Discrimination of Polygonatum Species via Polysaccharide Fingerprinting: Integrating Their Chemometrics, Antioxidant Activity, and Potential as Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Polysaccharides from Three Common Polygonatum Species
2.3. Monosaccharide Composition Analysis of Three Common Polygonatum spp. Polysaccharides
2.3.1. Identification of Individual Chemical Markers by GC-MS
2.3.2. Global Fingerprint Profiling by IR
Establishing Method
Method Validation
2.4. In Vitro Antioxidant Activity
2.5. HCA, PCA, and OPLS-DA
2.6. Statistical Analysis
3. Results and Discussion
3.1. Comprehensive Evaluation of PSPs, PCPs, and PKPs
Monosaccharide Composition
3.2. Effects of PCPs, PSPs, and PKPs on Antioxidant Activity
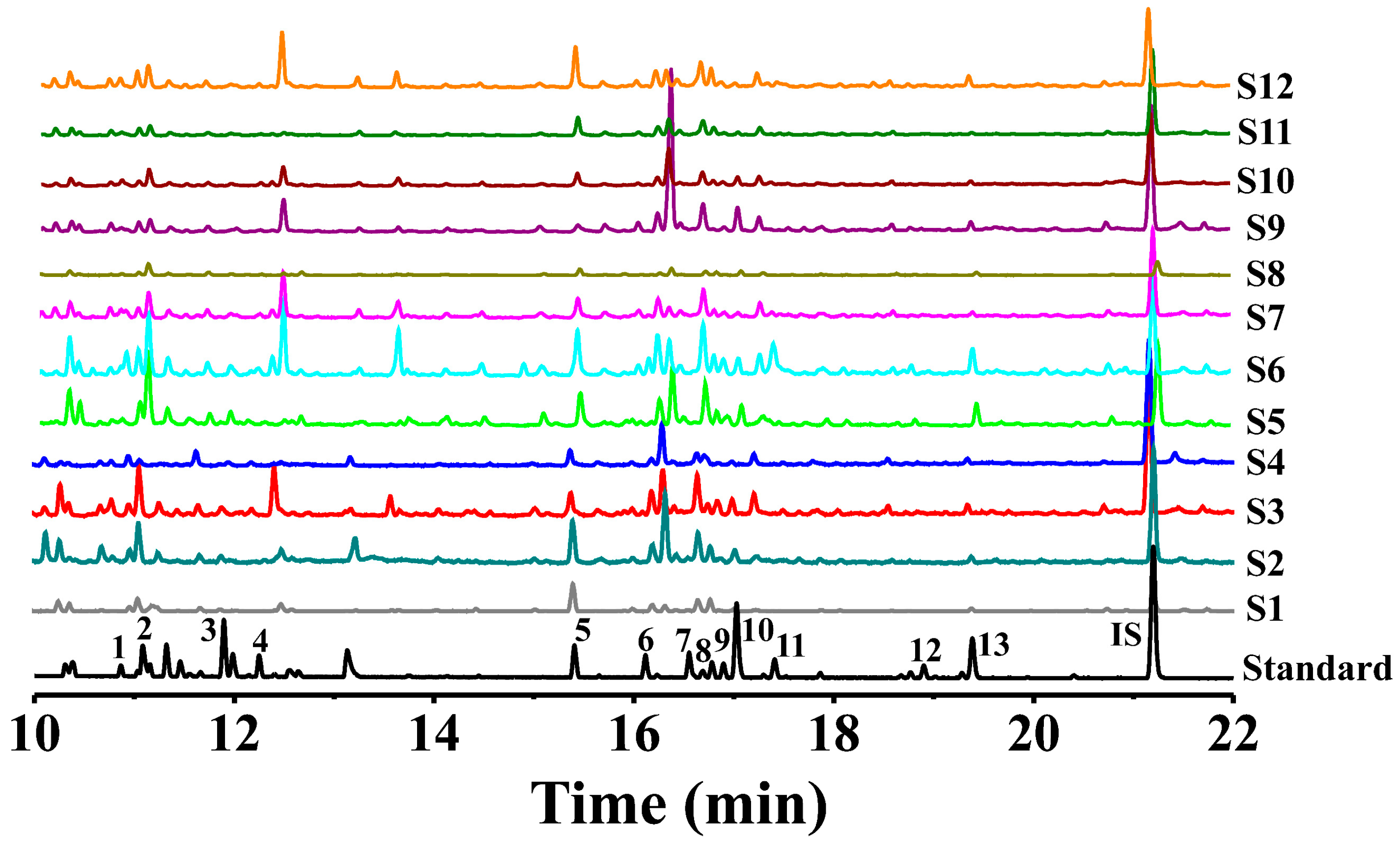
3.3. Quantitative Analysis for Polysaccharides from Three Common Polygonatum spp.
3.3.1. Method Validation
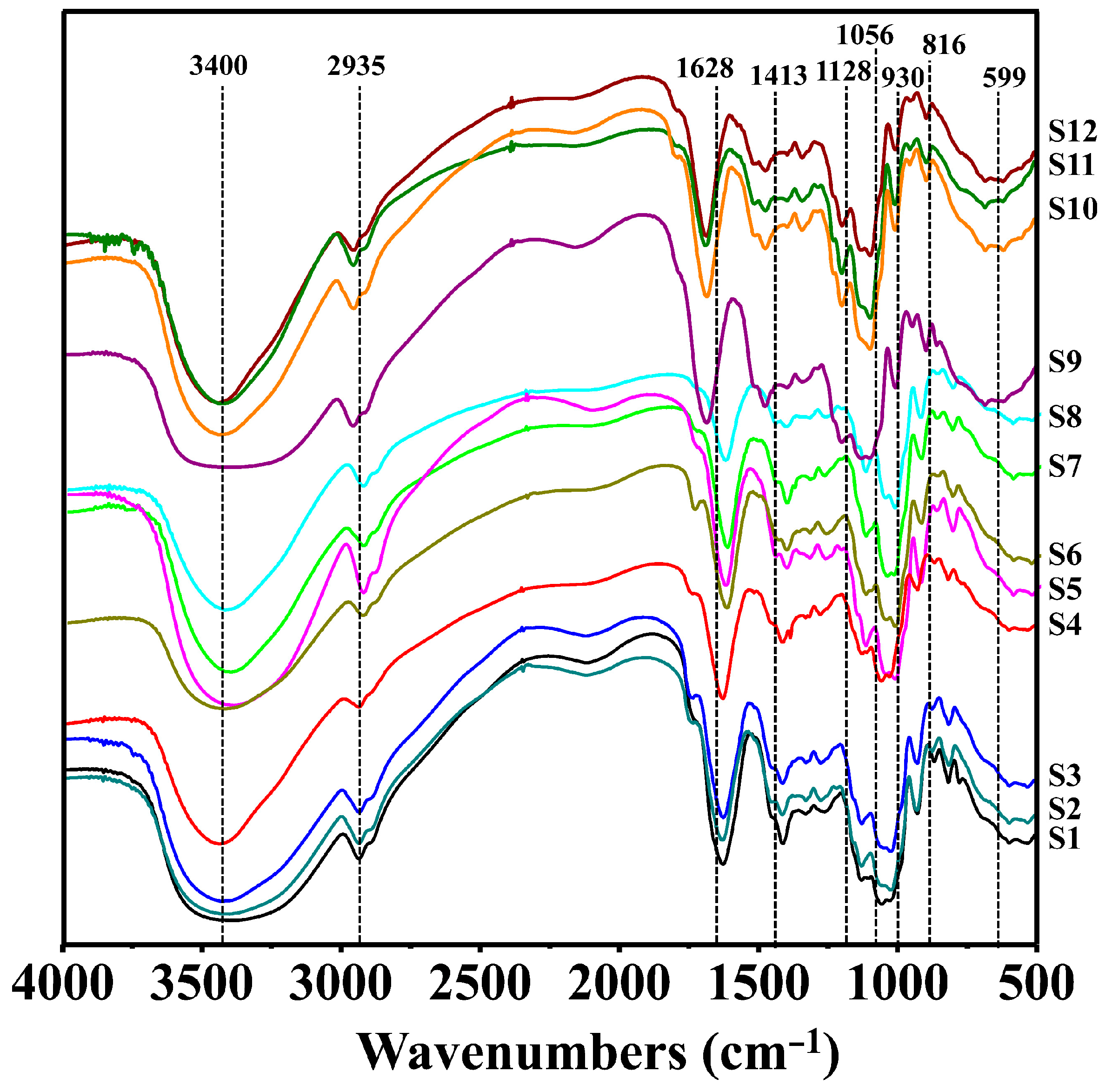
3.3.2. IR Spectrum Analysis
3.3.3. IR Spectrum Analysis
3.3.4. HCA
3.3.5. PCA
3.3.6. OPLS-DA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The Genus Polygonatum: A Review of Ethnopharmacology, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.Z.; Yang, W.Z.; Yang, M.Q.; Zhang, J.Y. Research Progress in Chemical Constituents in Plants of Polygonatum and Their Pharmacological Effects. Chin. J. Chin. Mater. Med. 2019, 44, 1989–2008. [Google Scholar]
- Jiang, C.X.; Zhang, T.J.; Chen, C.Q.; Li, X.K.; Liu, C.X. Research Progress in Polygonati rhizoma and Predictive Analysis on Q-marker. Chin. Tradit. Herb. Drugs 2017, 48, 1–16. [Google Scholar]
- Li, X.L.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Wang, S.Y.; Zhang, J.G.; Wei, Z.J. Evolutionary Research Trend of Polygonatum Species: A Comprehensive Account of Their Transformation from Traditional Medicines to Functional Foods. Crit. Rev. Food Sci. Nutr. 2022, 62, 1769–1798. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, L.L.; Yang, L.Z.; Ji, X.L. An Insight into Anti-Inflammatory Effects of Natural Polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wang, Y.J.; Bao, Q.W.; Qin, Y.M.; Li, P.P.; Wu, Q.Q.; Xia, C.K.; Wu, D.L.; Xie, S.Z. Polygonatum cyrtonema Hua Polysaccharide Alleviates Ulcerative Colitis via Gut Microbiota-Independent Modulation of Inflammatory Immune Response. Carbohydr. Polym. 2025, 356, 123387. