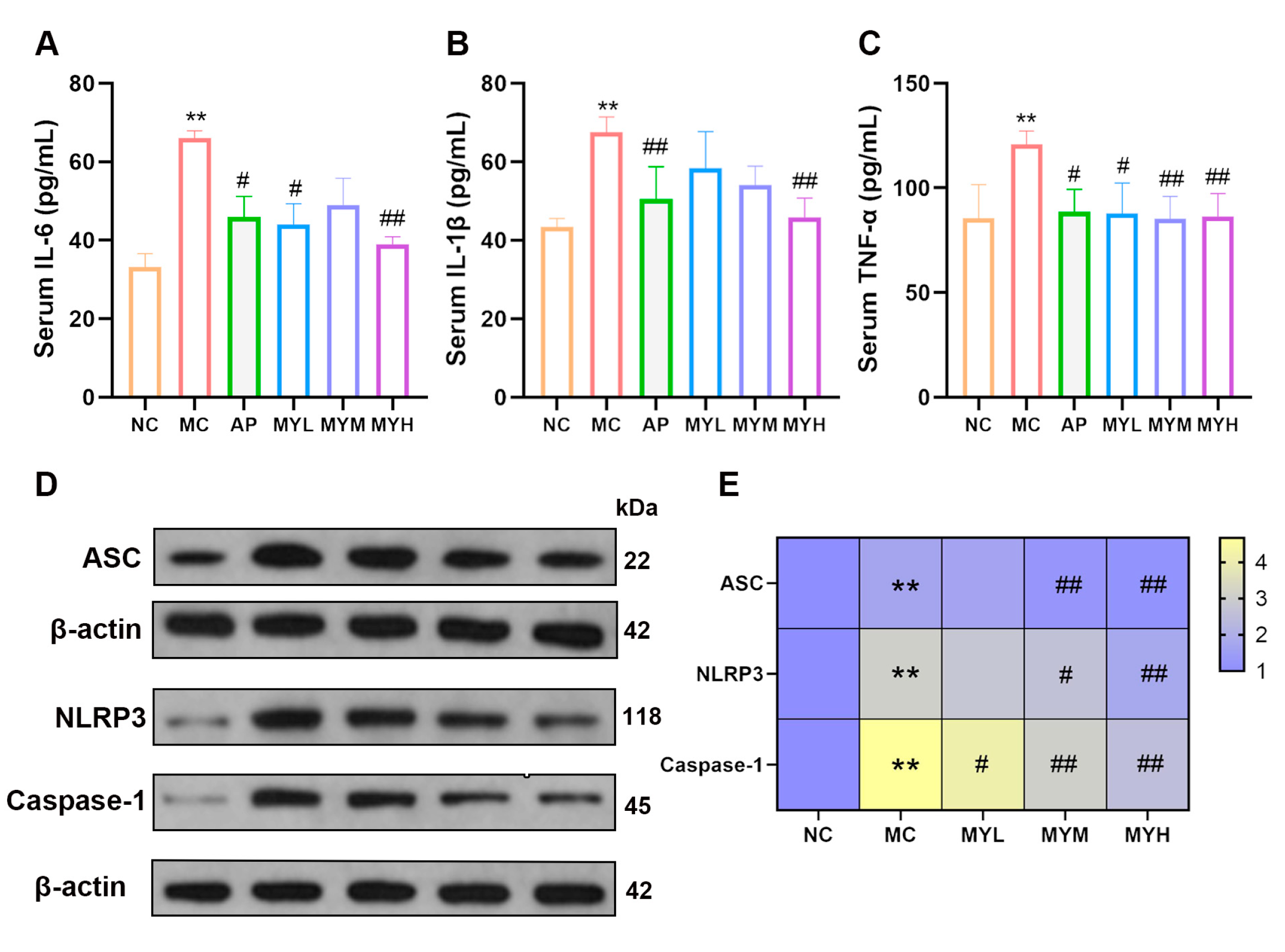
3.6. MYPs Attenuate Hyperuricemia-Induced Inflammation
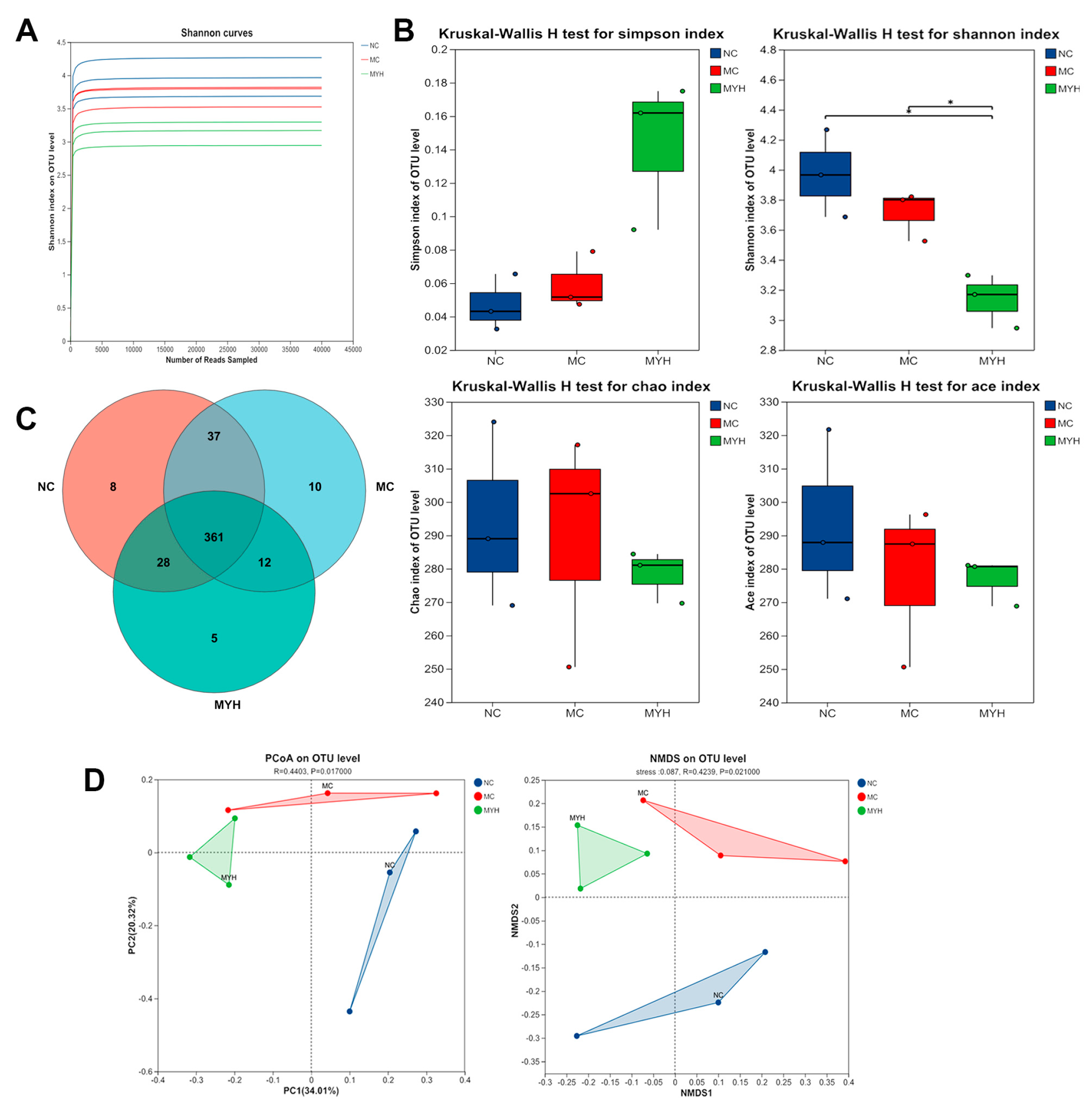
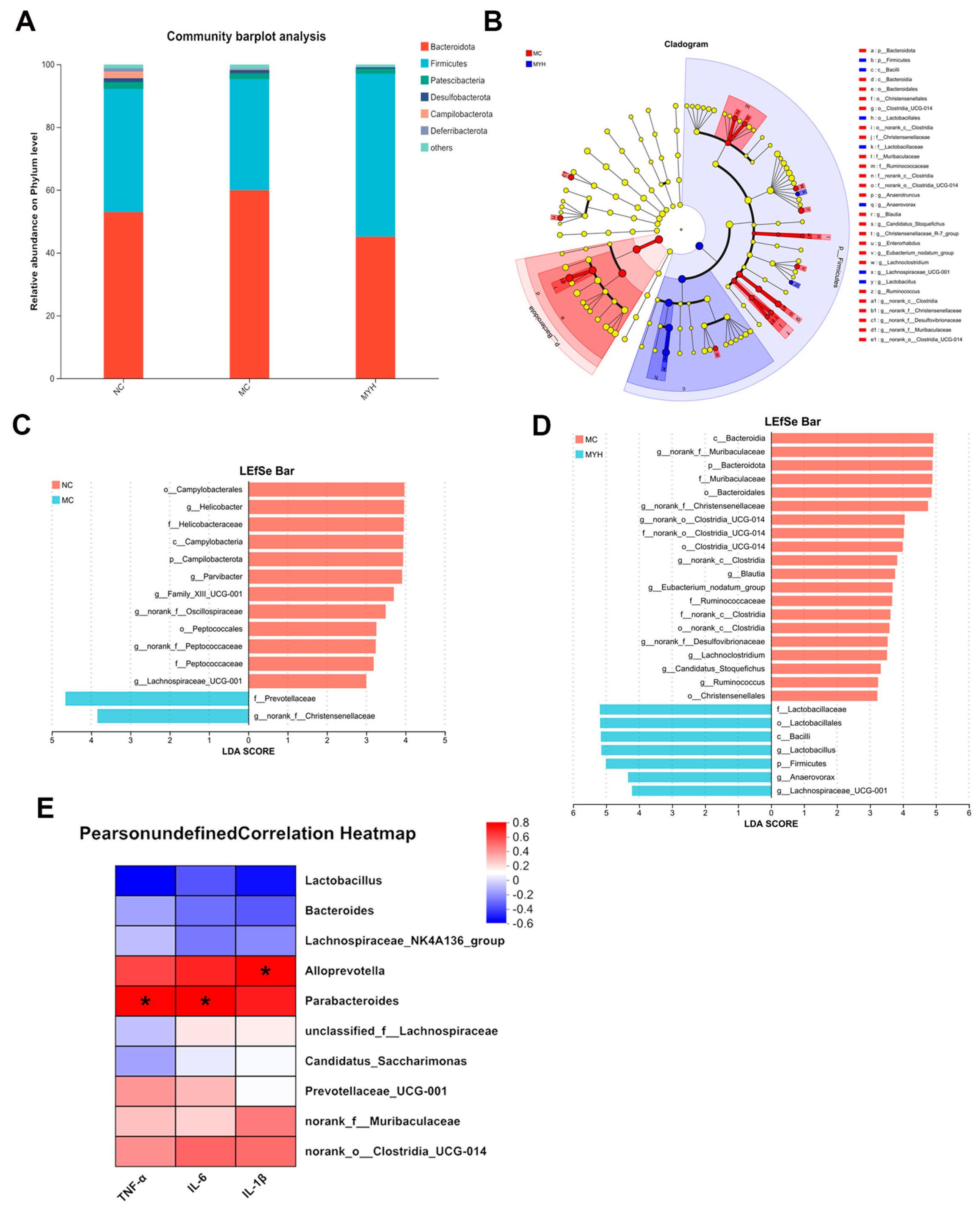
3.7. MYPs Modulate Gut Microbiota in Hyperuricemic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-Binding Cassette Subfamily G Member 2 |
| AK | Ankaflavin |
| ALT | Alanine Aminotransferase |
| AP | Allopurinol |
| ASC | Apoptosis-Associated Speck-Like Protein Containing a CARD |
| AST | Aspartate Aminotransferase |
| BUN | Blood Urea Nitrogen |
| Caspase-1 | Cysteine Aspartate Protease 1 |
| CAT | Catalase |
| CKD | Chronic Kidney Disease |
| CMC-Na | Carboxymethyl Cellulose Sodium |
| CRE | Creatinine |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GLUT9 | Glucose Transporter 9 |
| GPx/GSH-Px | Glutathione Peroxidase |
| H&E | Hematoxylin and Eosin |
| HX | Hypoxanthine |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| MC | Model Control |
| MDA | Malondialdehyde |
| MOPs | Monascus Orange Pigments |
| MPs | Monascus Pigments |
| mRNA | Messenger Ribonucleic Acid |
| MYPs | Monascus Yellow Pigments |
| MYH | High-dose Monascus Yellow Pigments |
| MYM | Medium-dose Monascus Yellow Pigments |
| MYL | Low-dose Monascus Yellow Pigments |
| MRPs | Monascus Red Pigments |
| MS | Monascin |
| NC | Normal Control |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| PBS | Phosphate-Buffered Saline |
| PO | Potassium Oxonate |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| SOD | Superoxide Dismutase |
| SUA | Serum Uric Acid |
| TNF-α | Tumor Necrosis Factor Alpha |
| UA | Uric Acid |
| URAT1 | Urate Transporter 1 |
| WB | Western Blot |
| XOD | Xanthine Oxidase |
References
- Adomako, E.A.; Moe, O.W. Uric Acid Transport, Transporters, and Their Pharmacological Targeting. Acta Physiol. 2023, 238, e13980. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, J.; Kim, G.-T. Prevalence of Hyperuricemia and Its Associated Factors in the General Korean Population: An Analysis of a Population-Based Nationally Representative Sample. Clin. Rheumatol. 2018, 37, 2529–2538. [Google Scholar] [CrossRef]
