Trans Fats in Spanish Pastries and Their Influence on Mesenchymal Stem Cell Behavior In Vitro and Related Health Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples’ Description
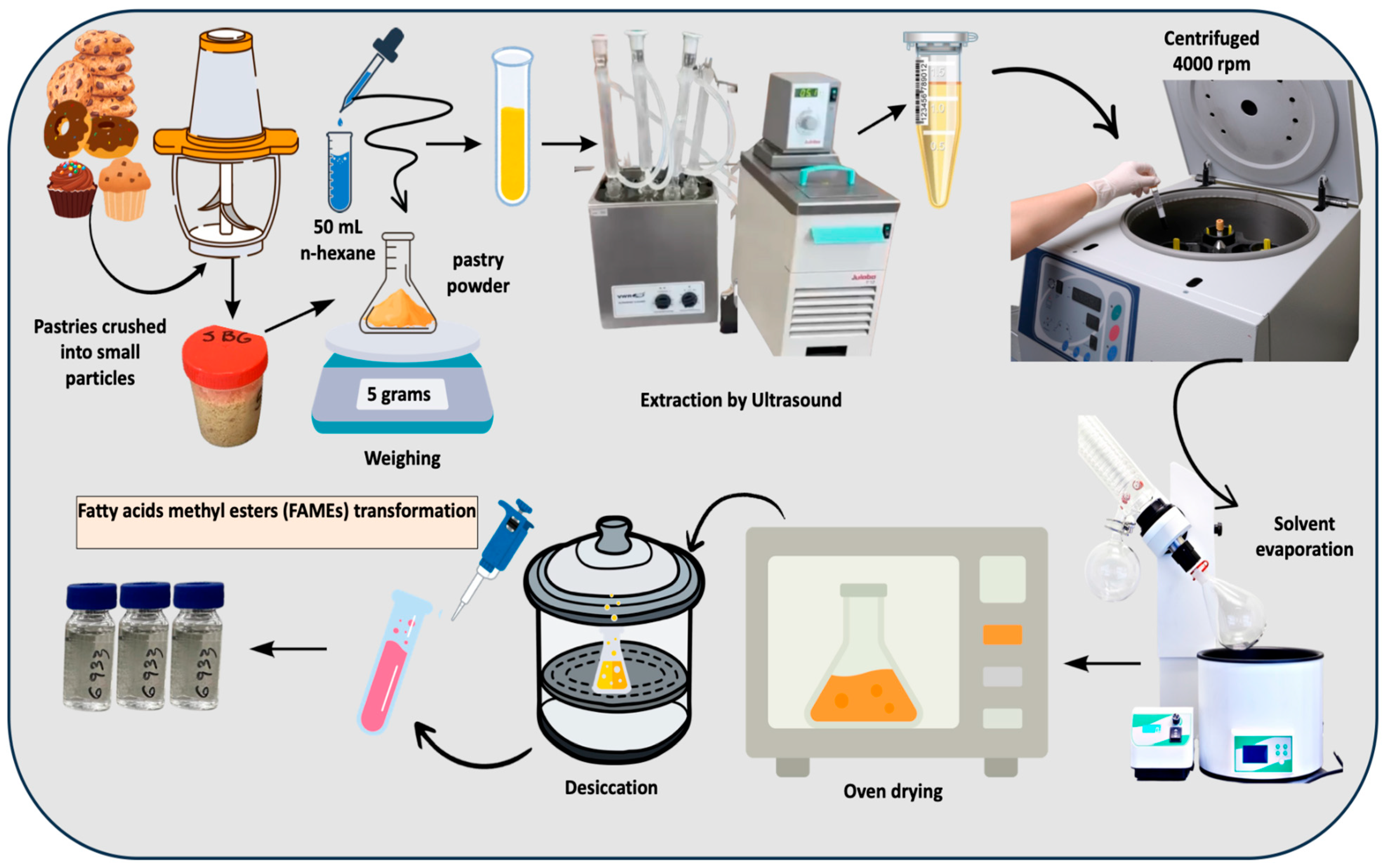
2.2. Fat Extraction Through Ultrasound
2.3. Fatty Acid Methyl Ester Preparation and Characterization Through Gas Chromatography-Mass Spectrometry (GC-MS)
2.4. Determination of Fatty Acid Quality and Composition Indices
2.4.1. Acid Value
2.4.2. Anisidine Value
2.4.3. Peroxide Value
2.4.4. Iodine Value
2.4.5. Saponification Value
2.5. Raman and FTIR Vibrational Spectroscopy Characterization
2.6. Cell Culture
2.6.1. Cytotoxic Effect of TFA
2.6.2. Morphology of MSCs with TFA
2.7. Statistical Analysis
3. Results
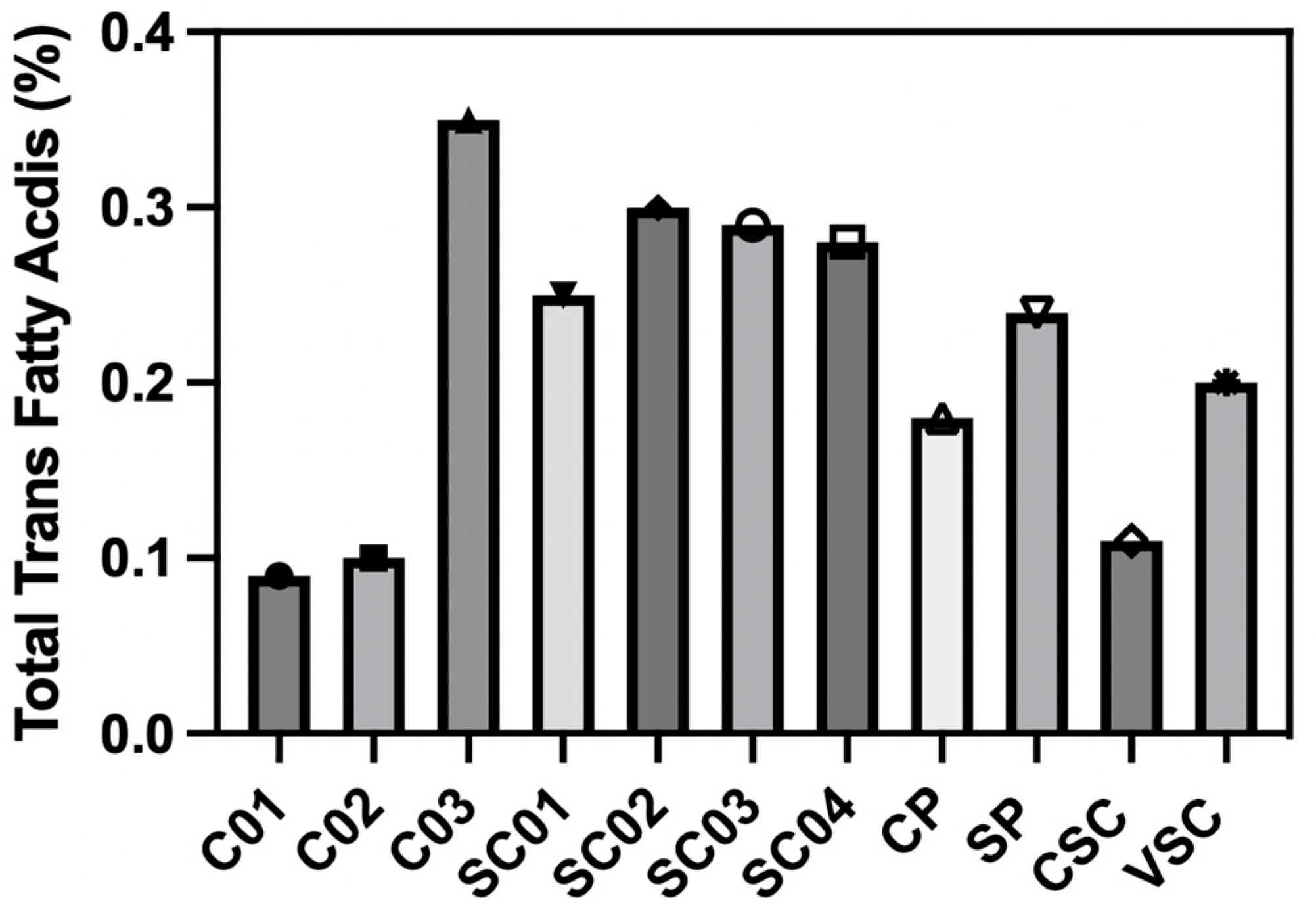
3.1. Fatty Acids’ Characterization
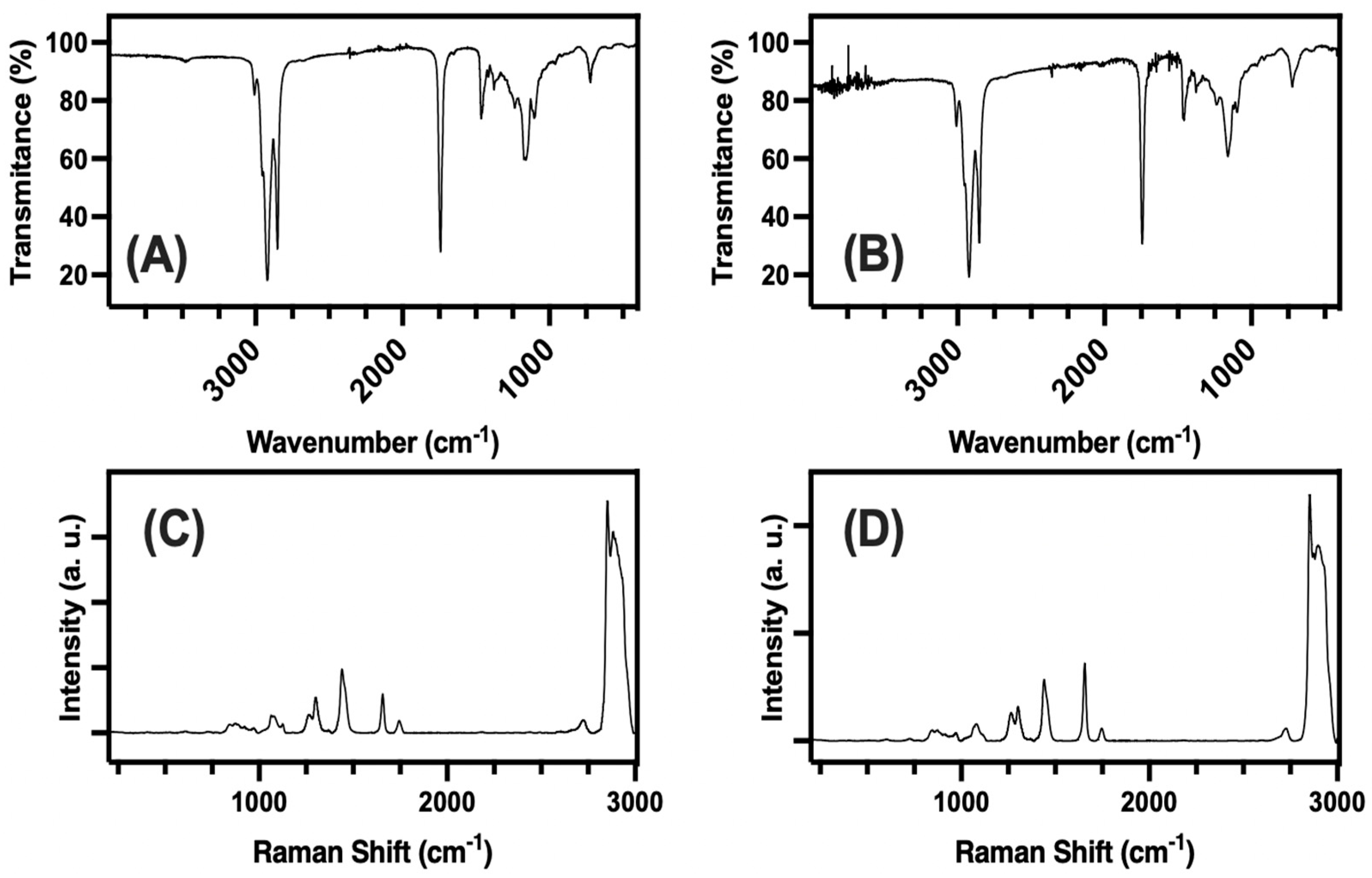
3.2. Raman and IR
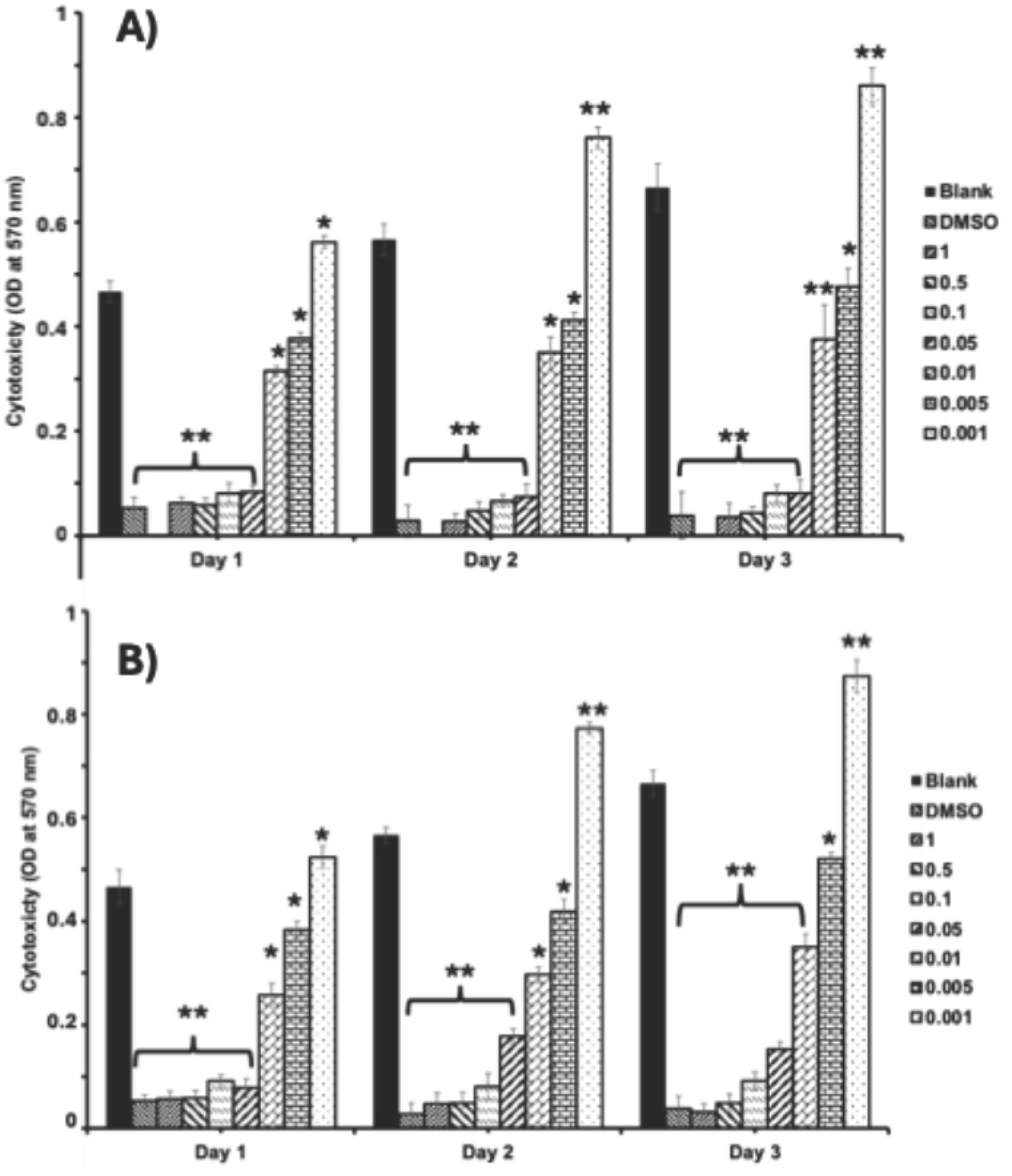
3.3. Cytotoxicity Assessment
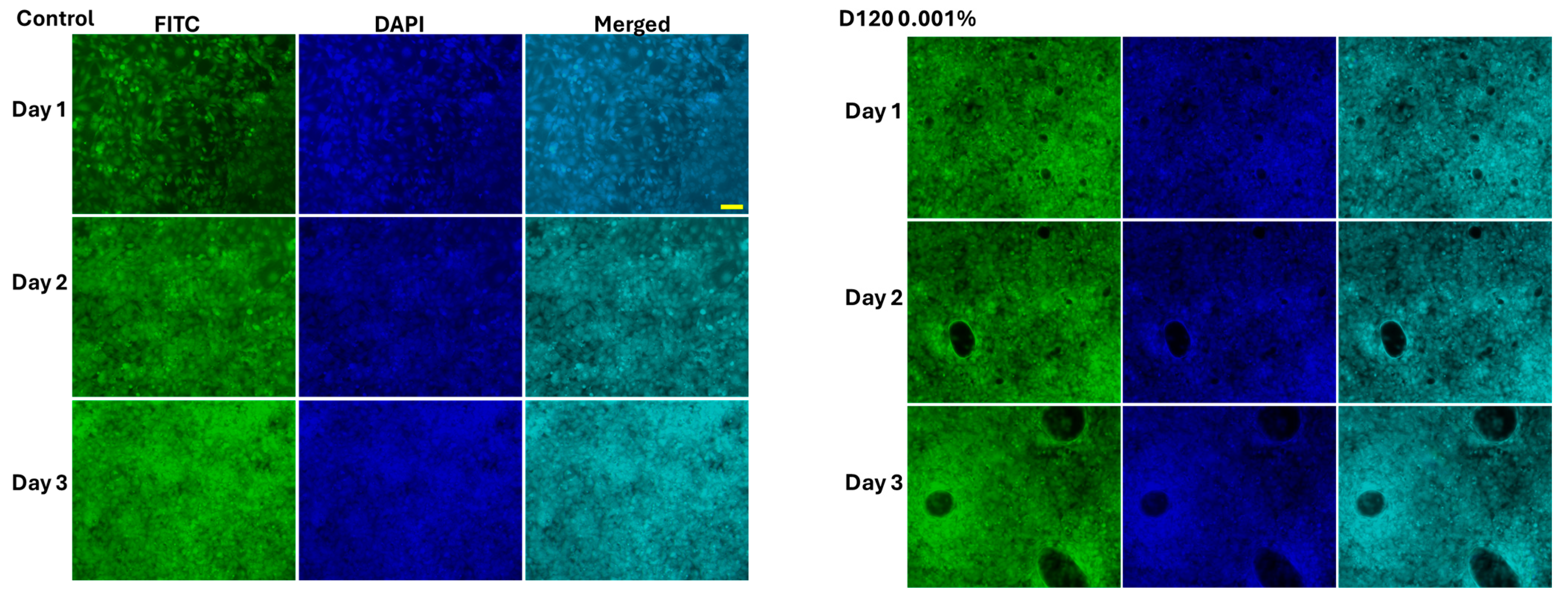
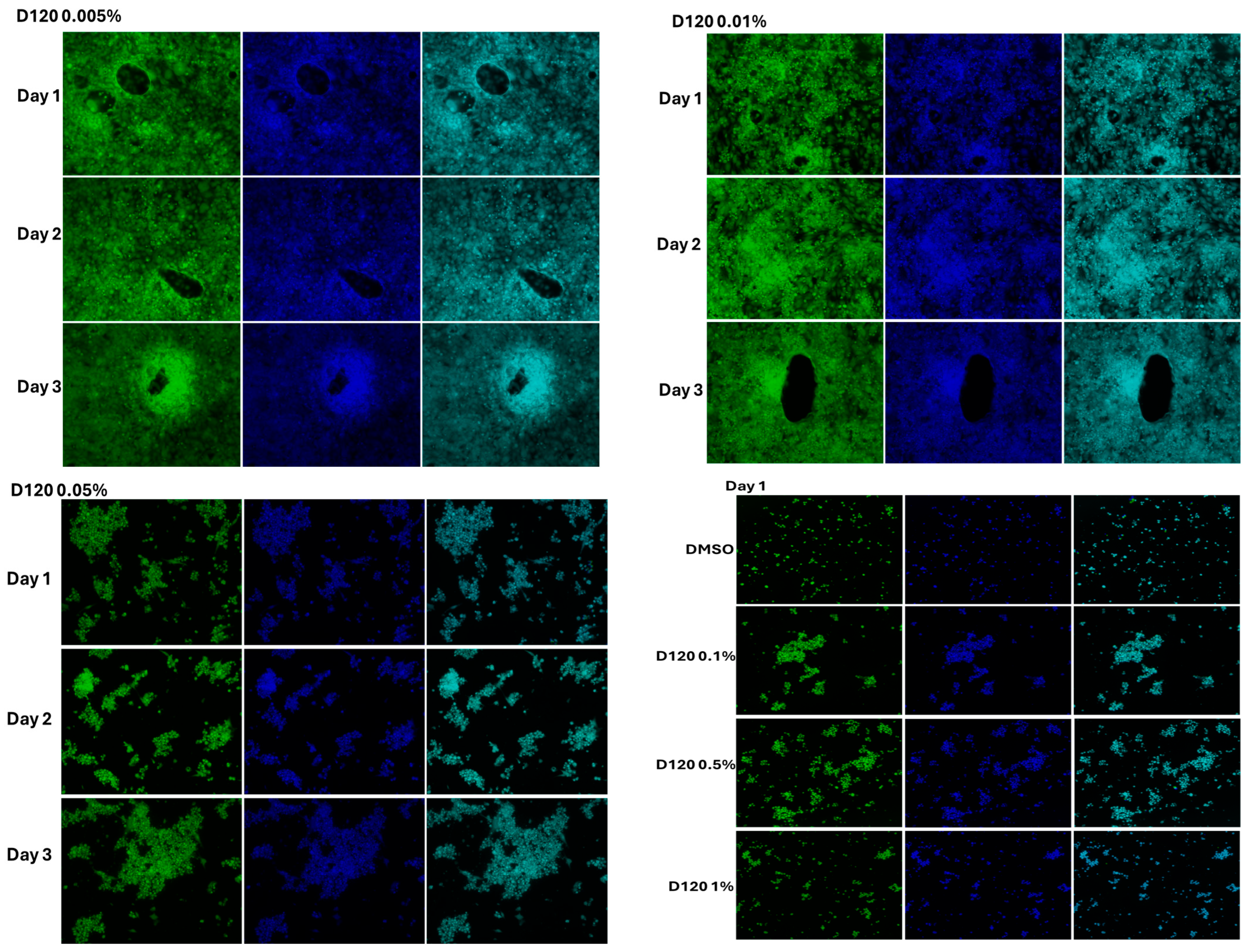
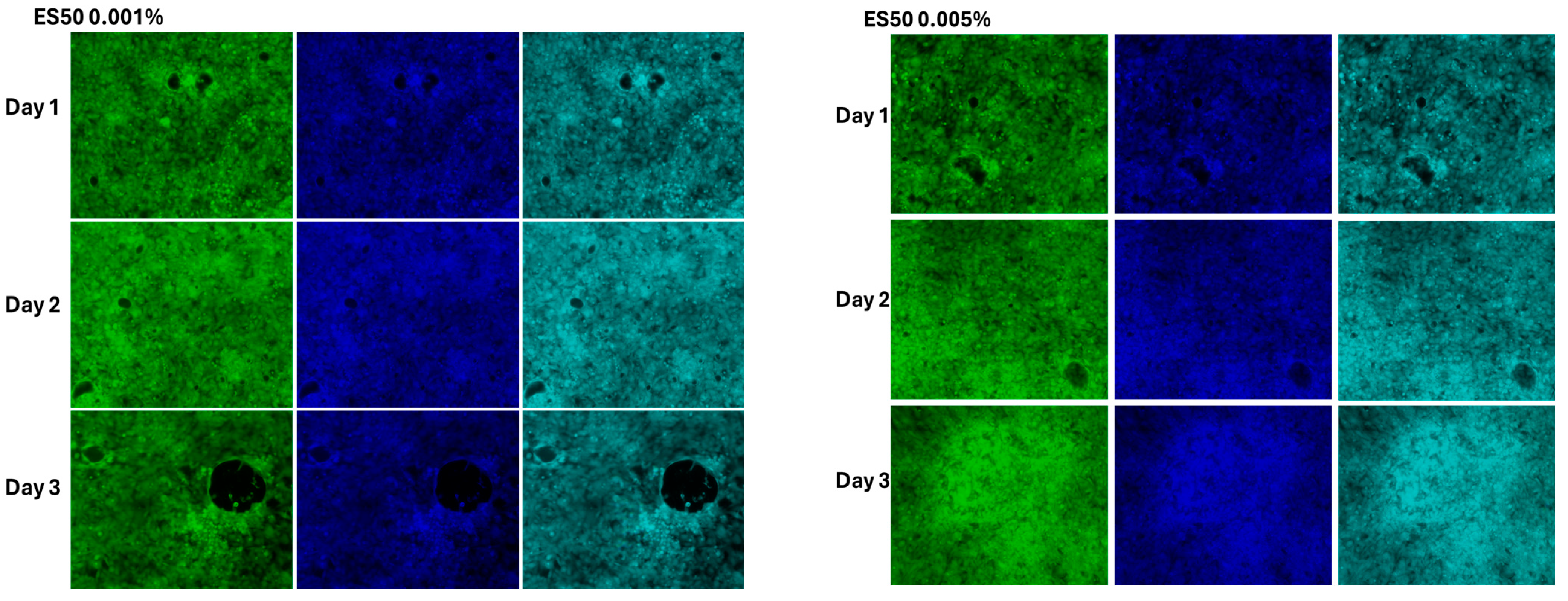
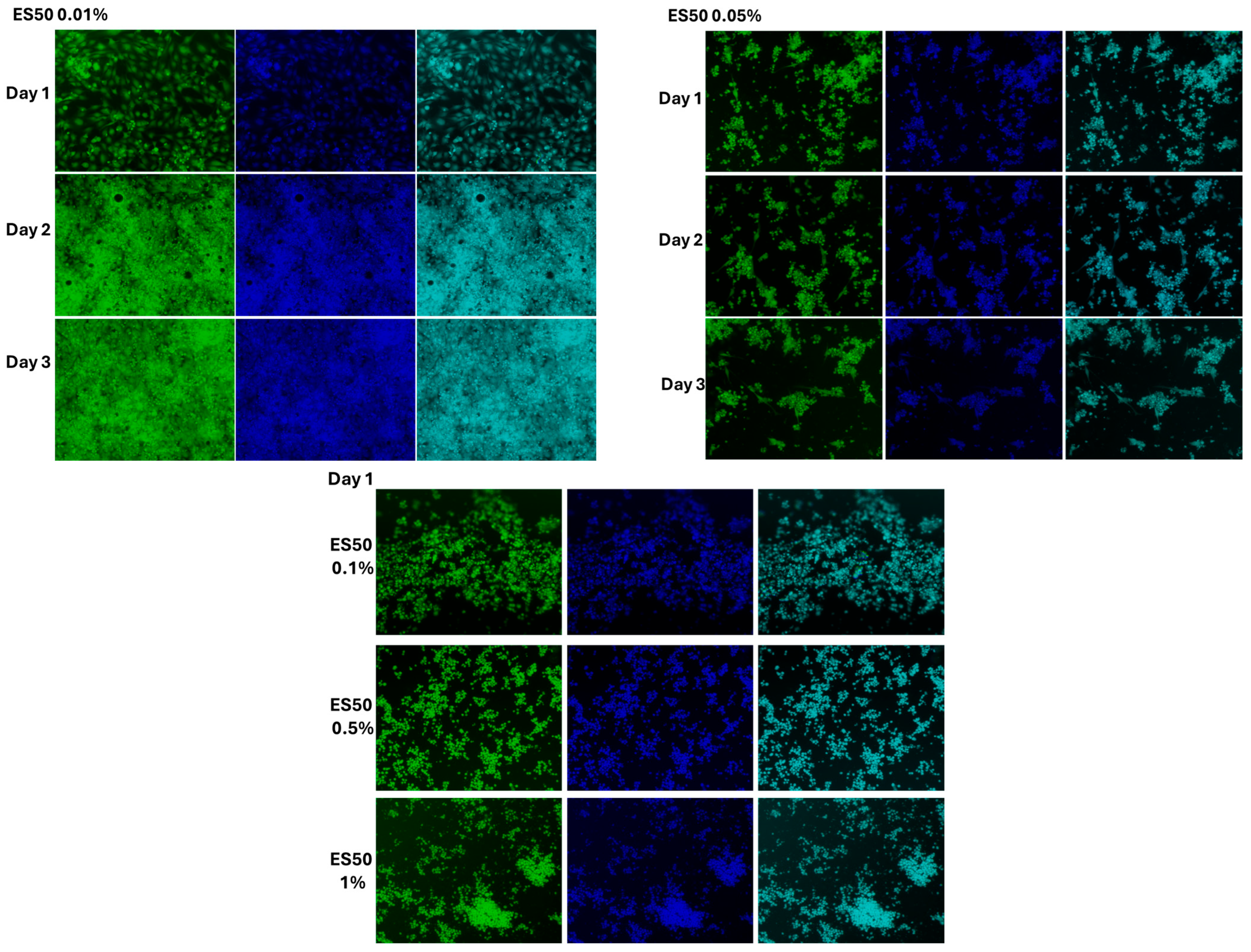
3.4. The Effect of Cell Morphology by TFA
4. Discussion
4.1. Fat and TFA Content in Pastries and Their Potential Impact on Health
4.2. FTIR and Raman
4.3. Cytotoxicity Assessment
4.4. Legal Analysis of Compliance with the Regulatory Framework for Trans Fatty Acids in Spain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spada, E.; De Cianni, R.; Di Vita, G.; Mancuso, T. Balancing Freshness and Sustainability: Charting a Course for Meat Industry Innovation and Consumer Acceptance. Foods 2024, 13, 1092. [Google Scholar] [CrossRef]