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Chen, Y.L.; Su, Y.; Yuan, W.Q.; Peng, D.; Guan, Z.W.; Chen, J.P.; Li, P.; Du, B. Identification of Carbohydrate in Polygonatum kingianum Coll. et Hemsl and Inhibiting Oxidative Stress. Int. J. Biol. Macromol. 2024, 261, 129760. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.Y.; Xiao, Y.; Yu, L.; Tang, Q.J.; Wang, Y.P.; Zhou, J.J. Structure Characterization of an Agavin-Type Fructan Isolated from Polygonatum cyrtonema and Its Effect on the Modulation of the Gut Microbiota In Vitro. Carbohydr. Polym. 2024, 330, 121829. [Google Scholar] [CrossRef]
- Shen, F.M.; Song, Z.J.; Xie, P.; Li, L.; Wang, B.; Peng, D.Y.; Zhu, G.Q. Polygonatum sibiricum Polysaccharide Prevents Depression-Like Behaviors by Reducing Oxidative Stress, Inflammation, and Cellular and Synaptic Damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Bai, J.B.; Ge, J.C.; Zhang, W.J.; Liu, W.; Luo, J.P.; Xu, F.Q.; Wu, D.L.; Xie, S.Z. Physicochemical, Morpho-Structural, and Biological Characterization of Polysaccharides from Three Polygonatum spp. RSC Adv. 2021, 11, 37952–37965. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and Saccharide Mapping of Polysaccharides from Four Common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, H.; Zhao, C.; Li, X.; Wang, Y.; Huang, L.; Gao, W. Purification, Characterization and Immunomodulatory Activity of Fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr. Polym. 2019, 214, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.R.; Shi, N.X.; Xie, P.X.; Zhang, G.F.; Liu, H.Y.; Ji, Y.H. Plastome Sequencing for Accurate and Effective Authentication of Polygonatum kingianum (Asparagaceae). Ind. Crops Prod. 2022, 184, 115056. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.Z.; Yang, M.Q.; Yang, W.Z.; Yang, S.B.; Zhang, J.Y. Identification and Evaluation of Polygonatum kingianum with Different Growth Ages Based on Data Fusion Strategy. Microchem. J. 2021, 160, 105662. [Google Scholar] [CrossRef]
- Hu, J.Y.; Cheng, H.; Xu, J.; Liu, J.L.; Xing, L.H.; Shi, S.Y.; Wang, R.; Wu, Z.D.; Yu, N.J.; Peng, D.Y. Determination and Analysis of Monosaccharides in Polygonatum cyrtonema Hua Polysaccharides from Different Areas by Ultra-High-Performance Liquid Chromatography Quadrupole Trap Tandem Mass Spectrometry. J. Sep. Sci. 2021, 44, 3506–3515. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Zhang, X.Q.; Jia, H.