- Roughley, M.J.; Belcher, J.; Mallen, C.D.; Roddy, E. Gout and Risk of Chronic Kidney Disease and Nephrolithiasis: Meta-Analysis of Observational Studies. Arthritis Res. Ther. 2015, 17, 90. [Google Scholar] [CrossRef]
- Browne, L.D.; Jaouimaa, F.-Z.; Walsh, C.; Perez-Ruiz, F.; Richette, P.; Burke, K.; Stack, A.G. Serum Uric Acid and Mortality Thresholds among Men and Women in the Irish Health System: A Cohort Study. Eur. J. Intern. Med. 2021, 84, 46–55. [Google Scholar] [CrossRef]
- Chao, T.-F.; Liu, C.-J.; Chen, S.-J.; Wang, K.-L.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Chen, T.-J.; et al. Hyperuricemia and the Risk of Ischemic Stroke in Patients with Atrial Fibrillation—Could It Refine Clinical Risk Stratification in AF? Int. J. Cardiol. 2014, 170, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, C.-X.; Guo, L.; Zhang, X.-X.; Xia, D.-Z. Effects of Polysaccharides-Riched Prunus Mume Fruit Juice Concentrate on Uric Acid Excretion and Gut Microbiota in Mice with Adenine-Induced Chronic Kidney Disease. Curr. Res. Food Sci. 2022, 5, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tu, C.; Niu, Y.; Li, F.; Yuan, L.; Li, N.; Xu, A.; Gao, L.; Li, L. Dual Actions of Norathyriol as a New Candidate Hypouricaemic Agent: Uricosuric Effects and Xanthine Oxidase Inhibition. Eur. J. Pharmacol. 2019, 853, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and Endothelial Function: From Molecular Background to Clinical Perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric Acid Transporters Hiding in the Intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Dinour, D.; Gray, N.K.; Campbell, S.; Shu, X.; Sawyer, L.; Richardson, W.; Rechavi, G.; Amariglio, N.; Ganon, L.; Sela, B.-A.; et al. Homozygous SLC2A9 Mutations Cause Severe Renal Hypouricemia. J. Am. Soc. Nephrol. 2010, 21, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Ho Cha, S.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular Identification of a Renal Urate–Anion Exchanger That Regulates Blood Urate Levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ge, H.