- Delgado-Alarcón, J.M.; Hernández Morante, J.J.; Aviles, F.V.; Albaladejo-Otón, M.D.; Morillas-Ruíz, J.M. Effect of the Fat Eaten at Breakfast on Lipid Metabolism: A Crossover Trial in Women with Cardiovascular Risk. Nutrients 2020, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, H.; Naz, S.; Panhwar, A.; Khaskheli, A.R.; Sidhu, A.R.; Siyal, F.J.; Mahesar, S.A.; Kara, H.; Ayyildiz, H.F.; Kandhro, A.A.; et al. Review on Health Concern: Trans Fatty Acids and Hydrogenation Process. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci. 2024, 67, 91–102. [Google Scholar]
- Morillas-Ruiz, J.M.; Delgado-Alarcon, J.M.; Rubio-Perez, J.M.; Albaladejo Oton, M.D. The Type of Fat Ingested at Breakfast Influences the Plasma Lipid Profile of Postmenopausal Women. BioMed Res. Int. 2014, 2014, 815915. [Google Scholar] [CrossRef] [PubMed]
- Miró-Colmenárez, P.J.; Illán-Marcos, E.; Díaz-Cruces, E.; Rocasolano, M.M.; Martínez-Hernandez, J.M.; Zamora-Ledezma, E.; Zamora-Ledezma, C. Current Insights into Industrial Trans Fatty Acids Legal Frameworks and Health Challenges in the European Union and Spain. Foods 2024, 13, 3845. [Google Scholar] [CrossRef]
- Mavlanov, U.; Czaja, T.P.; Nuriddinov, S.; Dalimova, D.; Dragsted, L.O.; Engelsen, S.B.; Khakimov, B. The Effects of Industrial Processing and Home Cooking Practices on Trans-Fatty Acid Profiles of Vegetable Oils. Food Chem. 2025, 469, 142571. [Google Scholar] [CrossRef]