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. Comparative Studies on Characterization, Saccharide Mapping and Antiglycation Activity of Polysaccharides from Different Polygonatum ssp. J. Pharm. Biomed. Anal. 2020, 186, 113243. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.H.; Gu, T.Y.; Schmitz, O.J.; Li, D.X. An Active Derivatization Detection Method for Inline Monitoring the Isolation of Carbohydrates by Preparative Liquid Chromatography. J. Chromatogr. A 2024, 1719, 464730. [Google Scholar] [CrossRef]
- Islam, M.A.; Lee, J.; Yoo, S.H. Effect of Oximation Reagents on Gas Chromatographic Separation of Eight Different Kinds of Mono- and Di-Saccharides. Food Chem. 2022, 386, 132797. [Google Scholar] [CrossRef]
- Visnupriyan, R.; Flanagan, B.M.; Harper, K.J.; Cozzolino, D. Near Infrared Spectroscopy Combined with Chemometrics as Tool to Monitor Starch Hydrolysis. Carbohydr. Polym. 2024, 324, 121469. [Google Scholar] [CrossRef]
- Yahya, L.A.; Vakh, C.; Dushna, O.; Kalisz, O.; Bocian, S.; Tobiszewski, M. Guidelines on the Proper Selection of Greenness and Related Metric Tools in Analytical Chemistry—A Tutorial. Anal. Chim. Acta 2025, 1357, 344052. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.L.; Tong, G.Y.; Li, Y.; Lei, M.N.; Wu, H.; Wang, B.; Hu, R.F. Development of a Novel UHPLC-UV Combined with UHPLC-QTOF/MS Fingerprint Method for the Comprehensive Evaluation of Nao-Luo-Xin-Tong: Multi-Wavelength Setting Based on Traditional Chinese Medicinal Prescription Composition. Anal. Methods 2019, 11, 6092–6102. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.; Wang, Y.L.; Chen, L.J.; Liu, H.; Wang, Z.; Wang, B. Screening the Q-markers of TCMs from RA Rat Plasma Using UHPLC-QTOF/MS Technique for the Comprehensive Evaluation of Wu-Wei-Wen-Tong Capsule. J. Mass Spectrom. 2021, 56, e4711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Cao, Y.X.; Zhang, T.T.; Guo, H.Y.; Wang, B. A Novel Method for Investigating the Mechanism of the Antirheumatoid Arthritis Activity of Angelicae pubescentis Radix by Integrating UHPLC–QTOF/MS and Network Pharmacology. Biomed. Chromatogr. 2022, 36, e5389. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.X.; Du, J.; Wang, Z.; Li, X.Y.; Fu, R.Z.; Liu, H.; Xu, N.; Zhu, G.Q.; Wang, B. Structural Characteristics and Biological Activity of a Water-Soluble Polysaccharide HDCP-2 from Camellia sinensis. Int. J. Biol. Macromol. 