-Z.; Lei, S.-S.; Jiang, Z.-T.; Su, J.; He, X.; Zheng, X.; Wang, H.-Y.; Yu, Q.-X.; Li, B.; et al. Dendrobium Officinalis Six Nostrum Ameliorates Urate Under-Excretion and Protects Renal Dysfunction in Lipid Emulsion-Induced Hyperuricemic Rats. Biomed. Pharmacother. 2020, 132, 110765. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Q.; Zhang, Y.; Xue, M.; Yan, H.; Qiu, X.; Tian, Y.; Zhang, H.; Liang, H. Fucoidan from Saccharina Japonica Alleviates Hyperuricemia-Induced Renal Fibrosis through Inhibiting the JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2023, 71, 11454–11465. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, Q.-L.; OuYang, S.-X.; Chen, Y.; Gong, Y.-T.; Liang, Y.-M. Uric Acid Regulates NLRP3/IL-1β Signaling Pathway and Further Induces Vascular Endothelial Cells Injury in Early CKD through ROS Activation and K+ Efflux. BMC Nephrol. 2019, 20, 319. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, Q.; Miao, X.; Xia, X.; Yang, G.; Peng, X.; Yan, C. Punicalagin Prevents Hepatic Steatosis through Improving Lipid Homeostasis and Inflammation in Liver and Adipose Tissue and Modulating Gut Microbiota in Western Diet--Fed Mice. Mol. Nutr. Food Res. 2021, 65, 2001031. [Google Scholar] [CrossRef]
- DeBosch, B.J.; Kluth, O.; Fujiwara, H.; Schürmann, A.; Moley, K. Early-Onset Metabolic Syndrome in Mice Lacking the Intestinal Uric Acid Transporter SLC2A9. Nat. Commun. 2014, 5, 4642. [Google Scholar] [CrossRef]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; et al. Abnormal Metabolism of Gut Microbiota Reveals the Possible Molecular Mechanism of Nephropathy Induced by Hyperuricemia. Acta Pharm. Sin. B 2020, 10, 249–261. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Q.; Ren, H.; Gao, L.; Zhao, P.; Yang, X.; Yang, G.; Xu, D.; Wang, G.; Yang, W.; et al. The Altered Gut Microbiota of High-Purine-Induced Hyperuricemia Rats and Its Correlation with Hyperuricemia. PeerJ 2020, 8, e8664. [Google Scholar] [CrossRef]