- Umayangani, S.; De Silva, I.; Munasinghe, D.; Deshika, C.; Chandrapala, J.; Silva, M. Elimination of Trans Fatty Acids from Foods Using Novel Technologies. Food Rev. Int. 2025, 1–29. [Google Scholar] [CrossRef]
- Hirata, Y. Trans-Fatty Acids as an Enhancer of Inflammation and Cell Death: Molecular Basis for Their Pathological Actions. Biol. Pharm. Bull. 2021, 44, 1349–1356. [Google Scholar] [CrossRef]
- Fillmore, N.; Huqi, A.; Jaswal, J.S.; Mori, J.; Paulin, R.; Haromy, A.; Onay-Besikci, A.; Ionescu, L.; Thébaud, B.; Michelakis, E.; et al. Effect of Fatty Acids on Human Bone Marrow Mesenchymal Stem Cell Energy Metabolism and Survival. PLoS ONE 2015, 10, e0120257. [Google Scholar] [CrossRef]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Brouwer, I.A.; Wanders, A.J.; Katan, M.B. Effect of Animal and Industrial Trans Fatty Acids on HDL and LDL Cholesterol Levels in Humans—A Quantitative Review. PLoS ONE 2010, 5, e9434. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of Saturated and Trans Unsaturated Fatty Acids and Risk of All Cause Mortality, Cardiovascular Disease, and Type 2 Diabetes: Systematic Review and Meta-Analysis of Observational Studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Hwalla, N.; Chehade, L.; O’Neill, L.M.; Kharroubi, S.; Kassis, A.; Cheikh Ismail, L.; Al Dhaheri, A.S.; Ali, H.I.; Ibrahim, S.; Chokor, F.A.Z.; et al. Total Usual Nutrient Intakes and Nutritional Status of United Arab Emirates Children (4 Years–12.9 Years): Findings from the Kids Nutrition and Health Survey (KNHS) 2021. Nutrients 2023, 15, 234. [Google Scholar] [CrossRef]