2024, 277, 134437. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.X.; Zhao, Y.Y.; Du, J. Synthesis of Naphthalimide-Type Chemsensor and Its Application in Quality Evaluation for Polygonatum sibiricum Red. Front. Chem. 2022, 10, 969014. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Fu, R.Z.; Ou, J.M.; Wang, B. Structural Characterization and Anti-Inflammatory Activity of a Novel Polysaccharide PKP2-1 from Polygonatum kingianum. Front. Nutr. 2023, 10, 1156798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, Z.; Fu, R.Z.; Xie, R.N.; Wang, B.; Li, Q.L. Structural Characterization and Antioxidant Activity of Processed Polysaccharides PCP-F1 from Polygonatum cyrtonema Hua. Front. Nutr. 2023, 10, 1272977. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.Y.; Chen, L.J.; Du, J.; Guo, H.Y.; Wang, B. A Novel Method for the Pre-Column Derivatization of Saccharides from Polygonatum cyrtonema Hua. by Integrating Lambert–Beer Law and Response Surface Methodology. Molecules 2023, 28, 2186. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, R.N.; Wang, B. Comprehensive Evaluation and Anti-Inflammatory Activity of “Zhi” Polygonatum cyrtonema Produced by the Classical Steaming Approach. Pharmacol. Res. Mod. Chin. Med. 2023, 6, 100229. [Google Scholar] [CrossRef]
- Du, J.; Zhou, X.; Chen, L.J.; Xu, L.; Wang, B. Simultaneous Determination of Naphthalimide-Labelled Monosaccharides in P. cyrtonema Hua. Polysaccharides Utilizing the HPLC-UV Technique. Anal. Methods 2025, 17, 1196. [Google Scholar] [CrossRef]
- Li, W.J.; Yu, L.B.; Fu, B.; Chu, J.; Chen, C.; Li, X.J.; Ma, J.H.; Tang, W. Protective Effects of Polygonatum kingianum Polysaccharides and Aqueous Extract on Uranium-Induced Toxicity in Human Kidney (HK-2) Cells. Int. J. Biol. Macromol. 2022, 202, 68–79. [Google Scholar] [CrossRef]
- Zong, X.Y.; Wang, Z.; Chen, S.K.; Li, S.; Xie, M.Y.; Nie, S.P.; Yin, J.Y. Optimized Acid Hydrolysis Conditions for Better Characterization the Structure of Inulin-Type Fructan from Polygonatum sibiricum. Int. J. Biol. Macromol. 2024, 256, 128030. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.Y.; Hu, X.B.; Wang, Y.J.; Wang, J.H.; Tang, F.Y.; Liu, Y. Structural Characterization and Anti-Inflammatory Activity of a Pectin Polysaccharide HBHP-3 from Houttuynia cordata. Int. J. Biol. Macromol. 2022, 210, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Kan, Y.J.; Huang, Y.T.; Jiang, C.; Zhao, L.; Hu, J.; Pang, W.S. Physicochemical Characteristics and Antidiabetic Properties of the Polysaccharides from Pseudostellaria heterophylla. Molecules 2022, 27, 3719. [Google Scholar] [CrossRef]
