The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review
Abstract
1. Introduction
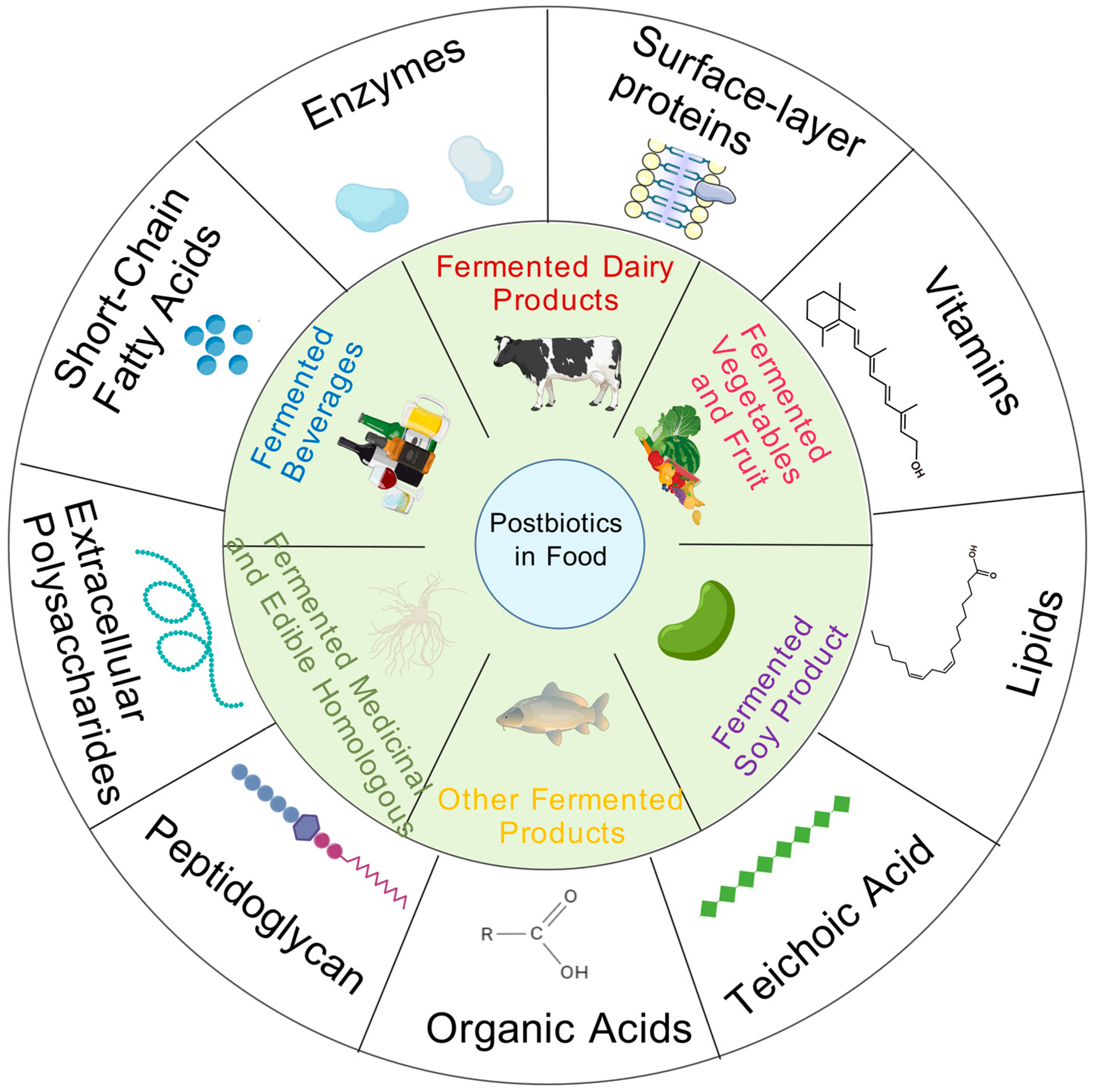
2. Composition of Postbiotics and Postbiotics in Food
2.1. Main Components of Postbiotics
- (1)
- Cellular components
- (2)
- Cellular metabolites
- (3)
- Other bioactive substances
2.2. Postbiotics in Food
- (1)
- Fermented dairy products
- (2)
- Fermented vegetables and fruits
- (3)
- Fermented soy products
- (4)
- Fermented beverages
- (5)
- Fermented medicinal and edible homologous plants
- (6)
- Other fermented products
3. Classification of Inactivation Methods
3.1. Thermal Inactivation
3.1.1. Traditional High-Temperature Sterilization Method
3.1.2. Pasteurization
3.1.3. Ohmic Heating
3.2. Non-Thermal Inactivation
3.2.1. Ultraviolet Inactivation
3.2.2. High-Pressure Inactivation
3.2.3. Ultrasonic Sterilization
3.2.4. Pulsed Electric Fields Inactivation
3.2.5. Other Non-Thermal Inactivation
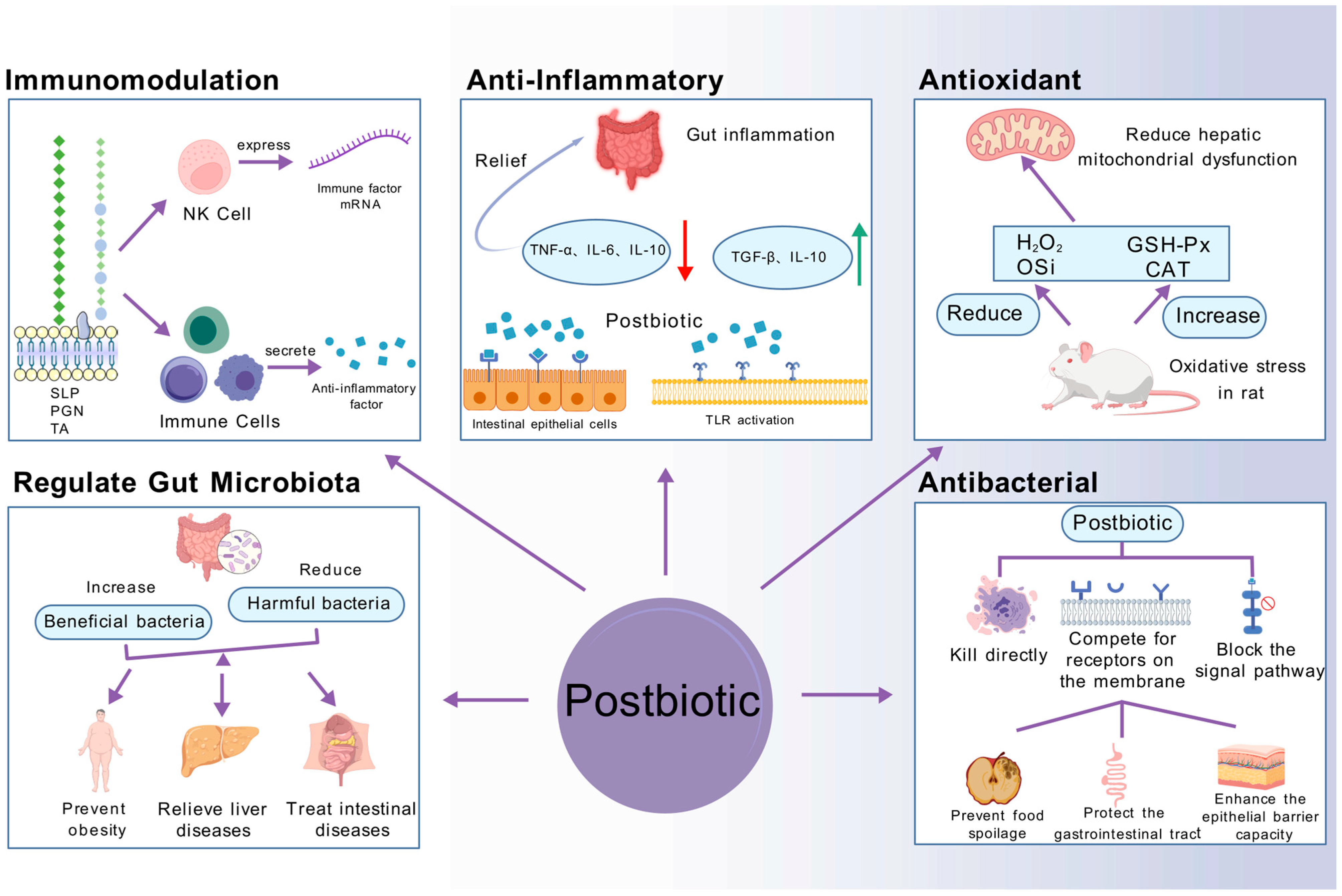
4. Changes in Postbiotic Bioactivity Under Different Inactivation Methods
4.1. Immunomodulation
4.2. Anti-Inflammatory
4.3. Antioxidation
4.4. Antibacterial Properties
4.5. Regulate Gut Microbiota
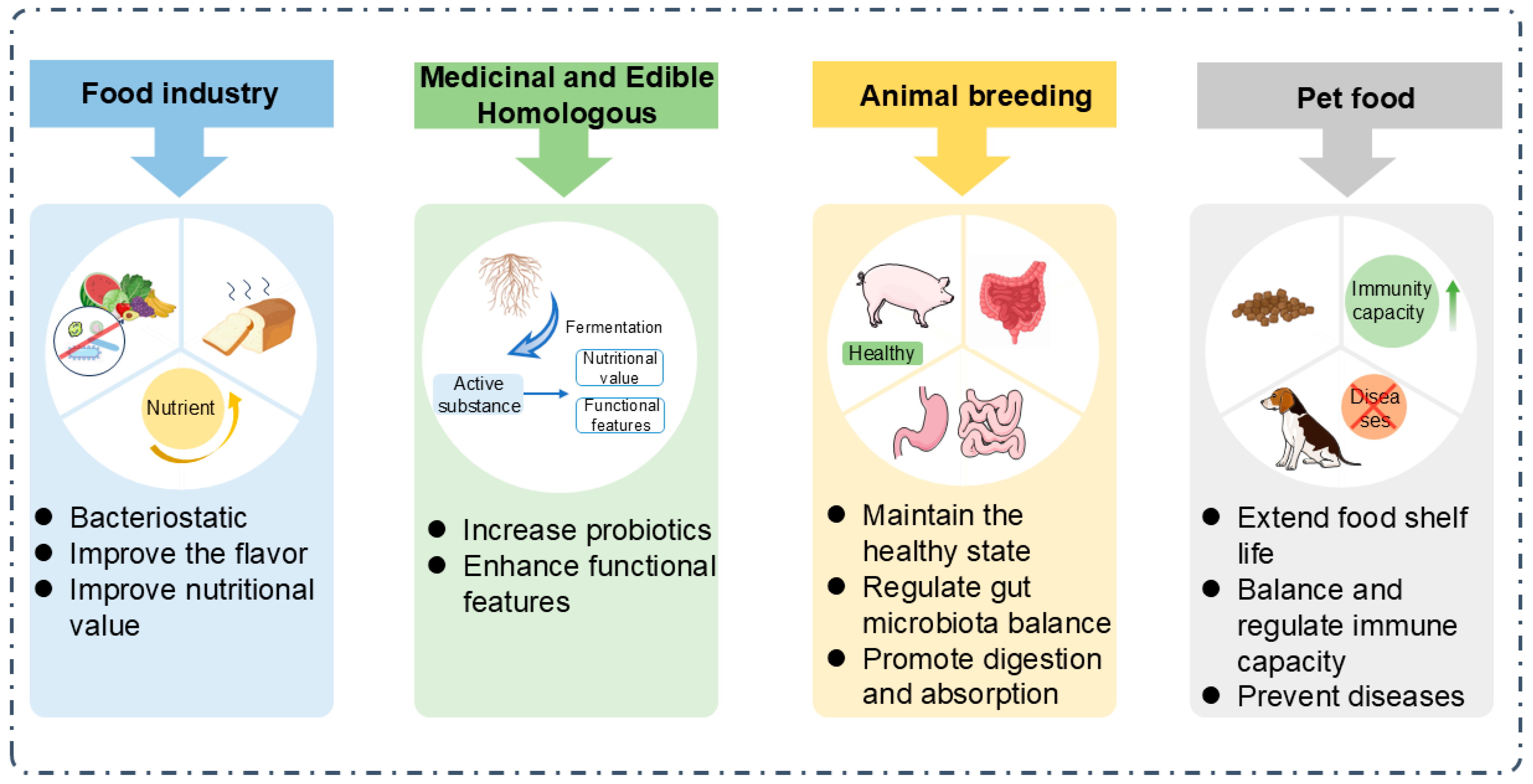
5. The Application of Postbiotics
5.1. Food Industry
5.2. Medicinal and Edible Homologous
5.3. Animal Breeding
5.4. Pet Food
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, A.; Rengasamy, K.R.R.; Di Minno, A.; Sacchi, R.; Daglia, M. The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach. Nutrients 2022, 14, 2751. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, X.; Sun, Q.; Qian, W.; Liu, X.; Li, J.; Long, Y.; Wan, X. Different Types and Functional Effects of Probiotics on Human Health through Regulating Glucose Homeostasis. J. Agric. Food Chem. 2021, 69, 14781–14791. [Google Scholar] [CrossRef]
- Zhao, N.; Huang, X.; Liu, Z.; Gao, Y.; Teng, J.; Yu, T.; Yan, F. Probiotic characterization of Bacillus smithii: Research advances, concerns, and prospective trends. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13308. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Marzban, H.; Goleij, P.; Sahebkar, A.; Morshedi, K.; Rezaei, S.; Mahjoubin-Tehran, M.; Tarrahimofrad, H.; Hamblin, M.R.; Mirzaei, H. Effects of therapeutic probiotics on modulation of microRNAs. Cell Commun. Signal. 2021, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Zhao, X.; Wan, F.; Gu, D.; Xie, W.; Gao, C. Intracellularly Gelated Macrophages Loaded with Probiotics for Therapy of Colitis. Nano Lett. 2024, 24, 13504–13512. [Google Scholar] [CrossRef]
- Wang, X.Z.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Xie, X.; Li, Q.Y.; Jia, L.F.; Yuan, H.; Guo, T.; Meng, T. Multishell Colloidosome Platform with Sequential Gastrointestinal Resistance for On-Demand Probiotic Delivery. Adv. Healthc. Mater. 2023, 12, 2202954. [Google Scholar] [CrossRef]
- Lin, Q.J.; Lin, S.Y.; Fan, Z.T.; Liu, J.; Ye, D.C.; Guo, P.T. A Review of the Mechanisms of Bacterial Colonization of the Mammal Gut. Microorganisms 2024, 12, 1026. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Huycke, M.M. Risks associated with enterococci as probiotics. Food Res. Int. 2020, 129, 108788. [Google Scholar] [CrossRef]
- Busanello, M.; Pozza, M.S.d.S.; Pozza, P.C.; Nunes, R.V.; Chambo, A.P.S.; Eckstein, I.I. Probiotics: Viable and inactivated cells on the performance, microflora and blood parameters of piglets. Rev. Bras. De Saúde E Produção Anim. 2015, 16, 387–396. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Li, Q.; Li, L.; Zhu, N.; Xiong, X.; Li, G. Peptidoglycan derived from Lactobacillus rhamnosus MLGA up-regulates the expression of chicken β-defensin 9 without triggering an inflammatory response. Innate Immun. 2020, 26, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Indira, M.; Venkateswarulu, T.C.; Peele, K.A.; Bobby, M.N.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech. 2019, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Saeed, M.; Afzal, Z.; Afzal, F.; Khan, R.U.; Elnesr, S.S.; Alagawany, M.; Chen, H.Y. Use of Postbiotic as Growth Promoter in Poultry Industry: A Review of Current Knowledge and Future Prospects. Food Sci. Anim. Resour. 2023, 43, 1111–1127. [Google Scholar] [CrossRef]
- Duysburgh, C.; Miclotte, L.; Green, J.B.; Watts, K.T.; Sardi, M.I.; Chakrabarti, A.; Khafipour, E.; Marzorati, M. Saccharomyces cerevisiae derived postbiotic alters gut microbiome metabolism in the human distal colon resulting in immunomodulatory potential in vitro. Front. Microbiol. 2024, 15, 1358456. [Google Scholar] [CrossRef]
- Freitas, A.d.S.; Barroso, F.A.L.; Campos, G.M.; Americo, M.F.; Viegas, R.C.d.S.; Gomes, G.C.; Vital, K.D.; Fernandes, S.O.A.; Carvalho, R.D.d.O.; Jardin, J.; et al. Exploring the anti-inflammatory effects of postbiotic proteins from Lactobacillus delbrueckii CIDCA 133 on inflammatory bowel disease model. Int. J. Biol. Macromol. 2024, 277, 134216. [Google Scholar] [CrossRef]
- Rezaie, N.; Aghamohammad, S.; Khiavi, E.H.A.G.; Talebi, M.; Pourshafie, M.R.; Rohani, M. The Analysis and Comparison of Anti-Inflammatory and Antioxidant Characteristics of Postbiotic and Paraprobiotic Derived From Novel Native Probiotic Cocktail in DSS-Induced Colitic Mice. Food Sci. Nutr. 2025, 13, e70034. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, N.; Ye, X.; Liu, M.; Wei, M.; Huang, Y. The postbiotic of hawthorn-probiotic ameliorating constipation caused by loperamide in elderly mice by regulating intestinal microecology. Front. Nutr. 2023, 10, 1103463. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Green, K.M.; Rawat, M. A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut health benefit and application of postbiotics in animal production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef]
- Gu, Z.; Meng, S.; Wang, Y.; Lyu, B.; Li, P.; Shang, N. A novel bioactive postbiotics: From microbiota-derived extracellular nanoparticles to health promoting. Crit. Rev. Food Sci. Nutr. 2023, 63, 6885–6899. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, Z.; Fang, B.; Hung, W.; Gao, H.; Zhao, W.; Lan, H.; Liu, M.; Zhao, L.; Zhang, M. Effect of Thermal Inactivation on Antioxidant, Anti-Inflammatory Activities and Chemical Profile of Postbiotics. Foods 2023, 12, 3579. [Google Scholar] [CrossRef]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Singh, V.; Muthuramalingam, K.; Kim, Y.M.; Park, S.; Kim, S.H.; Lee, J.; Hyun, C.; Unno, T.; Cho, M. Synbiotic supplementation with prebiotic Schizophyllum commune derived β-(1,3/1,6)-glucan and probiotic concoction benefits gut microbiota and its associated metabolic activities. Appl. Biol. Chem. 2021, 64, 1–10. [Google Scholar] [CrossRef]