- Shi, R.; Ye, J.; Fan, H.; Xiao, C.; Wang, D.; Xia, B.; Zhao, Z.; Zhao, B.; Dai, X.; Liu, X. Lactobacillus Plantarum LLY-606 Supplementation Ameliorates Hyperuricemia via Modulating Intestinal Homeostasis and Relieving Inflammation. Food Funct. 2023, 14, 5663–5677. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Xu, L.; Liu, H.; Qi, C.; Wang, C.; Chen, W. An Integrated Investigation Combining Network Pharmacology and Computer Simulation Dissects the Potential Mechanism of Anti-Obesity of Monascus Pigments (MPs). Food Biosci. 2024, 60, 104459. [Google Scholar] [CrossRef]
- Keith, M.P.; Gilliland, W.R. Improving the Use of Allopurinol in Chronic Gout: Monitoring Oxypurinol Levels to Guide Therapy. Clin. Pharmacol. Ther. 2011, 90, 363–364. [Google Scholar] [CrossRef]
- Becker, M.A.; Schumacher, H.R.; Espinoza, L.R.; Wells, A.F.; MacDonald, P.; Lloyd, E.; Lademacher, C. The Urate-Lowering Efficacy and Safety of Febuxostat in the Treatment of the Hyperuricemia of Gout: The CONFIRMS Trial. Arthritis Res. Ther. 2010, 12, R63. [Google Scholar] [CrossRef]
- Shin, H.J.; Takeda, M.; Enomoto, A.; Fujimura, M.; Miyazaki, H.; Anzai, N.; Endou, H. Interactions of Urate Transporter URAT1 in Human Kidney with Uricosuric Drugs. Nephrology 2011, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tausche, A.-K.; Alten, R.; Dalbeth, N.; Kopicko, J.; Fung, M.; Adler, S.; Bhakta, N.; Storgard, C.; Baumgartner, S.; Saag, K. Lesinurad Monotherapy in Gout Patients Intolerant to a Xanthine Oxidase Inhibitor: A 6 Month Phase 3 Clinical Trial and Extension Study. Rheumatology 2017, 56, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Cohen, R.E.; Pillinger, M.H. Asymptomatic Hyperuricemia: Is It Really Asymptomatic? Curr. Opin. Rheumatol. 2020, 32, 71–79. [Google Scholar] [CrossRef]
- Adin, S.N.; Gupta, I.; Panda, B.P.; Mujeeb, M. Monascin and Ankaflavin—Biosynthesis from Monascus Purpureus, Production Methods, Pharmacological Properties: A Review. Biotechnol. Appl. Biochem. 2023, 70, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, J.; Gan, Q.; Ran, Q.; Lou, G.; Xiong, H.; Peng, C.; Sun, J.; Yao, R.; Huang, Q. Impact of Red Yeast Rice on Metabolic Diseases: A Review of Possible Mechanisms of Action. J. Agric. Food Chem. 2020, 68, 10441–10455. [Google Scholar] [CrossRef]
