Limosilactobacillus fermentum MG4244 Protects Against Metabolic and Inflammatory Stress in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Worm Cultivation
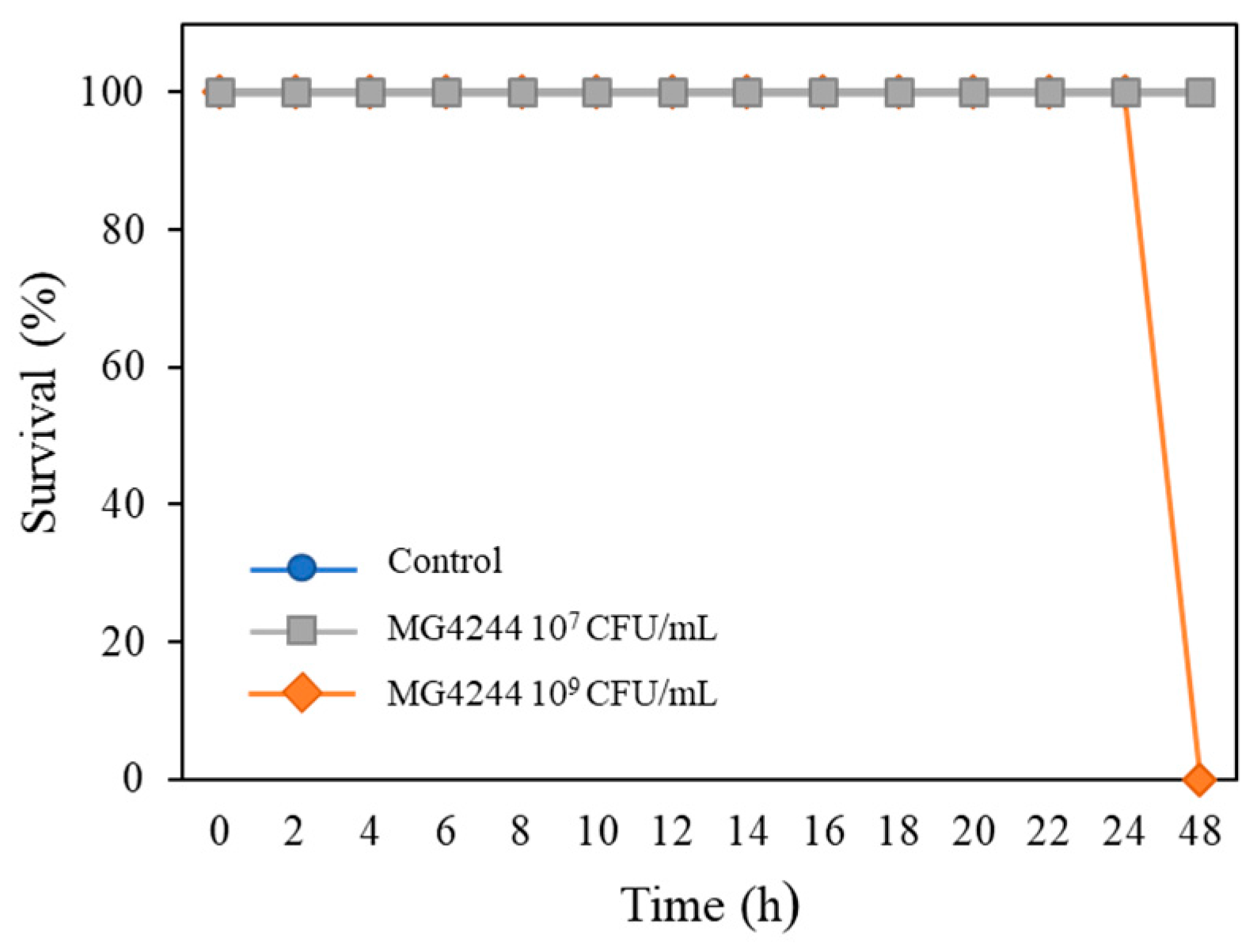
2.3. Acute Toxicity
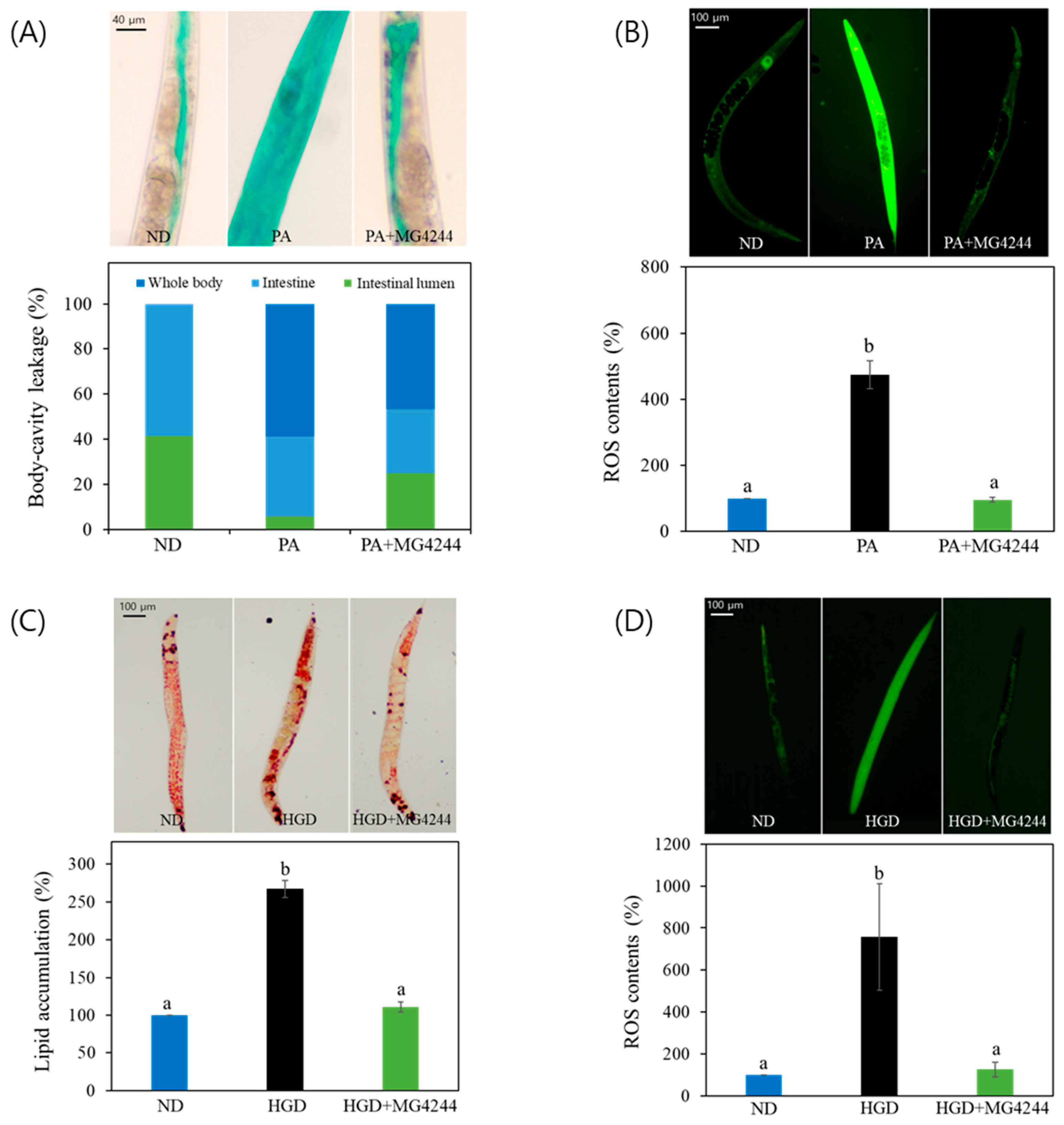
2.4. Measurement of Intestinal Permeability
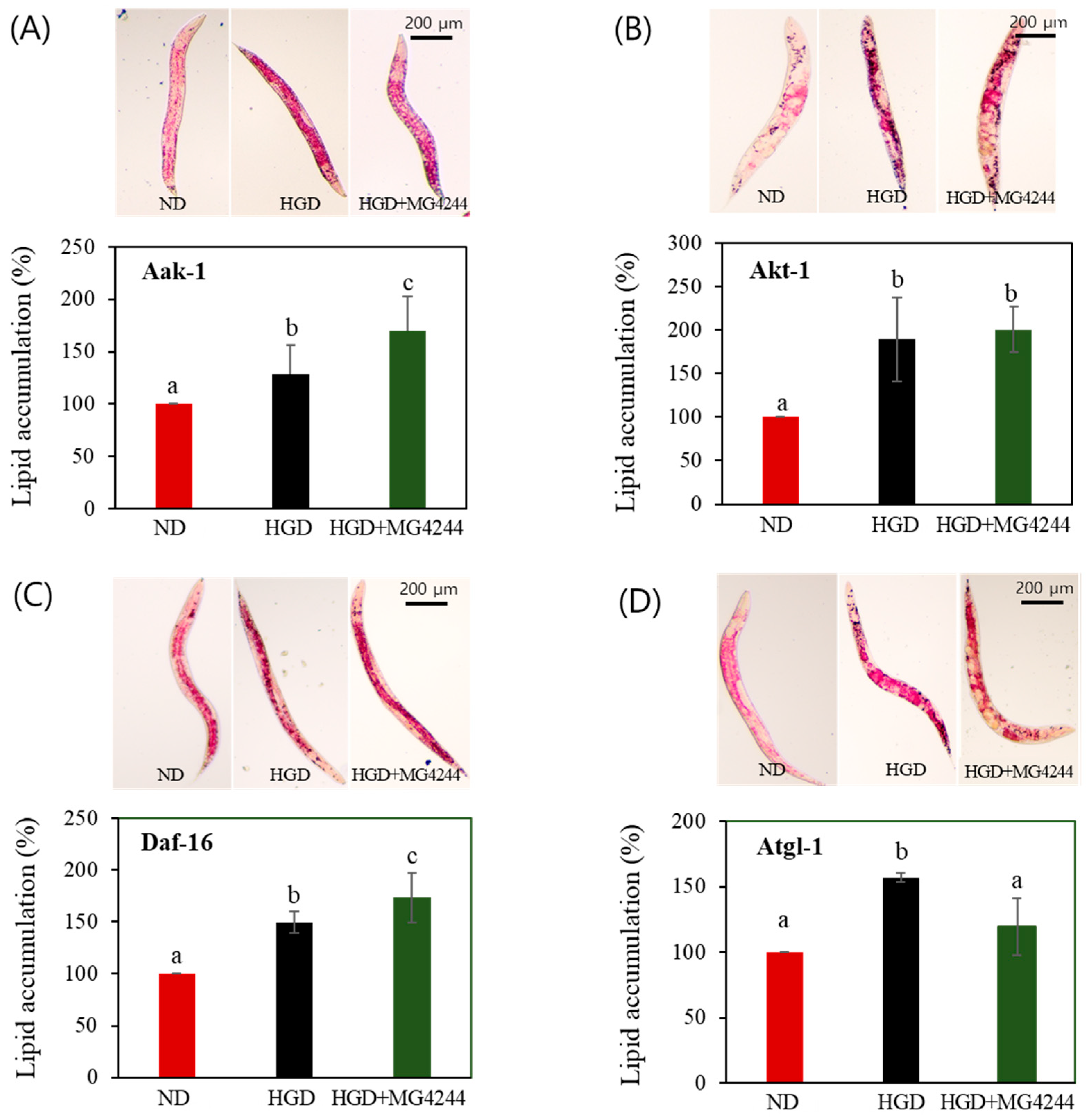
2.5. Oil Red O Staining
2.6. Measurement of Reactive Oxygen Species (ROS) Levels
2.7. Determination of Stress Resistance
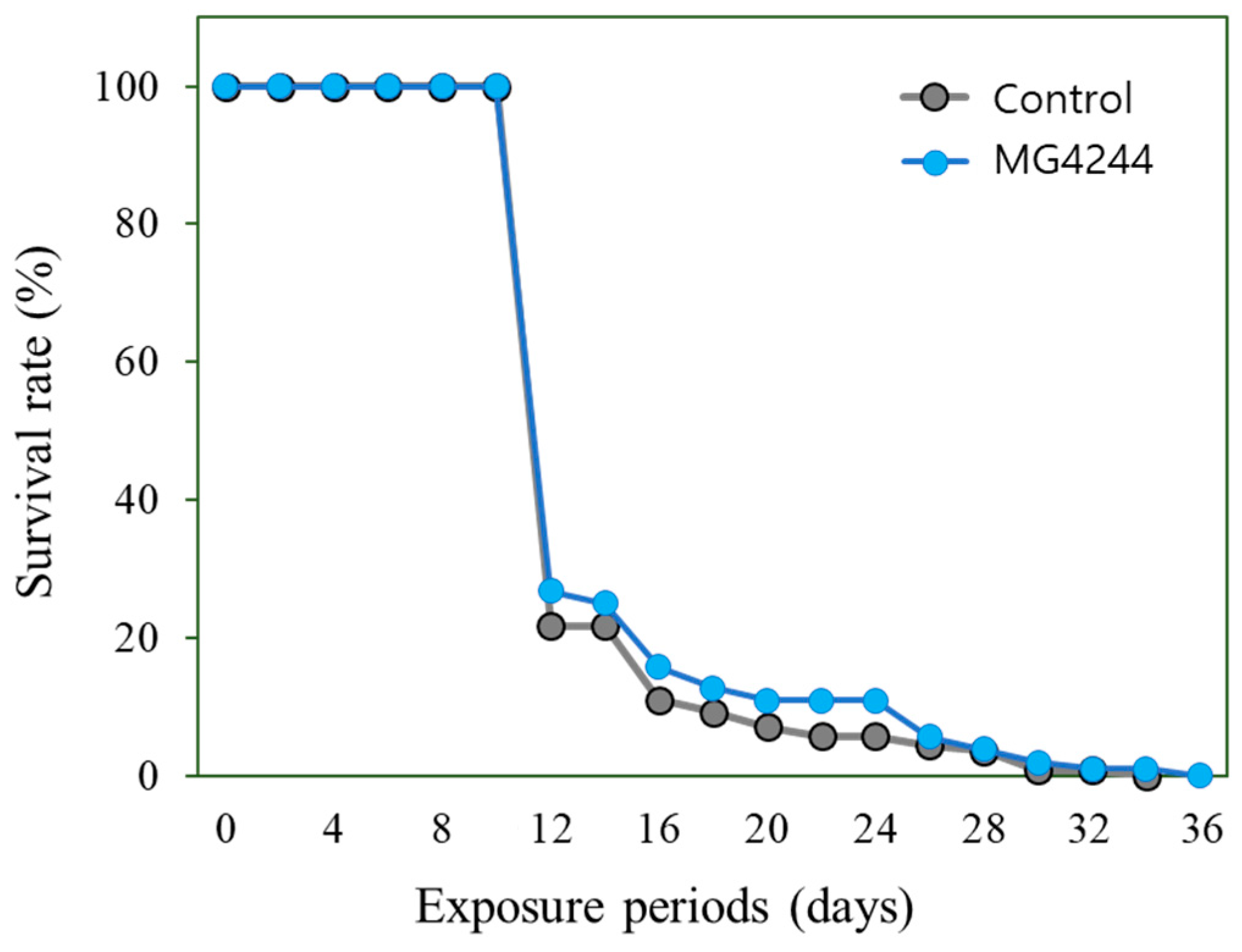
2.8. Lifespan Assay