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.G.; Lee, L.H. Exploring the Role and Potential of Probiotics in the Field of Mental Health: Major Depressive Disorder. Nutrients 2021, 13, 1728. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; Gul, M.; Awais, M.; Liang, Q.F.; Tufail, T.; Zhong, M.M.; Sun, Y.F.; Qayum, A.; El-Salam, E.A.; et al. Layer-by-layer concurrent encapsulation of probiotics and bioactive compounds with supplementation in intermediary layers: An establishing instrument for microbiome recharge, core safety, and targeted delivery. Food Hydrocoll. 2025, 161, 110873. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Gulati, G.; Avadhani, R.; Rashmi, H.M.; Soumya, K.; Kumari, A.; Gupta, A.; Dwivedi, D.; Kaushik, J.K.; Grover, S. Postbiotic Lipoteichoic acid of probiotic Lactobacillus origin ameliorates inflammation in HT-29 cells and colitis mice. Int. J. Biol. Macromol. 2023, 236, 123962. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Kojima, S.; Hosokawa, S.; Oda, Y.; Zenke, D.; Toura, Y.; Onohara, E.; Yokota, S.-I.; Nagaoka, M.; Kuroda, Y. Wall teichoic acid-dependent phagocytosis of intact cell walls of Lactiplantibacillus plantarum elicits IL-12 secretion from macrophages. Front. Microbiol. 2022, 13, 986396. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Y.-Y.; Hao, Y.-P. Protective function of surface layer protein from Lactobacillus casei fb05 against intestinal pathogens in vitro. Biochem. Biophys. Res. Commun. 2021, 546, 15–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopolysaccharide Produced by Lactobacillus plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Wen, X.; Xiaoyue, D.; Longkun, D.; Yue, X.; Man, Y.; Min, Z.; Liang, W.; Chengxue, Y.; Huaxi, X. Three main short-chain fatty acids inhibit the activation of THP-1 cells by Mycoplasma pneumoniae. Biosci. Biotechnol. Biochem. 2021, 85, 923–930. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. The Combinatorial Effect of Acetate and Propionate on High-Fat Diet Induced Diabetic Inflammation or Metaflammation and T Cell Polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, Y.; Li, Y.; Bao, Y.; Wang, X.; Piao, C. Co-production of nattokinase and α-amylase from Bacillus natto fermentation using okara. J. Food Process. Preserv. 2022, 46, e17130. [Google Scholar] [CrossRef]
- Patil, S.; Sawant, S.; Hauff, K.; Hampp, G. Validated Postbiotic Screening Confirms Presence of Physiologically-Active Metabolites, Such as Short-Chain Fatty Acids, Amino Acids and Vitamins in Hylak® Forte. Probiotics Antimicrob. Proteins 2019, 11, 1124–1131. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Hyun, I.K.; An, S.; Kim, D.; Kim, K.H.; Kang, S.-S. In vitro anti-inflammatory and antibiofilm activities of bacterial lysates from lactobacilli against oral pathogenic bacteria. Food Funct. 2022, 13, 12755–12765. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Han, J.R.; Zhao, X.; Zhao, X.L.; Li, P.; Gu, Q. Insight into the structure, biosynthesis, isolation method and biological function of teichoic acid in different gram-positive microorganisms: A review. Int. J. Biol. Macromol. 2023, 253, 126825. [Google Scholar] [CrossRef]
- Kwan, J.M.C.; Qiao, Y. Mechanistic Insights into the Activities of Major Families of Enzymes in Bacterial Peptidoglycan Assembly and Breakdown. Chembiochem 2023, 24, e202200693. [Google Scholar] [CrossRef]
- Kwan, J.M.C.; Liang, Y.Q.; Ng, E.W.L.; Sviriaeva, E.; Li, C.; Zhao, Y.; Zhang, X.L.; Liu, X.W.; Wong, S.H.; Qiao, Y. In silico MS/MS prediction for peptidoglycan profiling uncovers novel anti-inflammatory peptidoglycan fragments of the gut microbiota. Chem. Sci. 2024, 15, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, C.; Zhang, X.; Zhao, X.; Mahsa, G.C.; Ma, K.; Ji, F.; Azarpazhooh, E.; Ajami, M.; Rui, X.; et al. Effects of Lacticaseibacillus paracasei SNB-derived postbiotic components on intestinal barrier dysfunction and composition of gut microbiota. Food Res. Int. 2024, 175, 113773. [Google Scholar] [CrossRef]
- Farid, W.; Masud, T.; Sohail, A.; Ahmad, N.; Naqvi, S.M.S.; Khan, S.; Ali, A.; Khalifa, S.A.; Hussain, A.; Ali, S.; et al. Gastrointestinal transit tolerance, cell surface hydrophobicity, and functional attributes of Lactobacillus Acidophilus strains isolated from Indigenous Dahi. Food Sci. Nutr. 2021, 9, 5092–5102. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, D.; Yang, H.; Jiao, X.; Zhou, R.; Zheng, J.; Wu, C. Lactic acid bacteria-derived exopolysaccharide: Biosynthesis and antibacterial characterization. Trends Food Sci. Technol. 2025, 160, 105033. [Google Scholar] [CrossRef]
- Srutkova, D.; Kozakova, H.; Novotna, T.; Gorska, S.; Hermanova, P.P.; Hudcovic, T.; Svabova, T.; Sinkora, M.; Schwarzer, M. Exopolysaccharide from Lacticaseibacillus rhamnosus induces IgA production in airways and alleviates allergic airway inflammation in mouse model. Eur. J. Immunol. 2023, 53, e2250135. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Cotter, P.D.; Freitas, M.; Gueimonde, M.; Holscher, H.D.; Ruas-Madiedo, P.; Salminen, S.; Swanson, K.S.; Sanders, M.E.; Cifelli, C.J. Fermented foods: A perspective on their role in delivering biotics. Front. Microbiol. 2023, 14, 1196239. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef]
- Yerlikaya, O. A review of fermented milks: Potential beneficial effects on human nutrition and health. Afr. Health Sci. 2023, 23, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Niu, S.Z.; Wu, M.Q.; Li, H.L.; Yang, C.; Liu, C.Y.; Dai, C.H.; He, R.H.; Xu, Y.J. Research Progress on Quality Characteristics of Yoghurt and Its Fermentation Enhancement Technology: A Review. J. Food Process Eng. 2025, 48, e70042. [Google Scholar] [CrossRef]
- Li, D.Y.; Zheng, Y.; Kwok, L.Y.; Zhang, W.Y.; Sun, T.S. Metabolic footprinting revealed key biochemical changes in a brown fermented milk product using Streptococcus thermophilus. J. Dairy. Sci. 2020, 103, 2128–2138. [Google Scholar] [CrossRef]
- Yu, J.; Sun, M.Y.; Jiang, S.L.; Jiang, C.Q.; Mu, G.Q.; Tuo, Y. Oral Administration of Fermented Milk from Co-Starter Containing Lactobacillus plantarum Y44 Shows an Ameliorating Effect on Hypertension in Spontaneously Hypertensive Rats. Foods 2024, 13, 641. [Google Scholar] [CrossRef]
- Ganzorig, K.; Urashima, T.; Fukuda, K. Exploring Potential Bioactive Peptides in Fermented Bactrian Camel’s Milk and Mare’s Milk Made by Mongolian Nomads. Foods 2020, 9, 1817. [Google Scholar] [CrossRef]
- Bayarsaikhan, D.; Ohnishi-Kameyama, M.; Shirai, N.; Takahashi, Y.; Yamaki, K. Inhibition of Angiotensin-Converting Enzyme by Components of Traditional Mongolian Fermented Milk Products. Food Sci. Technol. Res. 2011, 17, 567–572. [Google Scholar] [CrossRef]
- Song, J.J.; Wang, Q.; Du, M.; Ji, X.M.; Mao, X.Y. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy. Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef]
- Estruch, R.; Lamuela-Raventós, R.M. Cardiovascular benefits of fermented foods and beverages: Still up for debate. Nat. Rev. Cardiol. 2023, 20, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Doriya, K.; Kumar, D.S.; Thorat, B.N. A systematic review on fruit-based fermented foods as an approach to improve dietary diversity. J. Food ProcessPreserv. 2022, 46, e16994. [Google Scholar] [CrossRef]
- Yang, S.Q.; Tao, Y.; Maimaiti, X.Y.D.; Su, W.; Liu, X.L.; Zhou, J.Z.; Fan, L.L. Investigation on the exopolysaccharide production from blueberry juice fermented with lactic acid bacteria: Optimization, fermentation characteristics and Vis-NIR spectral model. Food Chem. 2024, 452, 139589. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Xiao, L.; Qu, L.; Zhang, X.; Wei, Y. Advancing Insights into Probiotics during Vegetable Fermentation. Foods 2023, 12, 3789. [Google Scholar] [CrossRef] [PubMed]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and metabolic characterization of organic artisanal sauerkraut fermentation and study of gut health-promoting properties of sauerkraut brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Guney, D.; Sengun, M.G.B.B.I. Probiotic characterisation of lactic acid bacteria isolated from pickles and their potential application as presumptive probiotic starter culture in cucumber pickles. J. Food Meas. Charact. 2025, 19, 2077–2097. [Google Scholar] [CrossRef]