- Wang, T.Y.; Guo, S.; Ren, X.M.; Du, J.F.; Bai, L.; Cui, X.Q.; Ho, C.T.; Bai, N.S. Simultaneous Quantification of 18 Bioactive Constituents in Ziziphus jujuba Fruits by HPLC Coupled with a Chemometric Method. Food Sci. Hum. Wellness 2022, 11, 771–780. [Google Scholar] [CrossRef]
- Kim, M.; Hong, J.; Lee, D.; Kim, S.; Chun, H.S.; Cho, Y.H.; Kim, B.H.; Ahn, S. Discriminant Analysis of the Geographical Origin of Asian Red Pepper Powders Using Second-Derivative FT-IR Spectroscopy. Foods 2021, 10, 1034. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Yan, Y.Z.; Shi, M.M.; Liu, Y.Q. Comparison of Structural Characterization and Antioxidant Activity of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Lei, J.; Li, W.; Fu, M.X.; Wang, A.Q.; Wu, D.T.; Guo, H.; Hu, Y.C.; Gan, R.Y.; Zou, L.; Liu, Y. Pressurized Hot Water Extraction, Structural Properties, Biological Effects, and In Vitro Microbial Fermentation Characteristics of Sweet Tea Polysaccharide. Int. J. Biol. Macromol. 2022, 222, 3215–3228. [Google Scholar] [CrossRef]
- Li, M.Y.; Cheng, S.C.; Li, D.; Wang, S.N.; Huang, A.M.; Sun, S.Q. Structural Characterization of Steam-Heat Treated Tectona grandis Wood Analyzed by FT-IR and 2D-IR Correlation Spectroscopy. Chin. Chem. Lett. 2015, 26, 221–225. [Google Scholar] [CrossRef]
- Qu, L.; Chen, J.B.; Zhang, G.J.; Sun, S.Q.; Zheng, J. Chemical Profiling and Adulteration Screening of Aquilariae Lignum Resinatum by Fourier Transform Infrared (FT-IR) Spectroscopy and Two-Dimensional Correlation Infrared (2D-IR) Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 174, 177–182. [Google Scholar] [CrossRef] [PubMed]






| Sample | Mean Value | 95% Confidence Interval (Lower Limit~Upper Limit) |
|---|---|---|
| S1 | 0.15076 | 0.117888~0.183632 |
| S2 | 0.112358 | 0.042594~0.182122 |
| S3 | 0.326436 | 0.168262~0.484610 |
| S4 | 0.10799 | 0.048957~0.167023 |
| S5 | 0.22898 | 0.108162~0.349798 |
| S6 | 0.083622 | 0.009179~0.158065 |
| S7 | 0.084396 | 0.009953~0.158839 |
| S8 | 0.052144 | 0.016793~0.087495 |