2.9. Statistical Analyses
3. Results
3.1. Safety of MG4244
3.2. MG4244 Modulates PA- and HGD-Induced Health Deterioration in C. elegans
3.3. Effect on the Lifespan of MG4244 Under Normal Conditions
3.4. Effect on the Lifespan of MG4244 Under Oxidative and Thermal Conditions
3.5. The Effect of MG4244 on Improving Lipid Metabolism Is Mediated by AMPK Related Factors
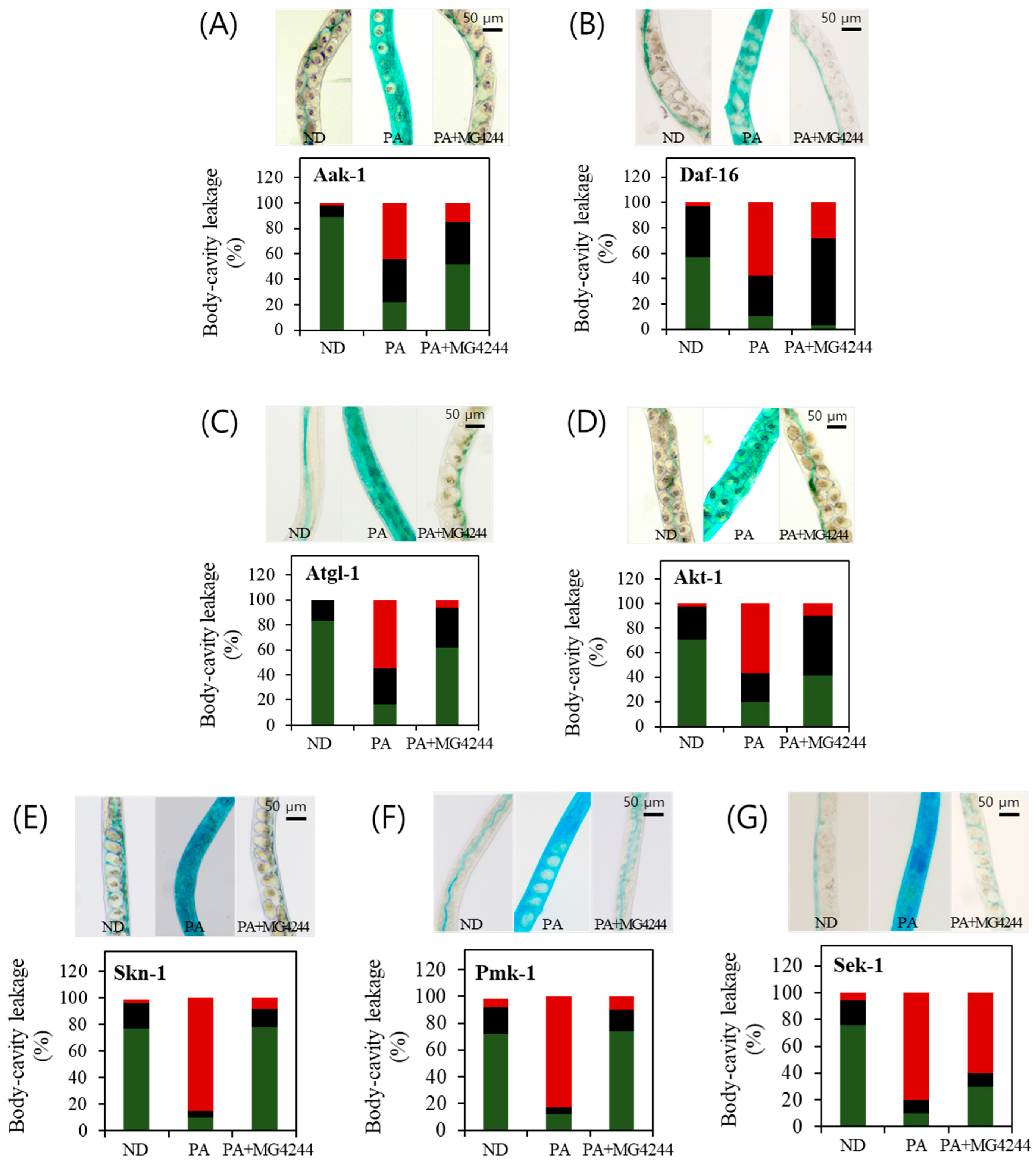
3.6. PA-Induced Intestinal Permeability Improvement Effect of MG4244 Is Related to Sek-1 and Daf-16
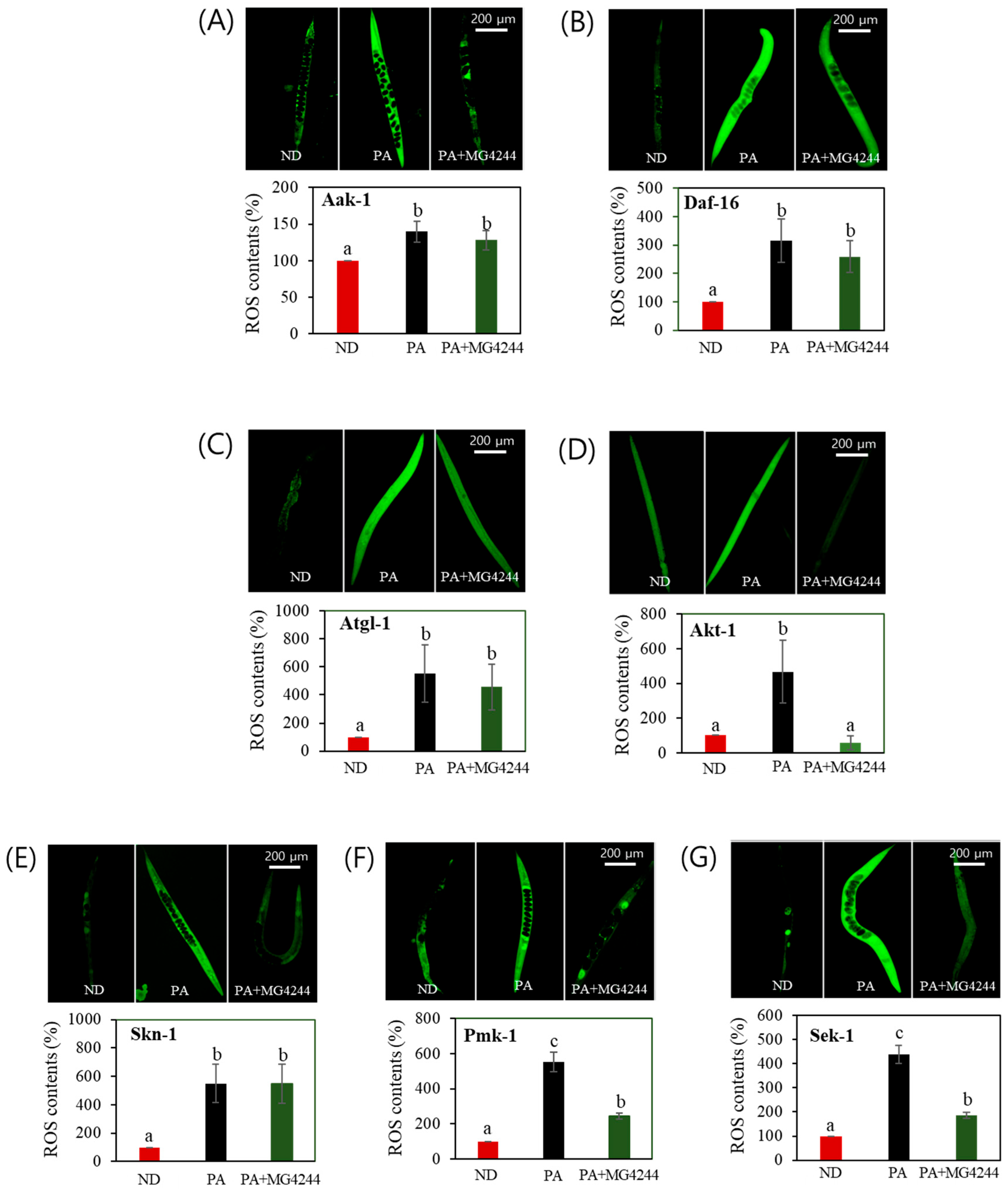