- Delgado-Alarcón, J.M.; Hernández Morante, J.J.; Morillas-Ruiz, J.M. Modification of Breakfast Fat Composition Can Modulate Cytokine and Other Inflammatory Mediators in Women: A Randomized Crossover Trial. Nutrients 2023, 15, 3711. [Google Scholar] [CrossRef]
- Charlebois, S.; Latif, N.; Ilahi, I.; Sarker, B.; Music, J.; Vezeau, J. Digital Traceability in Agri-Food Supply Chains: A Comparative Analysis of OECD Member Countries. Foods 2024, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Rahmania, H.; Indrayanto, G.; Windarsih, A.; Fernando, D.; Bakar, N.K.A.; Rohman, A. Standard Review of the Application of Molecular Spectroscopic and Chromatographic-Based Methods for Determination of Trans Fatty Acids in Food Products. Appl. Food Res. 2025, 5, 100657. [Google Scholar] [CrossRef]
- Sarwar, S.; Shaheen, N.; Ashraf, M.M.; Bahar, N.; Akter, F.; Hossain, M.M.; Mridha, M.K.; Kajal, M.A.M.; Rasul, S.S.B.; Khondker, R.; et al. Fatty Acid Profile Emphasizing Trans-Fatty Acids in Commercially Available Soybean and Palm Oils and Its Probable Intake in Bangladesh. Food Chem. Adv. 2024, 4, 100611. [Google Scholar] [CrossRef]
- Da Silva, T.L.T.; Martini, S. Recent Advances in Lipid Crystallization in the Food Industry. Annu. Rev. Food Sci. Technol. 2024, 15, 355–379. [Google Scholar] [CrossRef]
- Katan, M.B.; Zock, P.L.; Mensink, R.P. Trans Fatty Acids and Their Effects on Lipoproteins in Humans. Annu. Rev. Nutr. 1995, 15, 473–493. [Google Scholar] [CrossRef]
- World Health Organization Trans Fat. Available online: https://www.who.int/news-room/fact-sheets/detail/trans-fat (accessed on 21 April 2025).