- Hwang, H.M.; Park, Y.M.; Lee, H.Y.; Shin, D.Y.; Kim, J.G.; Bae, J.S.; Haam, D.S.; Yang, H.J.; Kim, M.J.; Kim, M.S.; et al. Immunostimulatory Effects of Red Beet Ferment Extract on Cyclophosphamide-Induced Immunosuppression in Wistar Rats. Appl. Sci. 2024, 14, 1013. [Google Scholar] [CrossRef]
- David, N.; Aphinya, T.; Ervan, S.; Hartati, H.; Jinseok, O.; Ji-Gyeol, J.; Rachadaporn, B.; Reggie, S. Immunoenhancing and antioxidant potentials of kimchi, an ethnic food from Korea, as a probiotic and postbiotic food. J. Ethn. Foods 2024, 11, 1–9. [Google Scholar] [CrossRef]
- Lutfi, A.; Bimo, S.R.H.; Dandy, Y.; Lutfi, A.; Fathi, R.M. Exploring tempoyak, fermented durian paste, a traditional Indonesian indigenous fermented food: Typical of Malay tribe. J. Ethn. Foods 2023, 10, 1–13. [Google Scholar] [CrossRef]
- Xiao, X.; Li, X.; Bai, J.; Fan, S.; Daglia, M.; Li, J.; Ding, Y.; Zhang, Y.; Zhao, Y. Changes in the structural, physicochemical and functional properties and in vitro fecal fermentation characteristics of barley dietary fiber fermented by Lactiplantibacillus plantarum dy-1. Food Funct. 2024, 15, 4276–4291. [Google Scholar] [CrossRef]
- Heng, X.Y.; Chen, H.Y.; Lu, C.X.; Feng, T.; Li, K.Y.; Gao, E.B. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases. LWT-Food Sci. Technol. 2022, 154, 112626. [Google Scholar] [CrossRef]
- Tuly, J.A.; Ma, H.L. Bioconversion of food industrial waste okara by microbial fermentation: Scope of omics study and possibility. Trends Food Sci. Technol. 2024, 146, 104391. [Google Scholar] [CrossRef]
- Liu, D.D.; Guo, Y.T.; Liu, X.S.; Ma, H.L.; Ashokkumar, M. Production of value-added peptides from soybean meal during natural solid-state fermentation: Metabolites and bacterial communities. Food Biosci. 2023, 56, 103266. [Google Scholar] [CrossRef]
- Liu, D.D.; Guo, Y.T.; Zhu, J.S.; Yolandani; Wang, Y.C.; Ma, H.L. Peptide production through enhanced solid-state fermentation of soybean meal with Bacillus subtilis SBM_1: Fermentation process and product characteristics. Ind. Crops Prod. 2024, 219, 119184. [Google Scholar] [CrossRef]
- Kitagawa, M.; Shiraishi, T.; Yamamoto, S.; Kutomi, R.; Ohkoshi, Y.; Sato, T.; Wakui, H.; Itoh, H.; Miyamoto, A.; Yokota, S.I. Novel antimicrobial activities of a peptide derived from a Japanese soybean fermented food, Natto, against Streptococcus pneumoniae and Bacillus subtilis group strains. AMB Express 2017, 7, 127. [Google Scholar] [CrossRef]
- Hu, X.C.; Liu, W.M.; Luo, M.M.; Ren, L.J.; Ji, X.J.; Huang, H. Enhancing Menaquinone-7 Production by Bacillus natto R127 Through the Nutritional Factors and Surfactant. Appl. Biochem. Biotechnol. 2017, 182, 1630–1641. [Google Scholar] [CrossRef]
- Mulaw, G.; Gebregziabher, T.; Tesfay, T. A review on the microbiology of Ethiopian traditional fermented beverage products. Front. Nutr. 2025, 12, 1519547. [Google Scholar] [CrossRef]
- Murali, R.D.; Vishnu Priya, S.; Swetha; Antony, U. Traditionally Fermented Foods and Beverages for Nutritional Security and Global Acceptance. In Food Production, Diversity, and Safety Under Climate Change; Chakraborty, R., Mathur, P., Roy, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 77–87. [Google Scholar]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Vīna, I.; Semjonovs, P.; Linde, R.; Deniņa, I. Current evidence on physiological activity and expected health effects of kombucha fermented beverage. J. Med. Food. 2014, 17, 179–188. [Google Scholar] [CrossRef]
- Jung, W.-H.; Kim, G.-W.; Kang, G.-O.; Shim, J.-Y.; Son, J.-Y. Effect of Lactic Acid Bacteria Inoculation on Functional Component and Antioxidant Activity of Makgeolli. Foodserv. Ind. J. 2021, 17, 129–141. [Google Scholar] [CrossRef]
- Kim, B.K.; Kang, M.S.; Jeon, M.-J.; Lee, S.-H.; Kim, M. Effects of Makgeolli and Makgeolli precipitate on Hepatotoxicity and Serum Lipid Content in Rats. J. Life Sci. 2013, 23, 282–289. [Google Scholar] [CrossRef]
- Das, S.; Tamang, J.P. Fermentation Dynamics of Naturally Fermented Palm Beverages of West Bengal and Jharkhand in India. Ferment. Basel 2023, 9, 301. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.C.; Zhang, Z.M.; Xia, B.H.; Li, Y.M.; Lin, Y.; Li, M.J.; Wu, P.; Lin, L.M. Recent advances in medicinal and edible homologous plant polysaccharides: Preparation, structure and prevention and treatment of diabetes. Int. J. Biol. Macromol. 2024, 258, 128873. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiao, X.; Ji, T.; Wang, X.; Xu, Y.; Xiao, J.; Cao, H.; Chen, Z.; Liu, H.; Gao, Y.; et al. Reveal the pharmacodynamic substances and mechanism of an edible medicinal plant Rhodiola crenulate in DSS-induced colitis through plasma pharmacochemistry and metabolomics. Food Sci. Hum. Wellness 2024, 13, 2116–2131. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.T.; Ren, G.X.; Hu, Y.C.; Peng, L.X.; Zhao, J.L.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2023, 63, 657–673. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.; Li, X.; Hu, M. Effect of fermentation parameters and their optimization on the phytochemical properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2017, 11, 1462–1473. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, W.W.; Guan, Z.J.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Effect of fermentation methods on the quality and in vitro antioxidant properties of Lycium barbarum and Polygonatum cyrtonema compound wine. Food Chem. 2023, 409, 135277. [Google Scholar] [CrossRef]
- Li, X.; Shao, Y.W.; Hao, L.M.; Kang, Q.Z.; Wang, X.L.; Zhu, J.Q.; Zhao, C.C.; Shi, Y.L.; Lu, J.K.; Yi, J.J. The metabolic profiling of Chinese yam fermented by Saccharomyces boulardii and the biological activities of its ethanol extract in vitro. Food Sci. Hum. Wellness 2024, 13, 2718–2726. [Google Scholar] [CrossRef]
- Qu, Q.; Yang, F.; Zhao, C.; Liu, X.; Yang, P.; Li, Z.; Han, L.; Shi, X. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 2021, 267, 113594. [Google Scholar] [CrossRef]
- You, Y.; Liu, Y.L.; Ai, Z.Y.; Wang, Y.S.; Liu, J.M.; Piao, C.H.; Wang, Y.H. Lactobacillus fermentum KP-3-fermented ginseng ameliorates alcohol-induced liver disease in C57BL/6N mice through the AMPK and MAPK pathways. Food Funct. 2020, 11, 9801–9809. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Q.Q.; Zhao, F.; Jin, W.G.; Shi, T.; Xiong, Z.Y.; Gao, R.C. Dynamic changes and correlation analysis of protease activities, texture and flavour compounds during fish fermentation (suanyu) using mixed culture (Enterococcus rivorum and Enterococcus lactis). Int. J. Food Sci. Technol. 2023, 58, 2312–2324. [Google Scholar] [CrossRef]
- Tang, S.W.; Dong, S.Y.; Chen, M.; Gao, R.C.; Chen, S.J.; Zhao, Y.H.; Liu, Z.Y.; Sun, B.W. Preparation of a fermentation solution of grass fish bones and its calcium bioavailability in rats. Food Funct. 2018, 9, 4135–4142. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C. Ecology of exopolysaccharide formation by lactic acid bacteria: Sucrose utilization, stress tolerance and biofilm formation. Bact. Polysacch. Curr. Innov. Future Trends 2005, 263–278. [Google Scholar]