3.7. The Effect of MG4244 on Improving Oxidative Stress Is Mediated by AMPK, MAPK, and Related Factors
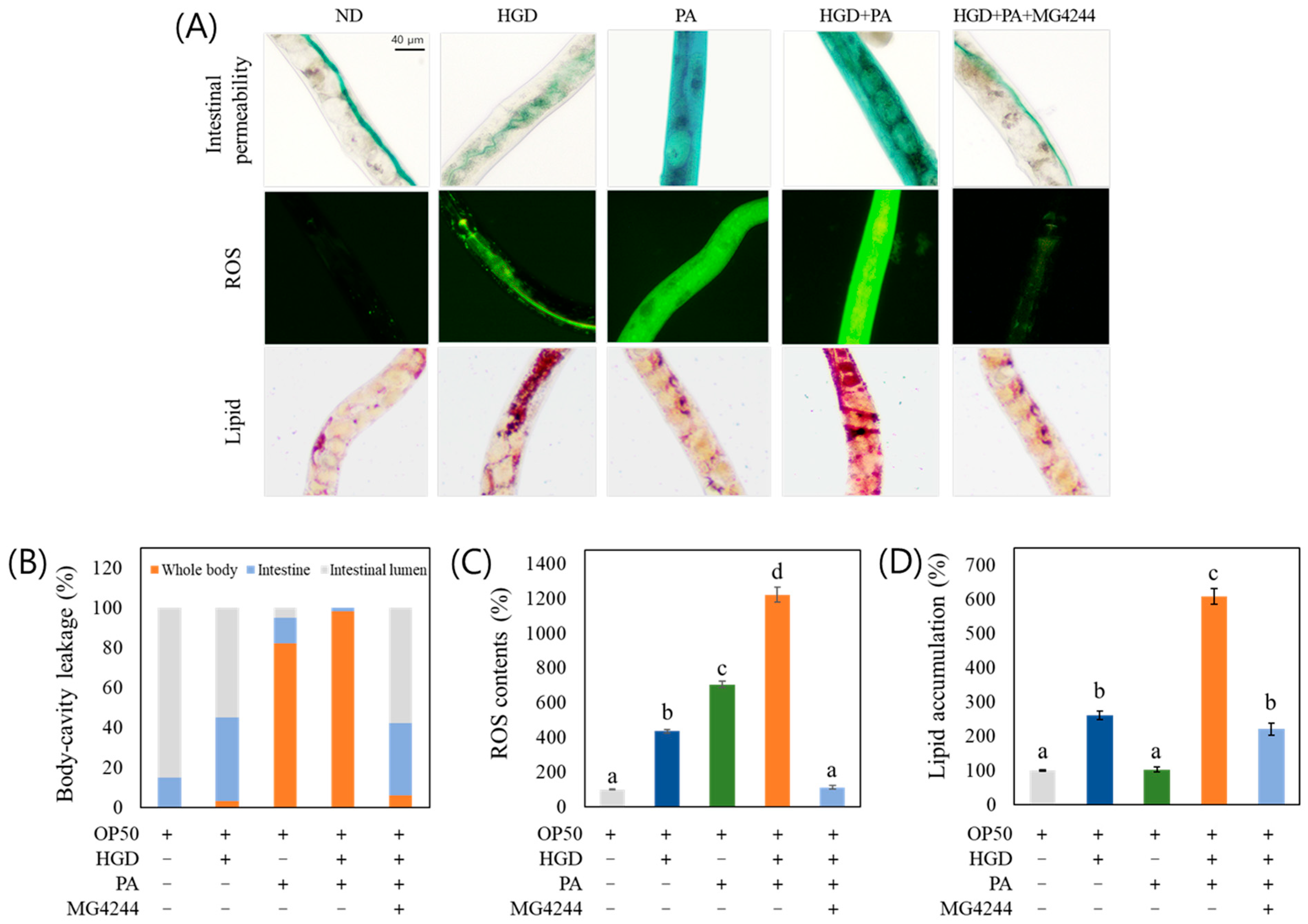
3.8. MG4244 Improves Intestinal Permeability, ROS, and Lipid Levels Under Combined Treatment with PA and an HGD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gieryńska, M.; Struzik, J.; Mielcarska, M.B.; Paulina, K. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota—A Mutual Relationship. Animals 2021, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- So, D.; Quigley, E.M.; Whelan, K. Probiotics