- Moreno Alcalde, S. Trans Fatty Acids Content in Foods Marketed in the Community of Madrid. Nutr. Hosp. 2014, 29, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Farinós, N.; Dal Re Saavedra, M.Á.; Villar Villalba, C.; Robledo De Dios, T. Trans-Fatty Acid Content of Food Products in Spain in 2015. Gac. Sanit. 2016, 30, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Official State Gazette Law 17/2011, of July 5, on Food Safety and Nutrition. 2011. Available online: https://www.boe.es/eli/es/l/2011/07/05/17/con (accessed on 23 June 2025).
- European Parliament Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 2011. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 23 June 2025).
- World Health Organization. Countdown to 2023: WHO Report on Global Trans-Fat Elimination 2022; World Health Organization: Genève, Switzerland, 2023.
- Juanéda, P.; Ledoux, M.; Sébédio, J. Analytical Methods for Determination of Trans Fatty Acid Content in Food. Eur. J. Lipid Sci. Technol. 2007, 109, 901–917. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Lin, L.J.; Li, J.H.; Lv, G.T.; Huang, M.F. Comparison of Four Different Extraction Methods of Oil from Macadamia integrifolia. Adv. Mater. Res. 2012, 610–613, 3382–3386. [Google Scholar] [CrossRef]
- European Union Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis 1991; European Union: Bruxelles Brussels, Belgium, 1991.
- ISO 12966-1:2014; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters (FAME). International Organization for Standardization: Genève, Switzerland, 2014.
- Iannone, A.; Carriera, F.; Passarella, S.; Fratianni, A.; Avino, P. There’s Something in What We Eat: An Overview on the Extraction Techniques and Chromatographic Analysis for PFAS Identification in Agri-Food Products. Foods 2024, 13, 1085. [Google Scholar] [CrossRef]
- World Health Organization. Eliminating Trans Fats in Europe: A Policy Brief; WHO Regional Office for Europe: København, Denmark, 2018.
- AOAC 940.28; Official Methods of Analysis, AOAC Official Method 940.28, Free Fatty Acids (Crude and Refined Oils). 18th ed. AOAC International: Gaithersburg, MD, USA, 2005.
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- AOAC 965.33; Official Methods of Analysis, AOAC Official Method 965.33, Peroxide Value of Oils and Fats. 18th ed. AOAC International: Gaithersburg, MD, USA, 2005.
- AOAC 920.158; Official Methods of Analysis, AOAC Official Method 920.158, Crude Fat in Flour. AOAC International: Gaithersburg, MD, USA, 2005.
- ISO 3961:2018; Animal and Vegetable Fats and Oils—Determination of Iodine Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- AOAC 920.160; Official Methods of Analysis, AOAC Official Method 920.160, Saponification Number (Koettstorfer Number) of Oils and Fats. AOAC International: Gaithersburg, MD, USA, 2005.
- ISO 3657:2020; Animal and Vegetable Fats and Oils—Determination of Saponification Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Elango, J. Proliferative and Osteogenic Supportive Effect of VEGF-Loaded Collagen-Chitosan Hydrogel System in Bone Marrow Derived Mesenchymal Stem Cells. Pharmaceutics 2023, 15, 1297. [Google Scholar] [CrossRef]
- Elango, J.; Salazar, K.; Velasquez, P.; Murciano, A.; De Aza, P.N.; Wu, W.; Granero Marín, J.M.; Mate Sanchez De Val, J.E. Fabrication of Novel Strontium-Coated Bioactive Ceramic-Glass (C2S(2P6)C2S) 3D-Porous Scaffold for the Proliferation and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Ceram. Int. 2024, 50, 16998–17010. [Google Scholar] [CrossRef]
- Parcerisa, J.; Codony, R.; Boatella, J.; Rafecas, M. Fatty Acids Including Trans Content of Commercial Bakery Products Manufactured in Spain. J. Agric. Food Chem. 1999, 47, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Echarte, A.; Ollé, R.; Astiasarán, I. 2012: No Trans Fatty Acids in Spanish Bakery Products. Food Chem. 2013, 138, 422–429. [Google Scholar] [CrossRef]
- Fernández-San Juan, P.M. Trans Fatty Acids (TFA): Sources and Intake Levels, Biological Effects and Content in Commercial Spanish Food. Nutr. Hosp. 2009, 24, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Hassan, M.F.; Rauf, A. Determination of Trans Fat in Selected Fast Food Products and Hydrogenated Fats of India Using Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy. J. Oleo Sci. 2017, 66, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Chen, H.; Yin, L.; Cheng, J.; Jin, J.; Zhao, H.; Liu, Z.; Dong, L.; Yu, H.; Lu, X. Rapid Fatty Acids Detection of Vegetable Oils by Raman Spectroscopy Based on Competitive Adaptive Reweighted Sampling Coupled with Support Vector Regression. Food Qual. Saf. 2022, 6, fyac053. [Google Scholar] [CrossRef]
- Afseth, N.K.; Dankel, K.; Andersen, P.V.; Difford, G.F.; Horn, S.S.; Sonesson, A.; Hillestad, B.; Wold, J.P.; Tengstrand, E. Raman and near Infrared Spectroscopy for Quantification of Fatty Acids in Muscle Tissue—A Salmon Case Study. Foods 2022, 11, 962. [Google Scholar] [CrossRef]
- Valasi, L.; Kokotou, M.G.; Pappas, C.S. GC-MS, FTIR and Raman Spectroscopic Analysis of Fatty Acids of Pistacia Vera (Greek variety “Aegina”) Oils from Two Consecutive Harvest Periods and Chemometric Differentiation of Oils Quality. Food Res. Int. 2021, 148, 110590. [Google Scholar] [CrossRef]
- Mossoba, M.M.; Azizian, H.; Kramer, J.K.G. Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids. Available online: https://www.aocs.org/resource/application-of-infrared-spectroscopy-to-the-rapid-determination-of-total-saturated-trans-monounsaturated-and-polyunsaturated-fatty-acids/ (accessed on 1 May 2025).