- Herranz, B.; Ordóñez, J.; De La Hoz, L.; Hierro, E.; Soto, E.; Cambero, M.I. Fatty acid composition of salami from different countries and their nutritional implications. Int. J. Food Sci. Nutr. 2008, 59, 607–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F. Microbes strengthened physicochemical indexes and volatile flavor components of salami, a western fermented sausage. Food Ferment. Ind. 2022, 48, 213–218. [Google Scholar]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B. Bacteriocin production by Lactobacillus curvatus MBSa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Cruz, A.G.; Pereira, E.; Almeida da Costa, W.K.; da Silva Rocha, R.; Targino de Souza Pedrosa, G.; Rocha, C.d.S.; Alves, J.M.; Alvarenga, V.O.; Sant’Ana, A.S.; et al. Postbiotics: An overview of concepts, inactivation technologies, health effects, and driver trends. Trends Food Sci. Technol. 2023, 138, 199–214. [Google Scholar] [CrossRef]
- Boyte, M.-E.; Benkowski, A.; Pane, M.; Shehata, H.R. Probiotic and postbiotic analytical methods: A perspective of available enumeration techniques. Front. Microbiol. 2023, 14, 1304621. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, C.; Ma, H.; Li, S.; Yagoub, A.E.A.; Abdualrahman, M.A.Y. Biological Effect and Inactivation Mechanism of Bacillus subtilis Exposed to Pulsed Magnetic Field: Morphology, Membrane Permeability and Intracellular Contents. Food Biophys. 2016, 11, 429–435. [Google Scholar] [CrossRef]
- Mote, R.D.; Laxmikant, V.S.; Singh, S.B.; Tiwari, M.; Singh, H.; Srivastava, J.; Tripathi, V.; Seshadri, V.; Majumdar, A.; Subramanyam, D. A cost-effective and efficient approach for generating and assembling reagents for conducting real-time PCR. J. Biosci. 2021, 46, 1–10. [Google Scholar] [CrossRef]
- Weidner, C.; Frenzel, J.; Bartsch, D.; Waiblinger, H.U.; Mankertz, J. Guideline for the verification of digital PCR methods in analytical GMO testing. J. Consum. Prot. Food Saf. 2024, 19, 335–337. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Rad, A.H.; Hosseini, S.; Pourjafar, H. Postbiotics as dynamic biological molecules for antimicrobial activity: A mini-review. Biointerface Res. Appl. Chem. 2022, 12, 6543–6556. [Google Scholar]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Müller, W.A.; Ferreira Marczak, L.D.; Sarkis, J.R. Microbial inactivation by ohmic heating: Literature review and influence of different process variables. Trends Food Sci. Technol. 2020, 99, 650–659. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Song, Y.; Zheng, B.; Wen, Z.; Gong, M.; Meng, L. Heat-Killed Lacticaseibacillus paracasei Ameliorated UVB-Induced Oxidative Damage and Photoaging and Its Underlying Mechanisms. Antioxidants 2022, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, G.H.; Hoang, T.H.; Lee, H.Y.; Kang, I.Y.; Chung, M.J.; Jin, J.S.; Chae, H.J. Heat-inactivated Lactobacillus plantarum nF1 promotes intestinal health in Loperamide-induced constipation rats. PLoS ONE 2021, 16, e0250354. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Kong, S.; Chen, N.; Hung, W.-L.; Zhao, W.; Zeng, Z.; Zhang, J.; Yang, Z. Lipoteichoic acid obtained from Lactobacillus paracasei via low-temperature pasteurization alleviates the macrophage inflammatory response by downregulating the NF-κB signaling pathway. J. Funct. Foods 2023, 107, 105673. [Google Scholar] [CrossRef]
- Seong, G.; Lee, S.; Min, Y.W.; Jang, Y.S.; Kim, H.S.; Kim, E.J.; Park, S.Y.; Kim, C.H.; Chang, D.K. Effect of Heat-killed Lactobacillus casei DKGF7 on a Rat Model of Irritable Bowel Syndrome. Nutrients 2021, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Ma, Y.; Yang, Y.; Cheng, Y.; Ma, H.; Ren, D.; Chen, P. Regulation of viable/inactivated/lysed probiotic Lactobacillus plantarum H6 on intestinal microbiota and metabolites in hypercholesterolemic mice. NPJ Sci. Food. 2022, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.H.; Chou, C.H.; Huang, T.Y.; Wang, H.L.; Chien, P.J.; Chang, W.W.; Lee, H.T. Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells 2021, 10, 3264. [Google Scholar] [CrossRef]
- Sugawara, T.; Sawada, D.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 2016, 27, 30259. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microbes 2018, 9, 855–864. [Google Scholar] [CrossRef]
- Blanchet, F.; Rault, L.; Peton, V.; Le Loir, Y.; Blondeau, C.; Lenoir, L.; Dubourdeaux, M.; Even, S. Heat inactivation partially preserved barrier and immunomodulatory effects of Lactobacillus gasseri LA806 in an in vitro model of bovine mastitis. Benef. Microbes 2021, 12, 95–106. [Google Scholar] [CrossRef]
- Shukla, G.; Kamboj, S.; Sharma, B. Comparative Analysis of Antigiardial Potential of Heat Inactivated and Probiotic Protein of Probiotic Lactobacillus rhamnosus GG in Murine Giardiasis. Probiotics Antimicrob. Proteins 2020, 12, 271–279. [Google Scholar] [CrossRef]
- Ben Othman, M.; Sakamoto, K. Effect of inactivated Bifidobacterium longum intake on obese diabetes model mice (TSOD). Food Res. Int. 2020, 129, 108792. [Google Scholar] [CrossRef]
- Barros, C.P.; Grom, L.C.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Almada, C.N.; Pimentel, T.C.; Venâncio, E.L.; Collopy Junior, I.; et al. Paraprobiotic obtained by ohmic heating added in whey-grape juice drink is effective to control postprandial glycemia in healthy adults. Food Res. Int. 2021, 140, 109905. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.Y.; Tan, Y.; Zhang, H. Heat-Killed Bifidobacterium bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation. Nutrients 2022, 14, 5233. [Google Scholar] [CrossRef]
- Stănciuc, N.; Borda, D.; Gurgu-Grigore, L.; Cotârleț, M.; Vasile, A.M.; Nistor, O.V.; Dumitrașcu, L.; Pihurov, M.; Păcularu-Burada, B.; Bahrim, G.E. Lactiplantibacillus plantarum MIUG BL21 paraprobiotics: Evidences on inactivation kinetics and their potential as cytocompatible and antitumor alternatives. Food Chem. X 2024, 21, 101114. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes. Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-C.; OuYang, C.-N.; Yuan, S.-N.; Lin, H.-C.; Huang, K.-Y.; Wu, P.-S.; Liu, C.-Y.; Tsai, K.-J.; Loi, L.-K.; Chen, Y.-J. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Evelyn; Silva, F.V.M. High pressure thermal processing for the inactivation of Clostridium perfringens spores in beef slurry. Innov. Food Sci. Emerg. Technol. 2016, 33, 26–31. [Google Scholar] [CrossRef]
- Karbowiak, M.; Galek, M.; Szydlowska, A.; Zielinska, D. The Influence of the Degree of Thermal Inactivation of Probiotic Lactic Acid Bacteria and Their Postbiotics on Aggregation and Adhesion Inhibition of Selected Pathogens. Pathogens 2022, 11, 1260. [Google Scholar] [CrossRef]
- Ashrafian, F.; Raftar, S.K.A.; Lari, A.; Shahryari, A.; Abdollahiyan, S.; Moradi, H.R.; Masoumi, M.; Davari, M.; Khatami, S.; Omrani, M.D.; et al. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb. Cell Factories 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Peng, L.Y.; Yin, Q.L.; Wang, X.W.; Zhong, Y.W.; Wang, Y.; Cai, W.T.; Zhou, R.S.; Chen, Y.; Hu, Y.; Cheng, Z.X.; et al. Pasteurized Akkermansia muciniphila Ameliorates Preeclampsia in Mice by Enhancing Gut Barrier Integrity, Improving Endothelial Function, and Modulating Gut Metabolic Dysregulation. Microorganisms 2024, 12, 131. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Kwok, L.-Y.; Wu, X.; Liu, H.; Shen, X.; Zhao, F.; Qi, H.; Ma, H.; Sun, Z. Improvement of sleep quality and sub-health conditions through pasteurized fermented milk consumption: A human intervention study. J. Funct. Foods 2024, 122, 106562. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Teixeira, S.; Bertolo, M.R.V.; Ranadheera, C.S.; Raices, R.S.L.; Russo, P.; Spano, G.; Bogusz, S., Jr.; Sant’Ana, A.S. Functional benefits of probiotic fermented dairy drink elaborated with sheep milk processed by ohmic heating. Food Biosci. 2024, 59, 110061. [Google Scholar] [CrossRef]