in irritable bowel syndrome and inflammatory bowel disease: Review of mechanisms and effectiveness. Curr. Opin. Gastroenterol. 2023, 39, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zheng, X.; Jiang, M.; Chen, Q.; Zhang, Y.; Wu, L. Probiotic Supplements Improve Blood Glucose and Insulin Resistance/Sensitivity among Healthy and GDM Pregnant Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. In Evidence-Based Complementary and Alternative Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; p. 9830200. [Google Scholar] [CrossRef]
- Mavrogeni, M.E.; Asadpoor, M.; Henricks, P.A.; Keshavarzian, A.; Folkerts, G.; Braber, S. Direct Action of Non-Digestible Oligosaccharides against a Leaky Gut. Nutrients 2021, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, O.C.; Hamamah, S.; Covasa, M. Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes. Nutrients 2024, 16, 3447. [Google Scholar] [CrossRef]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Mularczyk, M.; Bourebaba, Y.; Kowalczuk, A.; Marycz, K.; Bourebaba, L. Probiotics-rich emulsion improves insulin signalling in Palmitate/Oleate-challenged human hepatocarcinoma cells through the modulation of Fetuin-A/TLR4-JNK-NF-κB pathway. Biomed. Pharmacother. 2021, 139, 111560. [Google Scholar] [CrossRef]
- Yeboah, P.J.; Wijemanna, N.D.; Eddin, A.S.; Williams, L.L.; Ibrahim, S.A. Lactic Acid Bacteria: Review on the Potential Delivery System as an Effective Probiotic. 2023. Available online: https://drive.google.com/file/d/1lEkKU5FM2iM1I889gqaW8hkZlo9TxeSf/view (accessed on 9 April 2025).