- Vicario, I.M.; Griguol, V.; León-Camacho, M. Multivariate Characterization of the Fatty Acid Profile of Spanish Cookies and Bakery Products. J. Agric. Food Chem. 2003, 51, 134–139. [Google Scholar] [CrossRef]
- Hadian, Z.; Mousavi Khaneghah, A. Sugar, Fat, Saturated and Trans Fatty Acid Contents in Iranian Cereal-Based Baked Products. Food Sci. Technol. 2022, 42, e26724. [Google Scholar] [CrossRef]
- Nurshahbani, S.; Azrina, A. Trans Fatty Acids in Selected Bakery Products and its Potential Dietary Exposure. Int. Food Res. J. 2014, 21, 2175. [Google Scholar]
- Geng, L.; Liu, K.; Zhang, H. Lipid Oxidation in Foods and Its Implications on Proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef]
- Maire, M.; Rega, B.; Cuvelier, M.-E.; Soto, P.; Giampaoli, P. Lipid Oxidation in Baked Products: Impact of Formula and Process on the Generation of Volatile Compounds. Food Chem. 2013, 141, 3510–3518. [Google Scholar] [CrossRef]
- Mittakos, I.; Nathanailides, C.I.; Kokokiris, L.E.; Barbouti, A.; Bitchava, K.; Gouva, E.; Kolygas, M.N.; Terzidis, M.A.; Kontominas, M.G. Antioxidant Capacity, Lipid Oxidation, and Quality Traits of Slow- and Fast-Growing Meagre (Argyrosomus regius) Fillets During Cold Storage. Antioxidants 2025, 14, 124. [Google Scholar] [CrossRef]
- Scheim, D.E. Cytotoxicity of Unsaturated Fatty Acids in Fresh Human Tumor Explants: Concentration Thresholds and Implications for Clinical Efficacy. Lipids Health Dis. 2009, 8, 54. [Google Scholar] [CrossRef]
- Krogager, T.P.; Nielsen, L.V.; Kahveci, D.; Dyrlund, T.F.; Scavenius, C.; Sanggaard, K.W.; Enghild, J.J. Hepatocytes Respond Differently to Major Dietary Trans Fatty Acid Isomers, Elaidic Acid and Trans-Vaccenic Acid. Proteome Sci. 2015, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Kloetzel, M.; Ehlers, A.; Niemann, B.; Buhrke, T.; Lampen, A. Trans Fatty Acids Affect Cellular Viability of Human Intestinal Caco-2 Cells and Activate Peroxisome Proliferator-Activated Receptors. Nutr. Cancer 2013, 65, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Miyazawa, T. Newly Recognized Cytotoxic Effect of Conjugated Trienoic Fatty Acids on Cultured Human Tumor Cells. Cancer Lett. 2000, 148, 173–179. [Google Scholar] [CrossRef]
- Sarnyai, F.; Kereszturi, É.; Szirmai, K.; Mátyási, J.; Al-Hag, J.I.; Csizmadia, T.; Lőw, P.; Szelényi, P.; Tamási, V.; Tibori, K.; et al. Different Metabolism and Toxicity of TRANS Fatty Acids, Elaidate and Vaccenate Compared to Cis-Oleate in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 7298. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Doloresco, M.A. The Effects of Trans Fatty Acids on Fatty Acyl Δ5 Desaturation by Human Skin Fibroblasts. Lipids 1984, 19, 869–874. [Google Scholar] [CrossRef]
- Hirata, Y.; Kojima, R.; Ashida, R.; Nada, Y.; Kimura, S.; Sato, E.; Noguchi, T.; Matsuzawa, A. Industrially Produced Trans-Fatty Acids are Potent Promoters of DNA Damage-Induced Apoptosis. J. Toxicol. Sci. 2024, 49, 27–36. [Google Scholar] [CrossRef]