- Barros, C.P.; Pires, R.P.; Guimarães, J.T.; Abud, Y.K.; Almada, C.N.; Pimentel, T.C.; Sant’Anna, C.; De-Melo, L.D.B.; Duarte, M.C.K.; Silva, M.C. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Res. Int. 2021, 140, 110061. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef] [PubMed]
- Lado, B.H.; Yousef, A.E. Alternative food-preservation technologies: Efficacy and mechanisms. Microbes Infect. 2002, 4, 433–440. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, Y.; Yang, G.; Cui, L.; Wu, Z.; Zeng, X.; Pan, D.; Cai, Z. Structure and Anti-Inflammation Potential of Lipoteichoic Acids Isolated from Lactobacillus Strains. Foods 2022, 11, 1610. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Érix, C.N.; Costa, W.K.A.; Graça, J.S.; Cabral, L.; Noronha, M.F.; Gonçalves, A.; Santos, A.D.; Lollo, P.C.; Magnani, M.; et al. Wheat-durum pasta added of inactivated Bifidobacterium animalis decreases glucose and total cholesterol levels and modulates gut microbiota in healthy rats. Int. J. Food Sci. Nutr. 2021, 72, 781–793. [Google Scholar] [CrossRef]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef]
- Brandão, L.R.; de Brito Alves, J.L.; da Costa, W.K.A.; Ferreira, G.A.H.; de Oliveira, M.P.; Gomes da Cruz, A.; Braga, V.A.; Aquino, J.S.; Vidal, H.; Noronha, M.F.; et al. Live and ultrasound-inactivated Lacticaseibacillus casei modulate the intestinal microbiota and improve biochemical and cardiovascular parameters in male rats fed a high-fat diet. Food Funct. 2021, 12, 5287–5300. [Google Scholar] [CrossRef] [PubMed]
- Almada, C.N.; Almada-Érix, C.N.; Roquetto, A.R.; Santos-Junior, V.A.; Cabral, L.; Noronha, M.F.; Gonçalves, A.; Santos, P.D.; Santos, A.D.; Martinez, J.; et al. Paraprobiotics obtained by six different inactivation processes: Impacts on the biochemical parameters and intestinal microbiota of Wistar male rats. Int. J. Food Sci. Nutr. 2021, 72, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Ezzatpanah, H.; Gomez-Lopez, V.M.; Koutchma, T.; Lavafpour, F.; Moerman, F.; Mohammadi, M.; Raheem, D. New food safety challenges of viral contamination from a global perspective: Conventional, emerging, and novel methods of viral control. Compr. Rev. Food Sci. Food Saf. 2022, 21, 904–941. [Google Scholar] [CrossRef]
- Hinds, L.M.; O’Donnell, C.P.; Akhter, M.; Tiwari, B.K. Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innov. Food Sci. Emerg. Technol. 2019, 56, 102153. [Google Scholar] [CrossRef]
- Vallejo-Cordoba, B.; Castro-Lopez, C.; Garcia, H.S.; Gonzalez-Cordova, A.F.; Hernandez-Mendoza, A. Postbiotics and paraprobiotics: A review of current evidence and emerging trends. Adv. Food Nutr. Res. 2020, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure-based technologies in the food industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, Z.; Wu, D.; Fei, X.; Ei-Seedi, H.R.; Wang, C. High-pressure homogenization influences the functional properties of protein from oyster (Crassostrea gigas). LWT-Food Sci. Technol. 2021, 151, 112107. [Google Scholar] [CrossRef]
- Yan, H.; Cui, Z.; Manoli, T.; Zhang, H. Recent advances in non-thermal disinfection technologies in the food industry. Food Sci. Technol. Res. 2021, 27, 695–710. [Google Scholar] [CrossRef]
- Gibson, J.H.; Yong, D.H.N.; Farnood, R.R.; Seto, P. A Literature Review of Ultrasound Technology and Its Application in Wastewater Disinfection. Water Qual. Res. J. Can. 2008, 43, 23–35. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567. [Google Scholar] [CrossRef]
- Leong, S.Y.; Duque, S.M.M.; Conde, L.A.; Khrisanapant, P.; Oey, I. Addressing the opportunities of non-thermal food processing technologies in the ASEAN region context. Int. J. Food Sci. Technol. 2024, 59, 7739–7753. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, N.A.; Yagoub, A.A.; Chen, L.; Mustapha, A.T.; Yu, X.J.; Zhou, C.S. Ultrasound-assisted probiotics fermentation suspension treatment under mild heat to improve the storage quality of freshly cut lotus root. Food Chem. 2022, 397, 133823. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xie, B.J.; Sun, Z.D. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage. LWT-Food Sci. Technol. 2021, 137, 110438. [Google Scholar] [CrossRef]
- Li, J.H.; Shi, J.Y.; Huang, X.W.; Zou, X.B.; Li, Z.H.; Zhang, D.; Zhang, W.; Xu, Y.W. Effects of pulsed electric field on freeze-thaw quality of Atlantic salmon. Innov. Food Sci. Emerg. Technol. 2020, 65, 102454. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.-W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Lytras, F.; Psakis, G.; Gatt, R.; Hummerjohann, J.; Raso, J.; Valdramidis, V. Evaluation of strain variability of food microorganisms in response to decontamination by pulsed electric fields and thermal treatments. Innov. Food Sci. Emerg. Technol. 2024, 95, 103731. [Google Scholar] [CrossRef]
- Djukic-Vukovic, A.; Meglic, S.H.; Flisar, K.; Mojovic, L.; Miklavcic, D. Pulsed electric field treatment of Lacticaseibacillus rhamnosus and Lacticaseibacillus paracasei, bacteria with probiotic potential. LWT-Food Sci. Technol. 2021, 152, 103691. [Google Scholar] [CrossRef]
- Thamsuaidee, A.; Schaefer, E.; Schneider, D.; Siemer, C.; Valdramidis, V.P. Disentangling the effects of electroporation and heat during pulsed electric field (PEF) processing of oat-based milk alternative: A case study on Lactiplantibacillus plantarum inactivation. Innov. Food Sci. Emerg. Technol. 2024, 94, 103691. [Google Scholar] [CrossRef]
- Silva, E.K.; Alvarenga, V.O.; Bargas, M.A.; Sant’Ana, A.S.; Meireles, M.A.A. Non-thermal microbial inactivation by using supercritical carbon dioxide: Synergic effect of process parameters. J. Supercrit. Fluids 2018, 139, 97–104. [Google Scholar] [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Cappato, L.P.; Guimaraes, J.T.; Alvarenga, V.O.; Esmerino, E.A.; Portela, J.B.; Sant’Ana, A.S.; Freitas, M.Q.; et al. Dairy processing using supercritical carbon dioxide technology: Theoretical fundamentals, quality and safety aspects. Trends Food Sci. Technol. 2017, 64, 94–101. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Erix, C.N.; Bonatto, M.S.; Pradella, F.; dos Santos, P.; Abud, Y.K.D.; Farias, A.S.; Martinez, J.; Sant’Anna Filho, C.B.; Lollo, P.C.; et al. Obtaining paraprobiotics from Lactobacilus acidophilus, Lacticaseibacillus casei and Bifidobacterium animalis using six inactivation methods: Impacts on the cultivability, integrity, physiology, and morphology. J. Funct. Foods 2021, 87, 104826. [Google Scholar] [CrossRef]
- Thye, A.Y.K.; Law, J.W.F.; Tan, L.T.H.; Thurairajasingam, S.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647. [Google Scholar] [CrossRef]
