Study on Rapeseed Albumin Hydrolysis by PrtS Protease from Streptococcus thermophilus and Bioactivity Characterization of Resulting Hydrolysates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Bacterial Strains, Culture, and Growth Conditions
2.3. Specific Activity of Cell-Wall Protease PrtS
2.4. Hydrolysis of Rapeseed Albumins by PrtS Protease
2.5. Characterization of Hydrolysates by Size-Exclusion High-Performance Liquid Chromatography (SE-HPLC) Analysis
2.6. Evaluation of Bioactivities
2.6.1. Anti-Inflammatory Activity
2.6.2. ABTS Radical-Scavenging Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proteolytic Activity of PrtS Protease from S. thermophilus LMD-9 and S. thermophilus 4F44 Strains
3.2. Study of Rapeseed Albumin Hydrolysis Using Concentrated Cell Pellets of S. thermophilus 4F44 and LMD-9 Strains
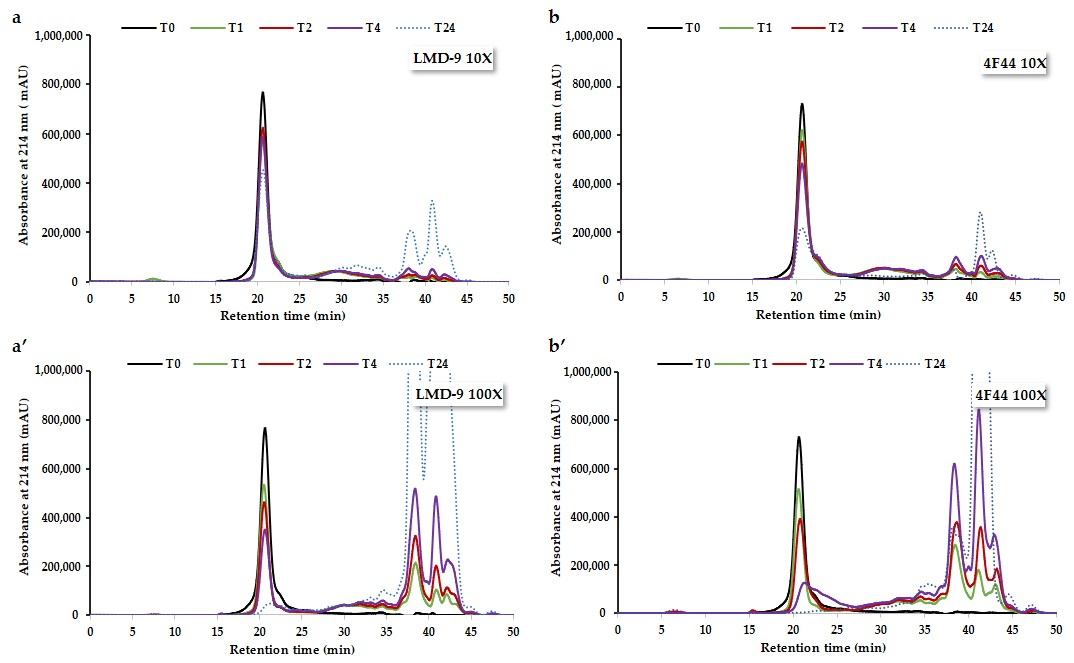
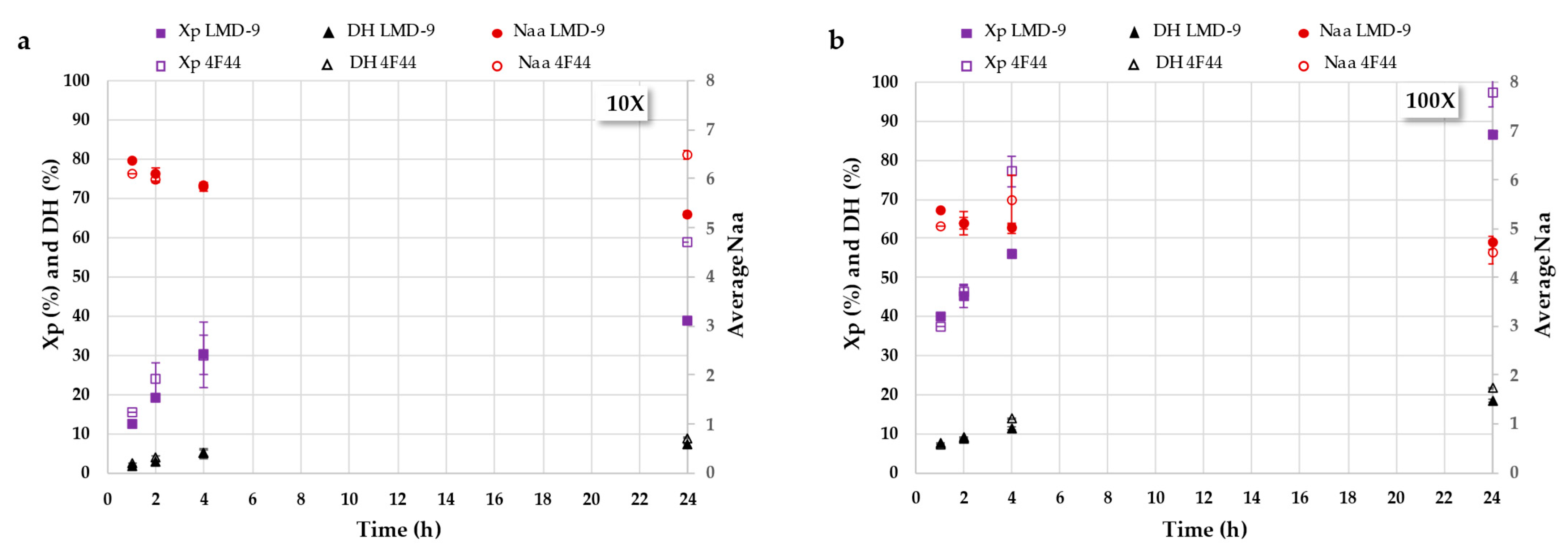
3.2.1. Hydrolysis Kinetics Study Using Size-Exclusion Chromatography Analysis
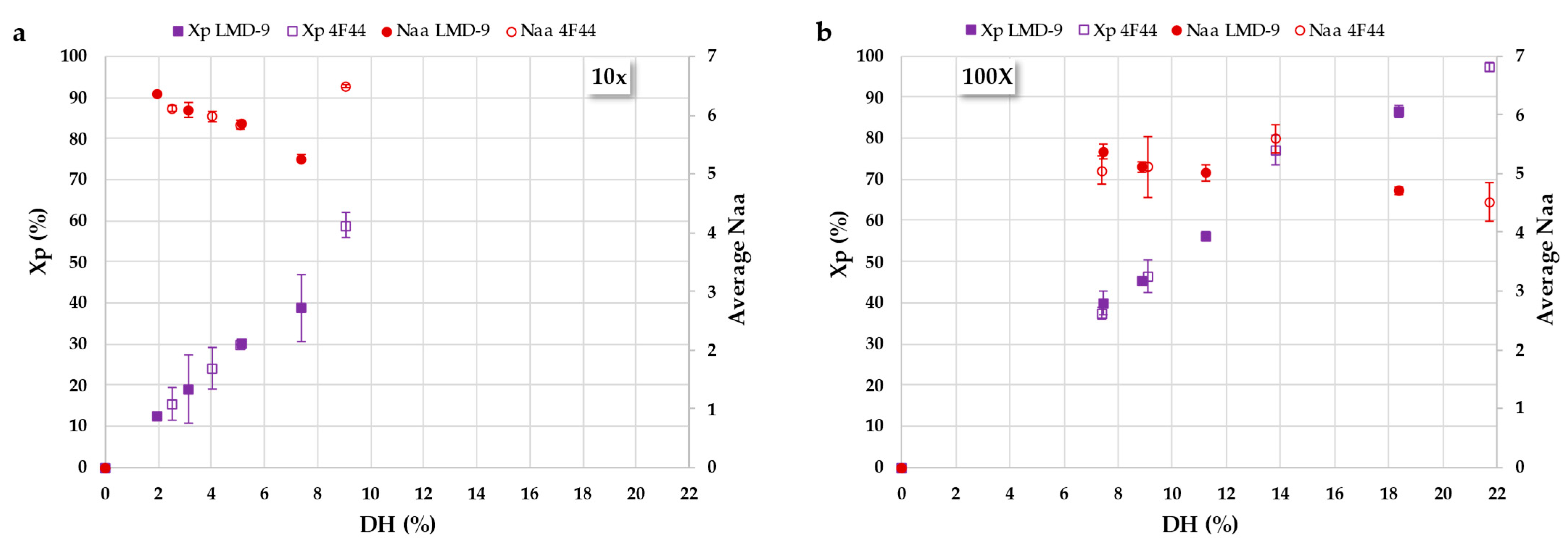