- European Union Commission Regulation (EU) 2019/649 of 24 April 2019 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Fat of Animal Origin (Text with EEA Relevance); European Union: Brussels, Belgium, 2019.
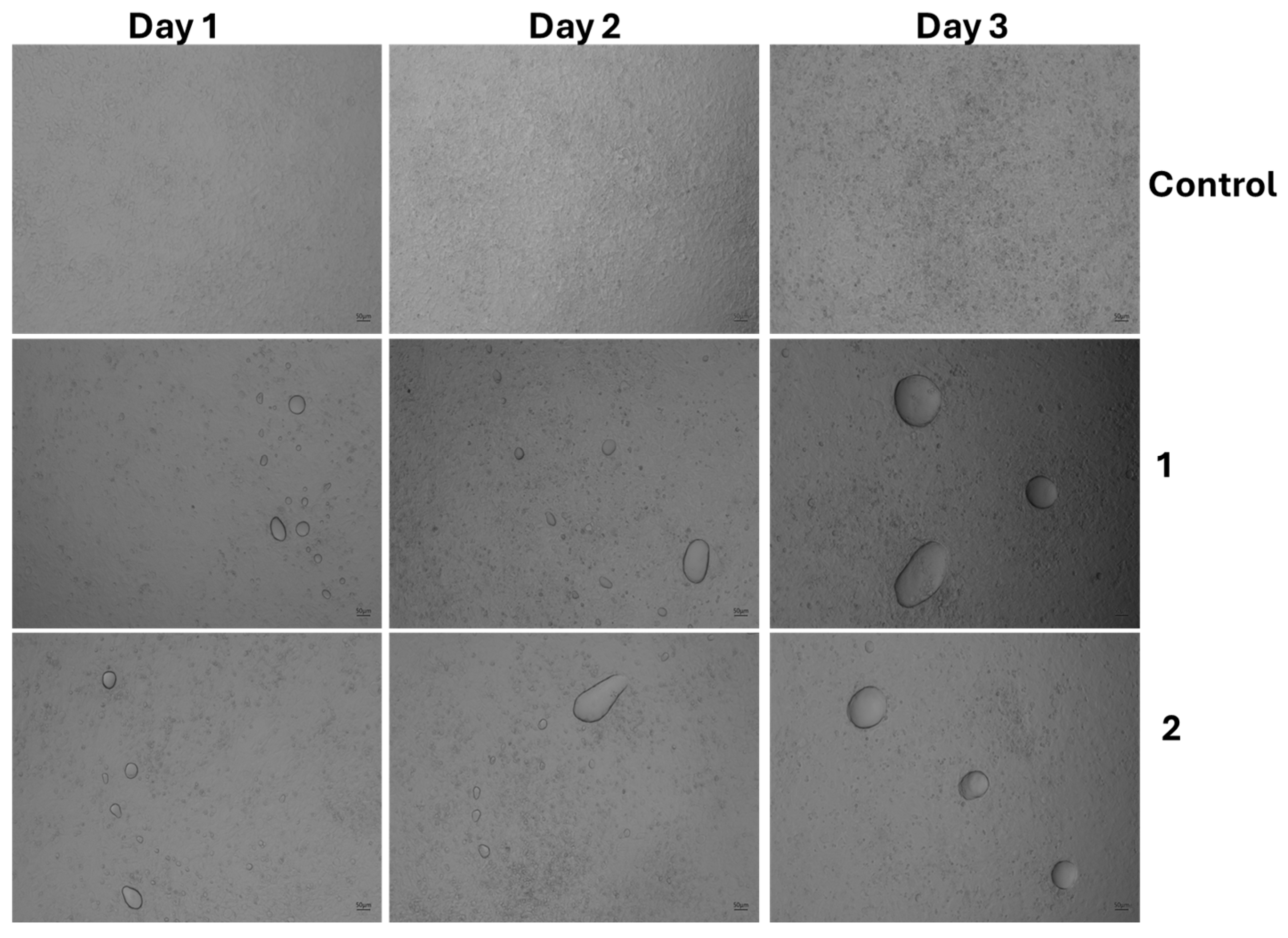
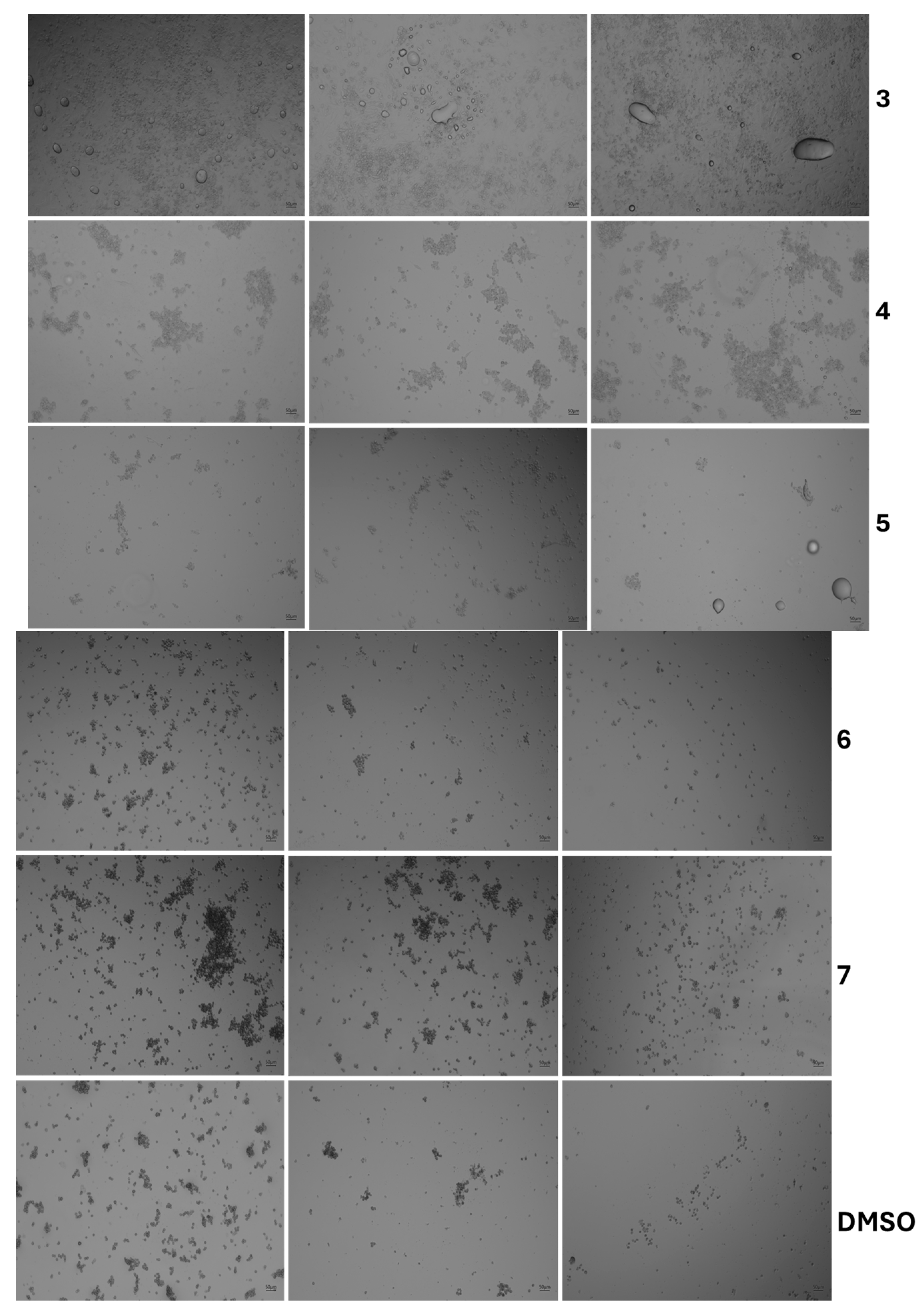
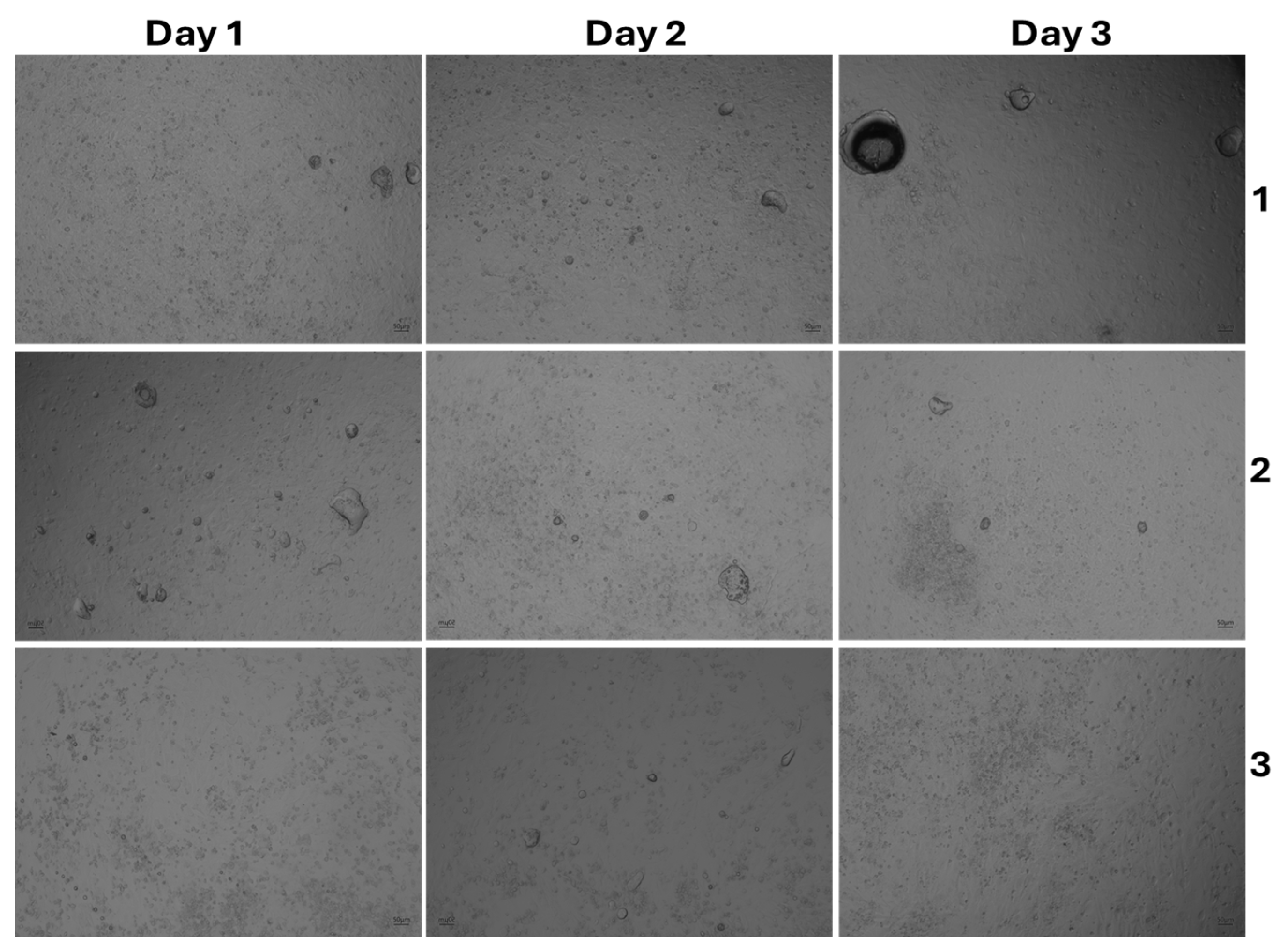
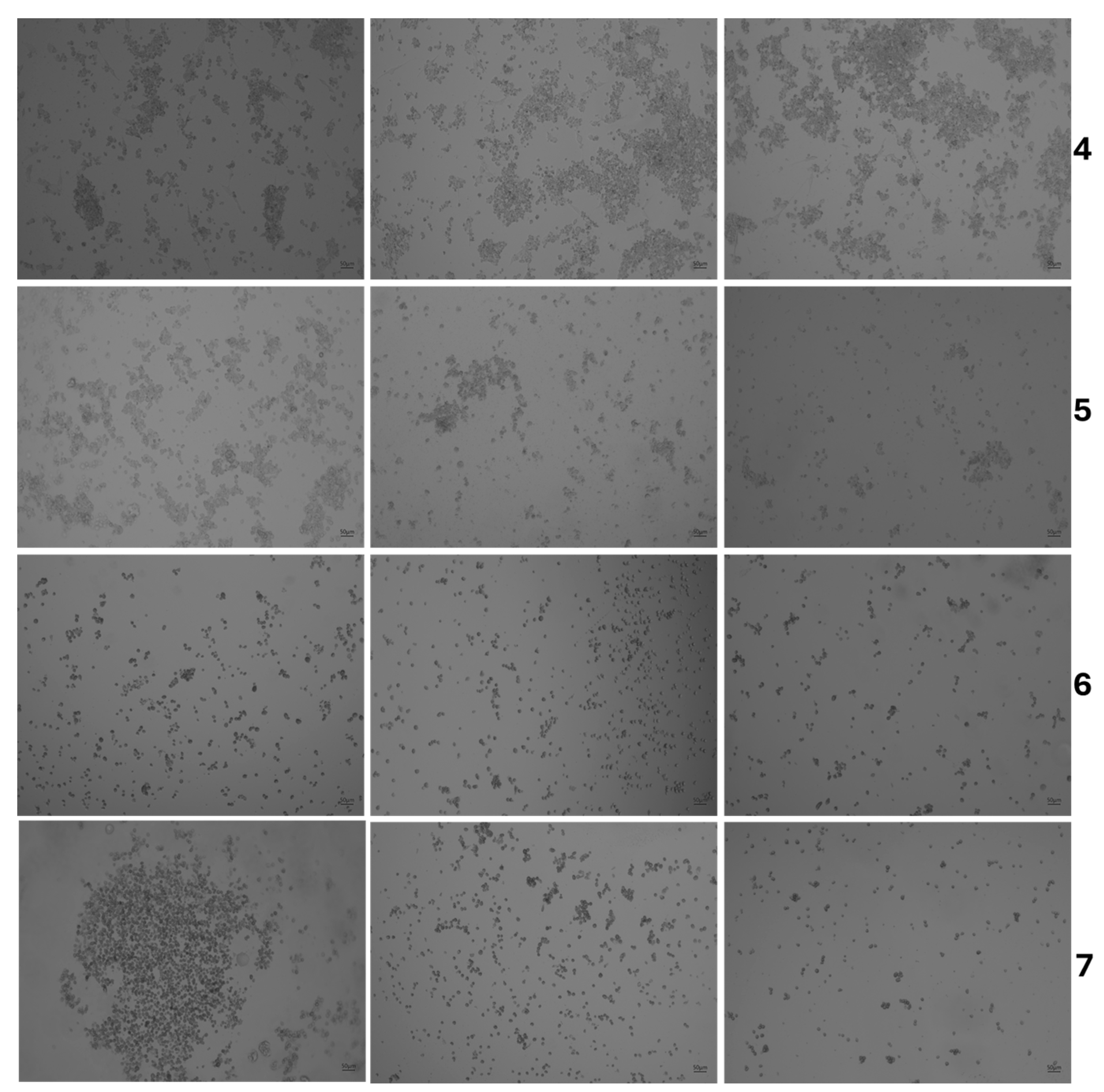
| Product Name | Code |
|---|---|
| Cookie-01 | C01 |
| Cookie-02 | C02 |
| Cookie-03 | C03 |
| Sponge Cake-01 | SC01 |
| Sponge Cake-02 | SC02 |
| Sponge Cake-03 | SC03 |
| Sponge Cake-04 | SC04 |
| Chocolate Palmier | CP |
| Sponge Cake Pastry | SP |
| Chocolate Sponge Cake | CSC |
| Valencia Sponge Cake | VSC |
| Fatty Acid | C01 | C02 | C03 | SC01 | SC02 | SC03 | SC04 | CP | SP | CSC | VSC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C-12:0 (Lauric) | 0.01 ± 0.01 | 0.12 ± 0.01 | 0.37 ± 0.02 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 17.19 ± 0.5 | 0.18 ± 0.01 | 15.68 ± 0.4 | 0.01 ± 0.01 |
| C-14:0 (Myristic) | 0.06 ± 0.01 | 0.13 ± 0.01 | 0.91 ± 0.03 | 0.33 ± 0.02 | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.27 ± 0.01 | 6.56 ± 0.2 | 0.67 ± 0.02 | 6.07 ± 0.2 | 0.08 ± 0.01 |
| C-16:0 (Palmitic) | 5.15 ± 0.10 | 5.90 ± 0.10 | 39.89 ± 0.5 | 22.65 ± 0.3 | 17.35 ± 0.2 | 22.22 ± 0.3 | 17.97 ± 0.2 | 28.91 ± 0.4 | 8.56 ± 0.10 | 8.74 ± 0.10 | 6.80 ± 0.10 |
| C-16:1 (Palmitoleic) | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 | 2.41 ± 0.05 | 2.11 ± 0.05 | 3.41 ± 0.08 | 2.43 ± 0.05 | 0.10 ± 0.01 | 0.42 ± 0.01 | 0.19 ± 0.01 | 0.24 ± 0.01 |
| C-17:0 (Margaric) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.10 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.18 ± 0.01 | 0.13 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| C-17:1 (Margaroleic) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| C-18:0 (Stearic) | 2.81 ± 0.05 | 2.99 ± 0.05 | 4.54 ± 0.08 | 5.71 ± 0.10 | 4.00 ± 0.08 | 5.11 ± 0.10 | 4.06 ± 0.08 | 10.15 ± 0.20 | 4.10 ± 0.05 | 11.32 ± 0.20 | 3.69 ± 0.05 |
| C-18:1 (Oleic) | 77.80 ± 1.0 | 75.60 ± 1.0 | 39.80 ± 0.5 | 44.41 ± 0.5 | 48.57 ± 0.5 | 39.42 ± 0.5 | 48.43 ± 0.5 | 22.43 ± 0.5 | 32.19 ± 0.5 | 20.90 ± 0.5 | 32.45 ± 0.5 |
| C-18:2 (Linoleic) | 11.85 ± 0.20 | 12.93 ± 0.20 | 12.86 ± 0.20 | 21.47 ± 0.30 | 23.20 ± 0.30 | 27.19 ± 0.30 | 22.43 ± 0.30 | 11.81 ± 0.20 | 52.03 ± 0.50 | 34.09 ± 0.50 | 55.04 ± 0.50 |
| C-20:0 (Arachidic) | 0.27 ± 0.01 | 0.28 ± 0.01 | 0.39 ± 0.01 | 0.18 ± 0.01 | 0.28 ± 0.01 | 0.05 ± 0.01 | 0.26 ± 0.01 | 0.32 ± 0.01 | 0.26 ± 0.01 | 0.29 ± 0.01 | 0.26 ± 0.01 |
| C-18:3 (Linolenic) | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.53 ± 0.01 | 1.94 ± 0.05 | 3.06 ± 0.08 | 1.65 ± 0.05 | 3.10 ± 0.08 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 |
| C-20:1 (Eicosenoic) | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.19 ± 0.01 | 0.43 ± 0.01 | 0.61 ± 0.01 | 0.33 ± 0.01 | 0.57 ± 0.01 | 0.10 ± 0.01 | 0.17 ± 0.01 | 0.11 ± 0.01 | 0.17 ± 0.01 |