- Ang, W.S.; Law, J.W.F.; Letchumanan, V.; Hong, K.W.; Wong, S.H.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H.; Tan, L.T.H. A Keystone Gut Bacterium Christensenella minuta-A Potential Biotherapeutic Agent for Obesity and Associated Metabolic Diseases. Foods 2023, 12, 2485. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Miyazawa, K.; Kubota, A.; Yoda, K.; Hiramatsu, M. Orally administered heat-killed Lactobacillus gasseri TMC0356 can upregulate cell-mediated immunity in senescence-accelerated mice. FEMS Microbiol. Lett. 2012, 326, 125–130. [Google Scholar] [CrossRef]
- Mehta, J.P.; Ayakar, S.; Singhal, R.S. The potential of paraprobiotics and postbiotics to modulate the immune system: A Review. Microbiol. Res. 2023, 275, 127449. [Google Scholar] [CrossRef] [PubMed]
- Luongo, D.; De Sena, V.; Maurano, F.; Rossi, M. Modulation of Mouse Dendritic Cells In Vitro by Lactobacillus gasseri Postbiotic Proteins. Probiotics Antimicrob. Proteins 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.D.; Leoni, J.; Paz, M.L.; Gonzalez Maglio, D.H. Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients 2022, 14, 723. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, L.; Zhang, Y.; Li, H.; Jiao, Y. Whole Peptidoglycan Extracts from the Lactobacillus paracasei subsp. paracasei M5 Strain Exert Anticancer Activity In Vitro. Biomed. Res. Int. 2018, 2018, 2871710. [Google Scholar] [CrossRef]
- Miyazawa, K.; He, F.; Kawase, M.; Kubota, A.; Yoda, K.; Hiramatsu, M. Enhancement of immunoregulatory effects of Lactobacillus gasseri TMC0356 by heat treatment and culture medium. Lett. Appl. Microbiol. 2011, 53, 210–216. [Google Scholar] [CrossRef]
- Eyal, R.; Daniel, R. Inactivated probiotic bacteria and methods of use thereof. US2005180962, 18 August 2005. [Google Scholar]
- Camba-Gómez, M.; Gualillo, O.; Conde-Aranda, J. New Perspectives in the Study of Intestinal Inflammation: Focus on the Resolution of Inflammation. Int. J. Mol. Sci. 2021, 22, 2605. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics Regulating Inflammation via NLRP3 Inflammasome Modulation: A Potential Therapeutic Approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D.M. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Gao, Y.A.; Davis, B.; Zhu, W.S.; Zheng, N.; Meng, D.; Walker, W.A. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G521–G530. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.-d.; Guo, Y.; Zeng, X. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef]
- Lee, G.A.; Chang, Y.W.; Lin, W.L.; Yang, Y.S.H.; Chen, W.J.; Huang, F.H.; Liu, Y.R. Modulatory Effects of Heat-Inactivated Streptococcus Thermophilus Strain 7 on the Inflammatory Response: A Study on an Animal Model with TLR3-Induced Intestinal Injury. Microorganisms 2023, 11, 278. [Google Scholar] [CrossRef]
- Jhong, J.H.; Tsai, W.H.; Yang, L.C.; Chou, C.H.; Lee, T.Y.; Yeh, Y.T.; Huang, C.H.; Luo, Y.H. Heat-Killed Lacticaseibacillus paracasei GMNL-653 Exerts Antiosteoporotic Effects by Restoring the Gut Microbiota Dysbiosis in Ovariectomized Mice. Front. Nutr. 2022, 9, 804210. [Google Scholar] [CrossRef]
- Chen, Q.; Fang, Z.Z.; Yang, Z.; Xv, X.; Yang, M.T.; Hou, H.J.; Li, Z.Z.; Chen, Y.Y.; Gong, A.H. Lactobacillus plantarum-Derived Extracellular Vesicles Modulate Macrophage Polarization and Gut Homeostasis for Alleviating Ulcerative Colitis. J. Agric. Food Chem. 2024, 72, 14713–14726. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. ESPEN 2019, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative Studies of Inhibitory and Antioxidant Activities, and Organic Acids Compositions of Postbiotics Produced by Probiotic Lactiplantibacillus plantarum Strains Isolated From Malaysian Foods. Front. Vet. Sci. 2020, 7, 602280. [Google Scholar] [CrossRef]
- Guerrero-Encinas, I.; González-González, J.N.; Santiago-López, L.; Muhlia-Almazán, A.; Garcia, H.S.; Mazorra-Manzano, M.A.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B(1)-Induced Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 1033–1043. [Google Scholar] [CrossRef]
- Dong, H.; Ren, X.; Song, Y.; Zhang, J.; Zhuang, H.; Peng, C.; Zhao, J.; Shen, J.; Yang, J.; Zang, J.; et al. Assessment of Multifunctional Activity of a Postbiotic Preparation Derived from Lacticaseibacillus paracasei Postbiotic-P6. Foods 2024, 13, 2326. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.Y.; Li, C.Z.; Al-Asmari, F.; Cui, H.Y.; Lin, L. Potential Application of Lactiplantibacillus plantarum in Food Bio-preservation-A Comprehensive Review with a Focus on the Antibacterial and Anti-Virulence Effects on Foodborne Pathogens. Food Rev. Int. 2024, 40, 2993–3019. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Huang, W.Y.; Toh, H.S.; Weng, T.C.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial Activity of Lactobacillus Species Against Carbapenem-Resistant Enterobacteriaceae. Front. Microbiol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Kutsukake, E.; Fukui, T.; Sato, I.; Shirai, T.; Kurihara, T.; Okada, N.; Danbara, H.; Toba, M.; Kohda, N.; et al. Oral administration of heat-killed Lactobacillus plantarum strain b240 protected mice against Salmonella enterica Serovar Typhimurium. Biosci. Biotechnol. Biochem. 2010, 74, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kuda, T.; An, C.; Kanno, T.; Takahashi, H.; Kimura, B. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 2012, 18, 19–24. [Google Scholar] [CrossRef]
- Huang, W.; Xu, H.; Pan, J.; Dai, C.; Mintah, B.K.; Dabbour, M.; Zhou, R.; He, R.; Ma, H. Mixed-Strain Fermentation Conditions Screening of Polypeptides from Rapeseed Meal and the Microbial Diversity Analysis by High-Throughput Sequencing. Foods 2022, 11, 3285. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Drago, L.; Ullah, H.; Di Minno, A.; Brindisi, G.; Brunese, F.P.; Dinardo, G.; Gori, A.; Indolfi, C.; Naso, M.; et al. Effects of the supplementation of single and multi-strain probiotics, alone or in combination with other treatments, on asthma in children: A systematic review of the randomized, placebo-controlled clinical studies. J. Funct. Foods 2024, 123, 106599. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes. Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Mizumachi, K.; Okamoto, T.; Sasaki, K.; Kurisaki, J. Influence of long-term consumption of a Lactococcus lactis strain on the intestinal immunity and intestinal flora of the senescence-accelerated mouse. Br. J. Nutr. 2009, 102, 181–185. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, X.; Zeng, X.; Guo, Y.; Zhang, T.; Fan, X.; Xu, J.; Wu, Z.; Pan, D. A protective mechanism of heat inactivation to enhance Levilactobacillus brevis PDD-2 against alcohol-induced chronic liver disease based on proteomic analysis. Food Funct. 2024, 15, 8356–8369. [Google Scholar] [CrossRef]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M.; Shang, A.; Mao, Q.Q.; Li, H.B. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods 2019, 8, 440. [Google Scholar] [CrossRef]
- Apalowo, O.E.; Adegoye, G.A.; Obuotor, T.M. Microbial-Based Bioactive Compounds to Alleviate Inflammation in Obesity. Curr. Issues Mol. Biol. 2024, 46, 1810–1831. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Song, Y.; Zhou, J.; Duan, Y.; Kong, T.; Ma, H.; Zhang, H. Recent progress of Lycium barbarum polysaccharides on intestinal microbiota, microbial metabolites and health: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2917–2940. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Yun, J.; Zhang, G.; Zhang, Y.; Zhao, M.; Zabed, H.M.; Ravikumar, Y.; Qi, X. d-Arabitol Ameliorates Obesity and Metabolic Disorders via the Gut Microbiota-SCFAs-WAT Browning Axis. J. Agric. Food Chem. 2023, 71, 522–534. [Google Scholar] [CrossRef]