- Feng, S.-S.; Li, W.; Hu, Y.-J.; Feng, J.-X.; Deng, J. The Biological Activity and Application of Monascus Pigments: A Mini Review. Int. J. Food Eng. 2022, 18, 253–266. [Google Scholar] [CrossRef]
- Yang, P.-X.; Hsu, Y.-W.; Pan, T.-M.; Lee, C.-L. Monascinol from Monascus Pilosus-Fermented Rice Exhibits Hypolipidemic Effects by Regulating Cholesterol and Lipid Metabolism. J. Funct. Foods 2024, 119, 106285. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, R.; Guo, W.; Hong, J.; Li, L.; Ni, L.; Sun, J.; Liu, B.; Rao, P.; Lv, X. Monascus Yellow, Red and Orange Pigments from Red Yeast Rice Ameliorate Lipid Metabolic Disorders and Gut Microbiota Dysbiosis in Wistar Rats Fed on a High-Fat Diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zou, B.; Zeng, H.; Zhang, L.; Chen, M.; Fu, G. Inhibitory Effect of Verbascoside on Xanthine Oxidase Activity. Int. J. Biol. Macromol. 2016, 93, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine Oxidoreductase Regulates Macrophage IL1β Secretion upon NLRP3 Inflammasome Activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef]
- Prieto-Moure, B.; Carabén-Redaño, A.; Aliena-Valero, A.; Cejalvo, D.; Toledo, A.H.; Flores-Bellver, M.; Martínez-Gil, N.; Toledo-Pereyra, L.H.; Lloris Carsí, J.M. Allopurinol in Renal Ischemia. J. Investig. Surg. 2014, 27, 304–316. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Ishaq, M.; Xin, W.; Zhao, L.; Wang, C.; Hossen, I.; Zhang, H.; Lian, Y.; Xu, M. Anti-Hyperuricemic Potential of Stevia (Stevia Rebaudiana Bertoni) Residue Extract in Hyperuricemic Mice. Food Funct. 2020, 11, 6387–6406. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Duan, S.; Yuan, X.; Liang, J.; Hou, S. Curcumin Attenuates Potassium Oxonate-Induced Hyperuricemia and Kidney Inflammation in Mice. Biomed. Pharmacother. 2019, 118, 109195. [Google Scholar] [CrossRef]