3.2.2. Deciphering Proteolysis Mechanism of Rapeseed Albumins by S. thermophilus PrtS Protease of Studied Strains
3.3. Characterization of Bioactivities of Rapeseed Albumin Hydrolysates Generated by PrtS Protease from S. thermophilus
3.3.1. Production of Rapeseed Albumin Hydrolysates Using Concentrated Cell Pellets of S. thermophilus LMD-9 Strain
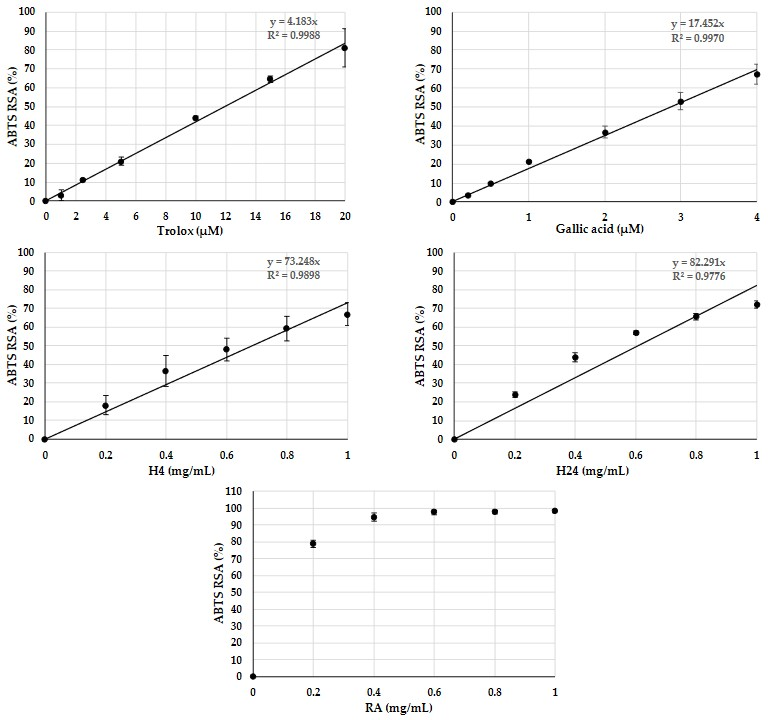
3.3.2. Antioxidant Activity of Rapeseed Albumin Hydrolysates
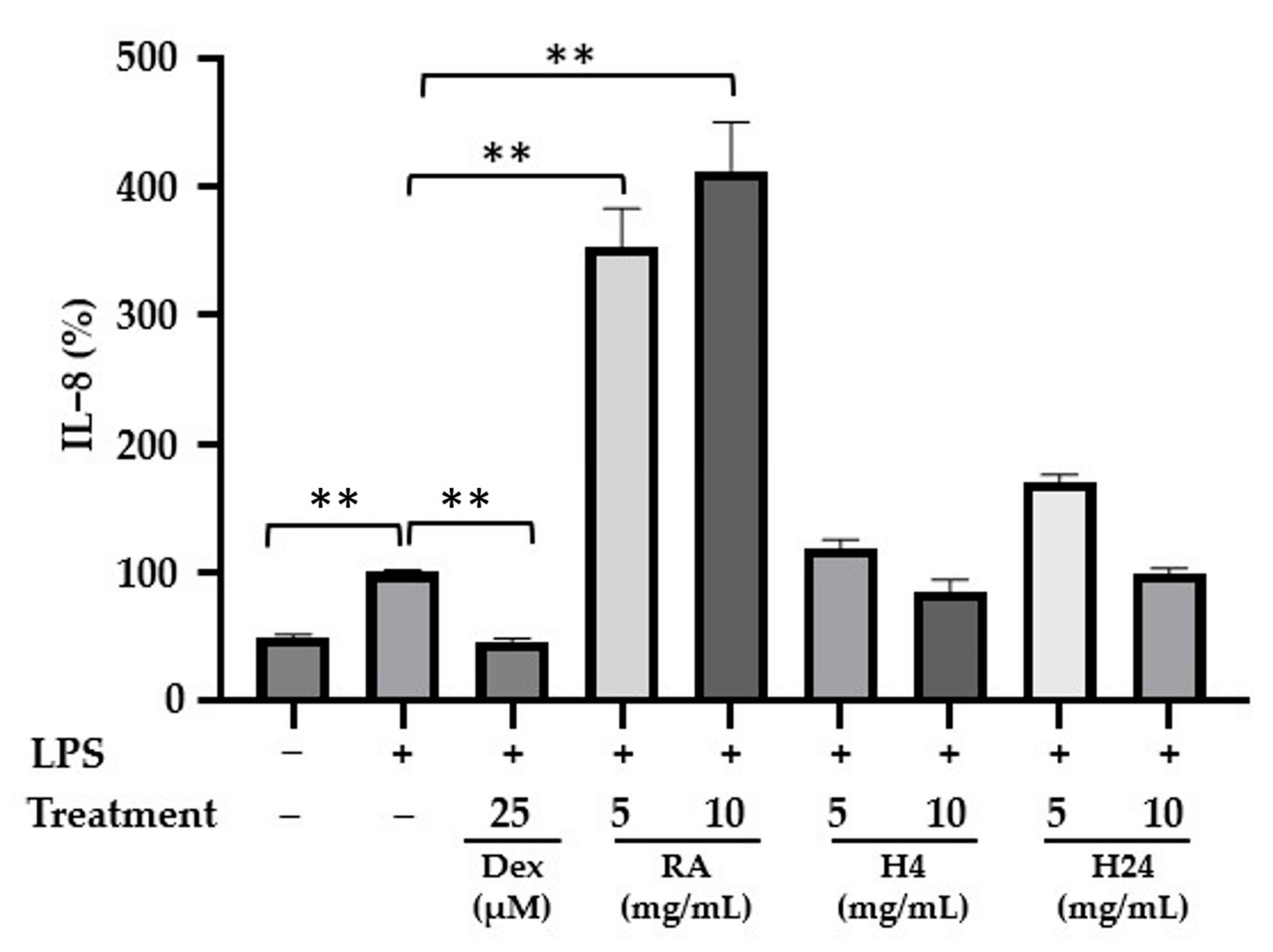
3.3.3. Anti-Inflammatory Activity of Rapeseed Albumin Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venter de Villiers, M.; Cheng, J.; Truter, L. The Shift Towards Plant-Based Lifestyles: Factors Driving Young Consumers’ Decisions to Choose Plant-Based Food Products. Sustainability 2024, 16, 9022. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Selinger, E.; Manuela, N.; Alina, K.; Jan, G.; Tilman, K.; Lukas, S.; Janett, B.; Schlesinger, S. Evidence of A Vegan Diet for Health Benefits and