| C-22:0 (Behenic) | 0.85 ± 0.01 | 0.86 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.01 | 0.17 ± 0.01 | 0.75 ± 0.01 | 0.51 ± 0.01 | 0.80 ± 0.01 |
| C-22:1 (Erucic) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.01 | 0.01 ± 0.01 | 0.07 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| C-24:0 (Lignoceric) | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.10 ± 0.01 | 0.27 ± 0.01 | 0.21 ± 0.01 | 0.29 ± 0.01 |
| T. Oleic (t-C18:1) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.06 ± 0.01 | 0.14 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 |
| T. Linoleic + Linolenic | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.28 ± 0.01 | 0.12 ± 0.01 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.01 |
| Total Fats | 9.04 ± 0.10 | 9.84 ± 0.10 | 8.09 ± 0.10 | 3.81 ± 0.10 | 2.83 ± 0.10 | 1.81 ± 0.10 | 2.76 ± 0.10 | 34.4 ± 0.5 | 27.9 ± 0.5 | 25.6 ± 0.5 | 25.3 ± 0.5 |
| Total Trans Fatty Acids | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.35 ± 0.01 | 0.25 ± 0.01 | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.28 ± 0.01 | 0.18 ± 0.01 | 0.24 ± 0.01 | 0.11 ± 0.01 | 0.20 ± 0.01 |
| Sample | Acidity Index (mg KOH/g) | Anisidine Index (mg/g) | Peroxide Value (meq O2/kg) | Iodine Value (g I2/100 g) | Saponification Value (mg KOH/g) |
|---|---|---|---|---|---|
| CP | 1.94 ± 0.06 | 18.23 ± 0.25 | 12.5 ± 0.3 | 80 ± 2 | 190 ± 2 |
| CSC | 1.71 ± 0.05 | 1.39 ± 0.05 | 2.4 ± 0.08 | 66 ± 2 | 187 ± 2 |
| SP | 1.49 ± 0.05 | 15.68 ± 0.20 | 10.2 ± 0.3 | 78 ± 2 | 194 ± 2 |
| VSC | 0.79 ± 0.03 | 11.72 ± 0.15 | 6.8 ± 0.2 | 84 ± 2 | 197 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Ledezma, C.; Martínez-Hernandez, J.M.; Elango, J.; Garcia-Garrido, J.; Morillas-Ruiz, J.M.; Díaz-Cruces, E.; Miró-Colmenárez, P.J.; Zamora-Ledezma, E. Trans Fats in Spanish Pastries and Their Influence on Mesenchymal Stem Cell Behavior In Vitro and Related Health Risks. Foods 2025, 14, 2247. https://doi.org/10.3390/foods14132247
Zamora-Ledezma C, Martínez-Hernandez JM, Elango J, Garcia-Garrido J, Morillas-Ruiz JM, Díaz-Cruces E, Miró-Colmenárez PJ, Zamora-Ledezma E. Trans Fats in Spanish Pastries and Their Influence on Mesenchymal Stem Cell Behavior In Vitro and Related Health Risks. Foods. 2025; 14(13):2247. https://doi.org/10.3390/foods14132247
Chicago/Turabian StyleZamora-Ledezma, Camilo, José Manuel Martínez-Hernandez, Jeevithan Elango, Judit Garcia-Garrido, Juana María Morillas-Ruiz, Eliana Díaz-Cruces, Pablo Javier Miró-Colmenárez, and Ezequiel Zamora-Ledezma. 2025. "Trans Fats in Spanish Pastries and Their Influence on Mesenchymal Stem Cell Behavior In Vitro and Related Health Risks" Foods 14, no. 13: 2247. https://doi.org/10.3390/foods14132247
APA StyleZamora-Ledezma, C., Martínez-Hernandez, J. M., Elango, J., Garcia-Garrido, J., Morillas-Ruiz, J. M., Díaz-Cruces, E., Miró-Colmenárez, P. J., & Zamora-Ledezma, E. (2025). Trans Fats in Spanish Pastries and Their Influence on Mesenchymal Stem Cell Behavior In Vitro and Related Health Risks. Foods, 14(13), 2247. https://doi.org/10.3390/foods14132247