- Bian, M.; Wang, J.; Wang, Y.; Nie, A.; Zhu, C.; Sun, Z.; Zhou, Z.; Zhang, B. Chicory Ameliorates Hyperuricemia via Modulating Gut Microbiota and Alleviating LPS/TLR4 Axis in Quail. Biomed. Pharmacother. 2020, 131, 110719. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Zhu, B.; Zhang, H.; Zhang, X.; Ding, X. Demographic, Regional and Temporal Trends of Hyperuricemia Epidemics in Mainland China from 2000 to 2019: A Systematic Review and Meta-Analysis. Glob. Health Action. 2021, 14, 1874652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, Y.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Zhang, S.; Wang, X.; Zhang, J.; Sun, S.; et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Hyperuricemia: The TCLSIH Cohort Study. Am. J. Med. 2023, 136, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Ye, P.; Lu, J.; Chen, B.; Li, N.; Zhang, H.; Bo, H.; Chen, X.; Liu, H.; Zhang, C.; et al. Prevalence and Related Factors of Hyperuricaemia in Chinese Children and Adolescents: A Pooled Analysis of 11 Population-Based Studies. Ann. Med. 2022, 54, 1608–1615. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Fan, Y.; Wu, D.; Meng, Y.; Qin, M. Agents for the Treatment of Gout: Current Advances and Future Perspectives. J. Med. Chem. 2023, 66, 14474–14493. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tan, M.-L.; Li, K.-K.; Leung, P.-C.; Ko, C.-H. Green Tea Polyphenols Decreases Uric Acid Level through Xanthine Oxidase and Renal Urate Transporters in Hyperuricemic Mice. J. Ethnopharmacol. 2015, 175, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, M.; Wu, J.-L.; Li, J.-X.; Ma, Z.-C. Effect of Lemon Water Soluble Extract on Hyperuricemia in a Mouse Model. Food Funct. 2019, 10, 6000–6008. [Google Scholar] [CrossRef]
- Chou, H.-W.; Chiu, H.-T.; Tsai, C.-W.; Ting, I.-W.; Yeh, H.-C.; Huang, H.-C.; Kuo, C.-C. CMUH Kidney Research Group Comparative Effectiveness of Allopurinol, Febuxostat and Benzbromarone on Renal Function in Chronic Kidney Disease Patients with Hyperuricemia: A 13-Year Inception Cohort Study. Nephrol. Dial. Transplant. 2018, 33, 1620–1627. [Google Scholar] [CrossRef]