- Bhat, M.I.; Kapila, S.; Kapila, R. Lactobacillus fermentum (MTCC-5898) supplementation renders prophylactic action against Escherichia coli impaired intestinal barrier function through tight junction modulation. LWT 2020, 123, 109118. [Google Scholar] [CrossRef]
- Kong, Y.; Olejar, K.J.; On, S.L.; Chelikani, V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef]
- Kaur, H.; Gupta, T.; Kapila, S.; Kapila, R. Lactobacillus fermentum (MTCC-5898) based fermented whey renders prophylactic action against colitis by strengthening the gut barrier function and maintaining immune homeostasis. Microb. Pathog. 2022, 173, 105887. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Prakash, S. Ferulic acid produced by Lactobacillus fermentum influences developmental growth through a dTOR-mediated mechanism. Mol. Biotechnol. 2019, 61, 1–11. [Google Scholar] [CrossRef]
- Hossain, T.J. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: Metabolic, probiotic and biotechnological perspectives. Heliyon 2022, 8, e11412. [Google Scholar] [CrossRef]
- Paulino do Nascimento, L.C.; Lacerda, D.C.; Ferreira, D.J.S.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum, current evidence on the antioxidant properties and opportunities to be exploited as a probiotic microorganism. Probiotics Antimicrob. Proteins 2022, 14, 960–979. [Google Scholar] [CrossRef]
- Paladino, L.; Rappa, F.; Barone, R.; Macaluso, F.; Zummo, F.P.; David, S.; Szychlinska, M.A.; Bucchieri, F.; Macario, A.J.; Cappello, F.; et al. NF-kB Regulation and the Chaperone System Mediate Restorative Effects of the Probiotic Lactobacillus fermentum LF31 in the Small Intestine and Cerebellum of Mice with Ethanol-Induced Damage. Biology 2023, 12, 1394. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.I.; Jang, M.; Jeong, Y.; Kang, C.H.; Kim, G.H. Anti-adipogenic effect of Lactobacillus fermentum MG4231 and MG4244 through AMPK pathway in 3T3-L1 preadipocytes. Food Sci. Biotechnol. 2020, 29, 1541–1551. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, S.I.; Jang, M.; Jeong, Y.A.; Kang, C.H.; Kim, G.H. Combination of Limosilactobacillus fermentum MG4231 and MG4244 attenuates lipid accumulation in high-fat diet-fed obese mice. Benef. Microbes 2021, 12, 479–492. [Google Scholar] [CrossRef]
- Wang, L.; Celik, C.; Abdul Khalid, A.T.; Thalappilly, S.; Xu, S.; Koh, J.H.; Lim, V.W.; Low, A.D.; Thibault, G. The unfolded protein response reverses the effects of glucose on lifespan in chemically-sterilized C. elegans. Nat. Commun. 2022, 13, 5889. [Google Scholar] [CrossRef]
- Cho, M.; Kim, Y.; You, S.; Hwang, D.Y.; Jang, M. Chlorogenic Acid of Cirsium japonicum Resists Oxidative Stress Caused by Aging and Prolongs Healthspan via SKN-1/Nrf2 and DAF-16/FOXO in Caenorhabditis elegans. Metabolites 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, Y. Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection. Nutrients 2019, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Walhout, A.J.; Ambros, V. A bacterial pathogen induces developmental slowing by high reactive oxygen species and mitochondrial dysfunction in Caenorhabditis elegans. Cell Rep. 2023, 42, 113189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, R.; Ilangovan, P.; Nongbri, D.; Suchiang, K. Probiotics Interactions and the Modulation of Major Signalling Pathways in Host Model Organism Caenorhabditis elegans. Indian J. Microbiol. 2021, 61, 404–416. [Google Scholar] [CrossRef]
- Berry, B.J.; Baldzizhar, A.; Nieves, T.O.; Wojtovich, A.P. Neuronal AMPK coordinates mitochondrial energy sensing and hypoxia resistance in C. elegans. FASEB J. 2020, 34, 16333. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, W.; Wang, B.; Gao, C.; Liu, X.; Song, Y.; Qi, H.; Liu, H.; Wu, T.; Wang, R.; et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023, 63, 102760. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Bang, E.; Ha, S.; Jung, H.J.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Organ-differential roles of Akt/FoxOs axis as a key metabolic modulator during aging. Aging Dis. 2021, 12, 1713. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Zhu, X.; Qin, Y.; Yu, C.; Jiang, N.; Li, S.; Liu, F.; Liu, Y. Luteolin promotes pathogen resistance in Caenorhabditis elegans via DAF-2/DAF-16 insulin-like signaling pathway. Int. Immunopharmacol. 2023, 115, 109679. [Google Scholar] [CrossRef]