| S9 | 0.168506 | 0.075545~0.261467 |
| S10 | 0.212622 | 0.121317~0.303927 |
| S11 | 0.159772 | 0.077289~0.242255 |
| S12 | 0.252788 | 0.134187~0.371389 |
| VC | 0.908248 | 0.892340~0.924156 |
| Component | Initial Eigenvalue | Load Sum of Squares | ||||
|---|---|---|---|---|---|---|
| Characteristic Value | Variance Contribution Rate (%) | Cumulative Variance Contribution Rate (%) | Characteristic Value | Variance Contribution Rate (%) | Cumulative Variance Contribution Rate (%) | |
| 1 | 5.278 | 58.643 | 58.643 | 5.278 | 58.643 | 58.643 |
| 2 | 2.236 | 24.849 | 83.492 | 2.236 | 24.849 | 83.492 |
| 3 | 0.683 | 7.591 | 91.083 | |||
| 4 | 0.361 | 4.013 | 95.096 | |||
| 5 | 0.208 | 2.313 | 97.409 | |||
| 6 | 0.137 | 1.523 | 98.932 | |||
| 7 | 0.055 | 0.610 | 99.542 | |||
| 8 | 0.025 | 0.279 | 99.820 | |||
| 9 | 0.016 | 0.180 | 100.000 | |||
| No. | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| S11 | 3.37 | 0.42 | 2.08 |
| S9 | 2.97 | 0.94 | 1.97 |
| S10 | 2.85 | 0.75 | 1.86 |
| S12 | 2.82 | −0.25 | 1.59 |
| S2 | −1.93 | 2.10 | −0.61 |
| S6 | −0.55 | −1.62 | −0.72 |
| S3 | −1.97 | 0.88 | −0.94 |
| S1 | −1.88 | 0.56 | −0.96 |
| S8 | −0.91 | −2.01 | −1.03 |
| S7 | −1.10 | −1.69 | −1.07 |
| S5 | −0.96 | −2.03 | −1.07 |
| S4 | −2.71 | 1.95 | −1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, W.; Wang, B. Discrimination of Polygonatum Species via Polysaccharide Fingerprinting: Integrating Their Chemometrics, Antioxidant Activity, and Potential as Functional Foods. Foods 2025, 14, 2385. https://doi.org/10.3390/foods14132385
Liu Z, Zhang W, Wang B. Discrimination of Polygonatum Species via Polysaccharide Fingerprinting: Integrating Their Chemometrics, Antioxidant Activity, and Potential as Functional Foods. Foods. 2025; 14(13):2385. https://doi.org/10.3390/foods14132385
Chicago/Turabian StyleLiu, Zhiguo, Wei Zhang, and Bin Wang. 2025. "Discrimination of Polygonatum Species via Polysaccharide Fingerprinting: Integrating Their Chemometrics, Antioxidant Activity, and Potential as Functional Foods" Foods 14, no. 13: 2385. https://doi.org/10.3390/foods14132385
APA StyleLiu, Z., Zhang, W., & Wang, B. (2025). Discrimination of Polygonatum Species via Polysaccharide Fingerprinting: Integrating Their Chemometrics, Antioxidant Activity, and Potential as Functional Foods. Foods, 14(13), 2385. https://doi.org/10.3390/foods14132385