- Wu, X.-H.; Ruan, J.-L.; Zhang, J.; Wang, S.-Q.; Zhang, Y.-W. Pallidifloside D, a Saponin Glycoside Constituent from Smilax Riparia, Resist to Hyperuricemia Based on URAT1 and GLUT9 in Hyperuricemic Mice. J. Ethnopharmacol. 2014, 157, 201–205. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Uric Acid Transport and Disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.-Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The Organic Anion Transporter (OAT) Family: A Systems Biology Perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Zhang, Q.-Y.; Wang, F.-M.; Kong, L.-D. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS ONE 2012, 7, e38285. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hsu, W.-H.; Chang, Y.-Y.; Kuo, H.-F.; Hsu, Y.-W.; Pan, T.-M. Ankaflavin: A Natural Novel PPARγ Agonist Upregulates Nrf2 to Attenuate Methylglyoxal-Induced Diabetes in Vivo. Free Radic. Biol. Med. 2012, 53, 2008–2016. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Wang, M.-X.; Ge, C.-X.; Wang, X.; Li, J.-M.; Kong, L.-D. Betaine Supplementation Protects against High-Fructose-Induced Renal Injury in Rats. J. Nutr. Biochem. 2014, 25, 353–362. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liao, W.; Huang, J.; Liu, Y.; Li, Z.; Tang, J. Gut Microbiota Remodeling: A Promising Therapeutic Strategy to Confront Hyperuricemia and Gout. Front. Cell. Infect. Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ji, S.; Chen, L.; Liu, X.; Deng, Y.; You, Y.; Wang, M.; He, Q.; Peng, B.; Yang, Y.; et al. Gut Microbiota Dysbiosis in Hyperuricaemia Promotes Renal Injury through the Activation of NLRP3 Inflammasome. Microbiome 2024, 12, 109. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, P.; Xiong, S.; Wei, B.; Du, T.; Huang, T.; Yu, Q.; Xie, M.; Xiong, T. Lacticaseibacillus Rhamnosus NCUH061012 Alleviates Hyperuricemia via Modulating Gut Microbiota and Intestinal Metabolites in Mice. Food Biosci. 2024, 58, 103699. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wei, W.; Zhang, Y.; Xiang, Z.; Peng, D.; Kasimumali, A.; Rong, S. Gut Microbial Metabolites SCFAs and Chronic Kidney Disease. J. Transl. Med. 2024, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Lu, L.; Zhao, M.; Luo, X.; Jia, F.; Feng, F.; Wang, J. Mulberry (Fructus Mori) Extract Alleviates Hyperuricemia by Regulating Urate Transporters and Modulating the Gut Microbiota. Food Funct. 2024, 15, 12169–12179. [Google Scholar] [CrossRef]