- Miao, C.; Wang, L.E.; Wang, H.B.; Shen, Y.; Man, C.X.; Zhang, W.; Zhang, Y.; Zhao, Q.Y.; Jiang, Y.J. Lacticaseibacillus plantarum postbiotics prepared by the combined technique of pasteurization and ultrasound: Effective measures to alleviate obesity based on the SCFAs-GPR41/GPR43 signaling pathway. Food Funct. 2024, 15, 11005–11019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, M.; Zhao, Y.; Zhu, Y.; Cui, S.; Xiao, X. Effects of Lactiplantibacillus plantarum dy-1 fermentation on multi-scale structure and physicochemical properties of barley starch. Food Funct. 2024, 15, 1923–1937. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, R.; Zhang, Q.; Tian, M.; Ren, X.; Wang, L.; Wang, X. Antifungal Activity of Cell-Free Supernatants from Lactobacillus pentosus 86 against Alternaria gaisen. Horticulturae 2023, 9, 911. [Google Scholar] [CrossRef]
- Molaee Parvarei, M.; Fazeli, M.R.; Mortazavian, A.M.; Sarem Nezhad, S.; Mortazavi, S.A.; Golabchifar, A.A.; Khorshidian, N. Comparative effects of probiotic and paraprobiotic addition on microbiological, biochemical and physical properties of yogurt. Food Res. Int. 2021, 140, 110030. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 2016, 79, 33–39. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Hamed, F.; Shaker, R. Rheological, textural, microstructural and sensory impact of exopolysaccharide-producing Lactobacillus plantarum isolated from camel milk on low-fat akawi cheese. Lwt-Food Sci. Technol. 2018, 87, 423–431. [Google Scholar] [CrossRef]
- Gezginc, Y.; Kara, U. The effect of exopolysaccharide producing Lactobacillus plantarum strain addition on sourdough and wheat bread quality. Qual. Assur. Saf. Crops Foods 2019, 11, 95–106. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.C.; Zheng, X.Y.; Zhang, Y.; Deng, K.W.; Wu, T.; Cheng, H. Analysis of physicochemical properties of exopolysaccharide from Leuconostoc mesenteroides strain XR1 and its application in fermented milk. LWT-Food Sci. Technol. 2021, 146, 111449. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, L.; Xiao, L.; Ye, Y.; Mo, W.; Zheng, Z.; Li, X. Monascus-fermented quinoa alleviates hyperlipidemia in mice by regulating the amino acid metabolism pathway. Food Funct. 2024, 15, 9210–9223. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Xie, C.; Li, L.; Lin, L.; Li, Q.; Li, L.; Chen, F.; Yang, X.; Yang, J.; et al. Fermented Gastrodia elata Bl. Alleviates Cognitive Deficits by Regulating Neurotransmitters and Gut Microbiota in D-Gal/AlCl3-Induced Alzheimer’s Disease-like Mice. Foods 2024, 13, 120315. [Google Scholar] [CrossRef]
- Wu, Y.F.; Xiao, Y.; Okoye, C.O.; Gao, L.; Chen, X.F.; Wang, Y.L.; Jiang, J.X. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid bacteria: A promising feed additive for sustainable agriculture. Ind. Crops Prod. 2025, 224, 585623. [Google Scholar] [CrossRef]
- Zhu, C.; Gong, L.; Huang, K.; Li, F.; Tong, D.; Zhang, H. Effect of Heat-Inactivated Compound Probiotics on Growth Performance, Plasma Biochemical Indices, and Cecal Microbiome in Yellow-Feathered Broilers. Front. Microbiol. 2020, 11, 585623. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Zulkifli, I.; Samsudin, A.A.; Mustapha, N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021, 100, 100908. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Humam, A.M. Postbiotic L. plantarum RG14 improves ruminal epithelium growth, immune status and upregulates the intestinal barrier function in post-weaning lambs. Sci. Rep. 2019, 9, 9938. [Google Scholar] [CrossRef]
- Li, J.Y.; Bai, J.; Yuan, J.; Fan, S.T.; Zhang, T.; Pan, T.; Zhao, Y.S.; Zhang, J.Y.; Xiao, X. Heterologous expression and characterization of an endoglucanase from Lactobacillus plantarum dy-1. Food Funct. 2023, 14, 3760–3768. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Dong, Y.; Xu, T.; Wu, F. Dietary supplementation with Lactobacillus plantarum dy-1 fermented barley suppresses body weight gain in high-fat diet-induced obese rats. J. Sci. Food Agric. 2016, 96, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- Mwakosya, A.W.; Limbu, S.M.; Majaliwa, N.; Zou, X.B.; Shi, J.Y.; Kibazohi, O. Aflatoxin B1 variations in animal feeds along the supply chain in Tanzania and its possible reduction by heat treatment. Food Agric. Immunol. 2022, 33, 192–206. [Google Scholar] [CrossRef]
- Boermans, H.J.; Leung, M.C. Mycotoxins and the pet food industry: Toxicological evidence and risk assessment. Int. J. Food Microbiol. 2007, 119, 95–102. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit. Rev. Food Sci. Nutr. 2014, 54, 64–83. [Google Scholar] [CrossRef]
- Moretti, A.F.; Pelaez, A.L.; Golowczyc, M.A. Protection capacity of Lactiplantibacillus plantarum 83114 against contamination of dry pet food with Aspergillus flavus. Anim. Feed. Sci. Technol. 2024, 316, 116085. [Google Scholar] [CrossRef]
- Koziol, S.A.; Oba, P.M.; Soto-Diaz, K.; Steelman, A.J.; Suchodolski, J.S.; Eckhardt, E.; Swanson, K.S. Effects of a Lactobacillus Fermentation Product on the Fecal Characteristics, Fecal Microbial Populations, Immune Function, and Stress Markers of Adult Dogs. J. Anim. Sci. 2023, 101, 102–103. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E. Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef]
- Wambacq, W.A.; Apper, E.; Le Bourgot, C.; Barbe, F.; Lyu, Y.; Pelst, M.; Broeckx, B.J.G.; Devriendt, B.; Cox, E.; Hesta, M. A new combination of a prebiotic and postbiotic mitigates immunosenescence in vaccinated healthy senior dogs. Front. Vet. Sci. 2024, 11, 1392985. [Google Scholar] [CrossRef]
- Wilson, S.M.; Oba, P.M.; Applegate, C.C.; Koziol, S.A.; Panasevich, M.R.; Norton, S.A.; Swanson, K.S. Effects of a Saccharomyces cerevisiae fermentation product-supplemented diet on fecal characteristics, oxidative stress, and blood gene expression of adult dogs undergoing transport stress. J. Anim. Sci. 2023, 101, skac378. [Google Scholar] [CrossRef]
| Source | Component | Specific Component | Function | Reference |
|---|---|---|---|---|
| Cellular components | Teichoic acid (TA) | Lipoteichoic acid (LTA) | Regulated inflammatory factors TNF-α and IL-10 levels. | [32] |
| Wall teichoic acid (WTA) | Stimulated macrophages and dendritic cells to secrete IL-12, modulating immune responses. | [33] | ||
| Peptidoglycan (PGN) | Cell wall components | Intervention regulated the functions of T lymphocytes and dendritic cells, alleviating Salmonella-induced inflammation in mice. | [34] | |
| Surface-layer proteins (SLPs) | Cell surface protein | Reduced adhesion of E. coli and Salmonella to HT-29 cells and inhibited pathogen-induced apoptosis. | [35] | |
| Metabolites | Extracellular polysaccharide (EPS) | Probiotic-secreted polysaccharides | Decreased NO production and reduced expression of the pro-inflammatory cytokine IL-6. | [36] |
| Short-chain fatty acids (SCFAs) | Propionate and butyrate | Inhibited the production of inflammatory factors such as IL-6 and IL-4, reduced reactive oxygen species (ROS) expression, and enhanced IL-10 and IFN-γ expression, alleviated cellular inflammation. | [37] | |