- Dhondt, I.; Petyuk, V.A.; Cai, H.; Vandemeulebroucke, L.; Vierstraete, A.; Smith, R.D.; Depuydt, G.; Braeckman, B.P. FOXO/DAF-16 activation slows down turnover of the majority of proteins in C. elegans. Cell Rep. 2016, 16, 3028–3040. [Google Scholar] [CrossRef]
- Matilainen, O.; Ribeiro, A.R.; Verbeeren, J.; Cetinbas, M.; Sood, H.; Sadreyev, R.I.; Garcia, S.M. Loss of muscleblind splicing factor shortens Caenorhabditis elegans lifespan by reducing the activity of p38 MAPK/PMK-1 and transcription factors ATF-7 and Nrf/SKN-1. Genetics 2021, 219, iyab114. [Google Scholar] [CrossRef]
- Yang, R.; Kang, Y.; Duan, J.; Zou, C.; Wu, Q. The p38 MAPK/PMK-1 Pathway Is Required for Resistance to Nocardia farcinica Infection in Caenorhabditis elegans. Pathogens 2022, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Suzuki, K.; Kunitomo, H.; Tomioka, M.; Iino, Y. Multiple p38/JNK mitogen-activated protein kinase (MAPK) signaling pathways mediate salt chemotaxis learning in C. elegans. G3 Genes Genomes Genet. 2023, 13, jkad129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, X.; Liu, J.; He, P.; Zhang, S.; Zhang, Y.; Gao, J.; Yang, S.; Kang, N.; Afridi, M.I.; et al. GABAergic synapses suppress intestinal innate immunity via insulin signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2021, 118, e2021063118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Yu, H.; Yin, X.; Nie, S.; Xie, M.; Chen, W.; Gong, J. Cell Signaling of Caenorhabditis elegans in Response to Enterotoxigenic Escherichia coli Infection and Lactobacillus zeae Protection. Front. Immunol. 2018, 9, 345588. [Google Scholar] [CrossRef]
- Peres, T.V.; Arantes, L.P.; Miah, M.R.; Bornhorst, J.; Schwerdtle, T.; Bowman, A.B.; Leal, R.B.; Aschner, M. Role of Caenorhabditis elegans AKT-1/2 and SGK-1 in Manganese Toxicity. Neurotox. Res. 2018, 34, 584–596. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The interplay between obesity and inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Deji-Oloruntoba, O.O.; Elufioye, T.O.; Adefegha, S.A.; Jang, M. Can Caenorhabditis elegans Serve as a Reliable Model for Drug and Nutraceutical Discovery? Appl. Biosci. 2025, 4, 23. [Google Scholar] [CrossRef]
| Conditions | Groups | Mean Lifespan (Days) | p-Value |
|---|---|---|---|
| Oxidative stress | Control | 5.39 ± 0.56 | <0.001 |
| MG4244 | 13.81 ± 1.24 | ||
| Thermal stress | Control | 11.53 ± 1.28 | 0.8186 |
| MG4244 | 11.70 ± 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Deji-Oloruntoba, O.O.; Choe, Y.; Lee, J.; Park, J.; Kim, B.; Choi, S.; Jang, M. Limosilactobacillus fermentum MG4244 Protects Against Metabolic and Inflammatory Stress in Caenorhabditis elegans. Foods 2025, 14, 1995. https://doi.org/10.3390/foods14111995
Kim Y, Deji-Oloruntoba OO, Choe Y, Lee J, Park J, Kim B, Choi S, Jang M. Limosilactobacillus fermentum MG4244 Protects Against Metabolic and Inflammatory Stress in Caenorhabditis elegans. Foods. 2025; 14(11):1995. https://doi.org/10.3390/foods14111995
Chicago/Turabian StyleKim, Yebin, Opeyemi O. Deji-Oloruntoba, Yunji Choe, Jiyeon Lee, Jeongyong Park, Byoungkook Kim, Sooim Choi, and Miran Jang. 2025. "Limosilactobacillus fermentum MG4244 Protects Against Metabolic and Inflammatory Stress in Caenorhabditis elegans" Foods 14, no. 11: 1995. https://doi.org/10.3390/foods14111995
APA StyleKim, Y., Deji-Oloruntoba, O. O., Choe, Y., Lee, J., Park, J., Kim, B., Choi, S., & Jang, M. (2025). Limosilactobacillus fermentum MG4244 Protects Against Metabolic and Inflammatory Stress in Caenorhabditis elegans. Foods, 14(11), 1995. https://doi.org/10.3390/foods14111995