| Reagent (mL) | GROUPS | |||
|---|---|---|---|---|
| Sample | Blank | Positive Control | Negative Control | |
| PBS | 1.6 | 1.95 | 1.75 | 2.1 |
| MYPs | 0.15 | 0.15 | 0 | 0 |
| Xanthin | 1.4 | 1.4 | 1.4 | 1.4 |
| XOD | 0.35 | 0 | 0.35 | 0 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| GAPDH | GGCAAGTTCAACGGCACAG | CGCCAGTAGACTCCACGACAT |
| URAT1 | CTCCATGCTGTGCTGGTTTG | CACAATCCCGATGAGTGCCT |
| GLUT9 | CGGCTCTTCTAACCGTCACA | ACGGAAACATGGGCTTTCTGA |
| ABCG2 | CCATCCAACAGGCCTAGAATCA | TCCTAGGAAGGCCGTTGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, F.; Zhu, R.; Li, J.; Liu, Z.; Niu, L.; Chen, W.; Wang, C.; Zheng, J. Monascus Yellow Pigments Ameliorate Hyperuricemia via Dual Mechanisms: Xanthine Oxidase Inhibition and Uric Acid Transporter Modulation (ABCG2, URAT1, and GLUT9). Foods 2025, 14, 2765. https://doi.org/10.3390/foods14162765
Xue F, Zhu R, Li J, Liu Z, Niu L, Chen W, Wang C, Zheng J. Monascus Yellow Pigments Ameliorate Hyperuricemia via Dual Mechanisms: Xanthine Oxidase Inhibition and Uric Acid Transporter Modulation (ABCG2, URAT1, and GLUT9). Foods. 2025; 14(16):2765. https://doi.org/10.3390/foods14162765
Chicago/Turabian StyleXue, Furong, Renqin Zhu, Jiaxing Li, Zheng Liu, Lidan Niu, Wei Chen, Chengtao Wang, and Jie Zheng. 2025. "Monascus Yellow Pigments Ameliorate Hyperuricemia via Dual Mechanisms: Xanthine Oxidase Inhibition and Uric Acid Transporter Modulation (ABCG2, URAT1, and GLUT9)" Foods 14, no. 16: 2765. https://doi.org/10.3390/foods14162765
APA StyleXue, F., Zhu, R., Li, J., Liu, Z., Niu, L., Chen, W., Wang, C., & Zheng, J. (2025). Monascus Yellow Pigments Ameliorate Hyperuricemia via Dual Mechanisms: Xanthine Oxidase Inhibition and Uric Acid Transporter Modulation (ABCG2, URAT1, and GLUT9). Foods, 14(16), 2765. https://doi.org/10.3390/foods14162765