Risks—An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Suttikhana, I. Plant-Based Bioactive Peptides: A Review of Their Relevant Production Strategies, In Vivo Bioactivities, Action Mechanisms and Bioaccessibility. Int. J. Food Sci. Technol. 2023, 58, 2228–2235. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds Beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Bermejo-Cruz, M.; Osorio-Ruiz, A.; Rodríguez-Canto, W.; Betancur-Ancona, D.; Martínez-Ayala, A.; Chel-Guerrero, L. Antioxidant Potential of Protein Hydrolysates from Canola (Brassica napus L.) Seeds. Biocatal. Agric. Biotechnol. 2023, 50, 102687. [Google Scholar] [CrossRef]
- Durand, E.; Beaubier, S.; Ilic, I.; Fine, F.; Kapel, R.; Villeneuve, P. Production and Antioxidant Capacity of Bioactive Peptides from Plant Biomass to Counteract Lipid Oxidation. Curr. Res. Food Sci. 2021, 4, 365–397. [Google Scholar] [CrossRef]
- Durand, E.; Beaubier, S.; Fine, F.; Villeneuve, P.; Kapel, R. High Metal Chelating Properties from Rapeseed Meal Proteins to Counteract Lipid Oxidation in Foods: Controlled Proteolysis and Characterization. Eur. J. Lipid Sci. Technol. 2021, 123, 2000380. [Google Scholar] [CrossRef]