| Organic acids | Lactic acid and acetic acid | Reduced HFD-induced inflammation and improved insulin sensitivity in diabetic mice. | [38] | |
| Enzymes | Digestive enzymes, synthetases, and transferases | The fermentation of soybean residue by Bacillus natto can produce nattokinase and α-amylase, which have the effects of lowering blood sugar and blood pressure. | [39] | |
| Vitamin | B vitamin | Under the condition of pH 2–4, the mycelia growth of Streptospora Geisenensis was inhibited, while antifungal activity was maintained up to 80%. | [40] | |
| Bacteriocins | Ribosomally synthesized antimicrobial peptides | Involved in cell signaling, possesses antimicrobial activity, and inhibits pathogen growth. | [41] | |
| Other bioactive substances | Bacterial lysates | Bioactive factors released by bacterial cell lysis | Reduced periodontitis and dental caries by modulating signaling pathways, which can help improving oral health. | [42] |
| Strain | Inactivation Method | Conditions | Target | Function | Reference |
|---|---|---|---|---|---|
| Lactobacillus paracasei | Thermal | 80 °C/20 min | UVB-induced skin cells (NHDF and B16F10) | Reduced DNA damage in NHDF and B16F10 cells, increased GSH content, antioxidant enzyme activity, and mRNA levels to alleviate UVB-induced oxidative damage, reduced UVB-induced photoaging in NHDF cells. | [109] |
| Lactiplantibacillus plantarum | Thermal | 100 °C/20 min | Mouse macrophages | Complete cell wall of L. plantarum induced IL-12 secretion via actin-dependent phagocytosis, modulating immune responses. | [33] |
| Lactobacillus plantarum | Thermal | 80 °C/10 min | Loperamide-induced constipated rats | Improved fecal pellet count, weight, water content, and intestinal contractility, increased mucosal layer thickness and goblet cell count, downregulated inflammatory cytokine levels. | [110] |
| Lactobacillus paracasei 6-1 | Low temperature | 65 °C/30 min | RAW 264.7 macrophages | Downregulated pro-inflammatory cytokines (TNF-α, IL-11, IL-6, IL-12), upregulated anti-inflammatory cytokine IL-10, repaired oxidative damage in colitis. | [111] |
| Lactobacillus casei DKGF7 | Thermal | 121 °C/15 min | IBS model rats | Reduced serum corticosterone levels, lowers colonic inflammatory cytokines, and increased tight junction protein expression. | [112] |
| Lactobacillus plantarum H-6 | Thermal | 90 °C water bath/30 min | Hypercholesterolemic mice | Reduced serum and liver lipid levels, improved glucose tolerance and insulin sensitivity, modulated gut microbiota. | [113] |
| Lactobacillus plantarum GMNL-6 and Lactobacillus paracasei GMNL-653 | Thermal | 121 °C/30 min | Tail-injured mice | Promoted wound healing, reduced fibrosis, and exhibited anti-fibrotic effects. | [114] |
| Lactobacillus gasseri CP2305 | Pasteurization | Below 90 °C | Male/female volunteers aged 20–70 | Increased defecation frequency was observed, accompanied by elevated abundance of beneficial gut microbiota and enhanced total power of autonomic nervous activity. | [115] |
| Lactobacillus paracasei MCC1849 | Thermal | Pasteurization in hot water | 241 adult healthy volunteers | The incidence of common colds, total symptom days, and symptom scores showed significant improvement, while stress-induced emotional deterioration was less pronounced. | [116] |
| Lactobacillus gasseri LA806 | Thermal | 70 °C/10 min | Bovine mammary epithelial Cells (bMEC) | Downregulated pro-inflammatory cytokine expression and demonstrated barrier-enhancing and immunomodulatory properties that prevented Staphylococcus aureus colonization in bovine mammary glands. | [117] |
| Lactobacillus rhamnosus GG MTCC 1048 | Thermal | 80 °C/20 min (Water bath) | LACA mice | Reduced the severity and duration of infection with Giardiasis, while elevating anti-Giardia IgA antibodies and nitric oxide levels in serum and intestinal fluid. | [118] |
| Bifidobacterium longum BR-108 | High pressure and thermal | 105 °C/20 min | Male obese TSOD mice | Significantly reduced body weight and blood sugar levels, while lowering levels of cholesterol, triglycerides, and NEFA. Serum and urine creatinine levels also decreased. | [119] |
| Lacticaseibacillus casei 01 | Ohmic Heating | 8 V/cm 95 °C/7 min, 60 Hz | 15 healthy subjects | Inhibited α-glucosidase and α-amylase activity, reduced blood glucose response. | [120] |
| Bifidobacterium bifidum B1628 | Thermal | 90 °C/15 min | Colitis induced by DSS in mice | Alleviated inflammatory severity and tissue damage while improving DSS-induced gut dysbiosis and remodeling intestinal microbiota composition. | [121] |
| Strain | Inactivation Method | Conditions | Target | Function | Reference |
|---|---|---|---|---|---|
| Lactobacillus plantarum A3, Lactobacillus reuteri DMSZ 8533, and Lactobacillus acidophilus CICC 6074 | Ultrasonic | Ice water bath ultrasonic for 30 min | LPS-induced RAW 264.7 macrophages and Caco-2 cells | LTA from the three strains significantly reduced inflammation, decreased TNF-α, IL-6, and IL-10 levels; LTA from L. reuteri DMSZ 8533 blocked LPS-triggered MAPK and NF-κB pathway expression. | [135] |
| Bifidobacterium animalis Bb-12 | Gamma irradiation | The cobalt-60 multifunctional irradiator is irradiated at 2.5 KGy | Male Wistar rats | Serum glucose and total cholesterol levels were reduced. The abundances of Firmicutes and Actinomycetes increased, while the abundances of Bacteroids decreased. | [136] |
| Lactobacillus plantarum L12, Lactobacillus reuteri DSM 20016, Bifidobacterium longum Bb46, Bifidobacterium infantis Bb02 | Ultrasonic | 130 W, 20 kHz, net power: 40, 60, and 80%, duration: 2, 4, and 6 min, pulse set to 2 s | Caco-2 cell | The adhesion of Lactobacillus reuteri DSM 20016 to Caco-2 cells was enhanced. | [137] |
| Lacticaseibacillus casei 01 | Ultrasonic | 20 kHz, 40 min, 792 W | Male obese rat | Lower cholesterol levels and control insulin resistance in obese rats. Increased beneficial bacteria and reduced harmful bacteria in the gut. | [138] |
| Bifidobacterium animalis subsp. lactis (B. lactis) | Supercritical carbon dioxide | 10 MPa, 40 °C, 180 min | Wistar male rat | Decreased serum total cholesterol level. Increased serum albumin and creatinine levels and decrease HDL-cholesterol levels. | [139] |
| Lacticaseibacillus casei subsp. paracasei 1 (L. casei) | Irradiation | Cobalt-60 multifunctional irradiator, dose: 2.5 KGy, 60 min | Wistar male rat | Decreased serum total cholesterol level. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Xiao, M.; Kang, T.; He, Y.; Zhang, J.; Zhao, Y.; Xiao, X. The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review. Foods 2025, 14, 2358. https://doi.org/10.3390/foods14132358
Zhu Y, Xiao M, Kang T, He Y, Zhang J, Zhao Y, Xiao X. The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review. Foods. 2025; 14(13):2358. https://doi.org/10.3390/foods14132358
Chicago/Turabian StyleZhu, Ying, Meiling Xiao, Tangying Kang, Yufeng He, Jiayan Zhang, Yansheng Zhao, and Xiang Xiao. 2025. "The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review" Foods 14, no. 13: 2358. https://doi.org/10.3390/foods14132358
APA StyleZhu, Y., Xiao, M., Kang, T., He, Y., Zhang, J., Zhao, Y., & Xiao, X. (2025). The Role of Inactivation Methods in Shaping Postbiotic Composition and Modulating Bioactivity: A Review. Foods, 14(13), 2358. https://doi.org/10.3390/foods14132358