- Pudel, F.; Tressel, R.P.; Düring, K. Production and Properties of Rapeseed Albumin. Lipid Technol. 2015, 27, 112–114. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S.; Mitra, P. Canola/Rapeseed Protein-Functionality and Nutrition. OCL 2016, 23, D407. [Google Scholar] [CrossRef]
- Barciszewski, J.; Szymanski, M.; Haertlé, T. Minireview: Analysis of Rape Seed Napin Structure and Potential Roles of the Storage Protein. J. Protein Chem. 2000, 19, 249–254. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Jez, J.M. Review: The Promise and Limits for Enhancing Sulfur-Containing Amino Acid Content of Soybean Seed. Plant Sci. 2018, 272, 14–21. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed Protein-Derived ACE Inhibitory Peptides LY, RALP and GHS Show Antioxidant and Anti-inflammatory Effects on Spontaneously Hypertensive Rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- Beaubier, S.; Durand, E.; Lenclume, C.; Fine, F.; Aymes, A.; Framboisier, X.; Kapel, R.; Villeneuve, P. Chelating Peptides from Rapeseed Meal Protein Hydrolysates: Identification and Evaluation of Their Capacity to Inhibit Lipid Oxidation. Food Chem. 2023, 422, 136187. [Google Scholar] [CrossRef]
- Delorme, C. Safety Assessment of Dairy Microorganisms: Streptococcus thermophilus. Int. J. Food Microbiol. 2008, 126, 274–277. [Google Scholar] [CrossRef]
- Iyer, R.; Tomar, S.K.; Uma Maheswari, T.; Singh, R. Streptococcus thermophilus Strains: Multifunctional Lactic Acid Bacteria. Int. Dairy J. 2010, 20, 133–141. [Google Scholar] [CrossRef]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From Yogurt Starter to A New Promising Probiotic Candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Dargahi, N.; Matsoukas, J.; Apostolopoulos, V. Streptococcus thermophilus ST285 Alters Pro-Inflammatory to Anti-Inflammatory Cytokine Secretion against Multiple Sclerosis Peptide in Mice. Brain Sci. 2020, 10, 126. [Google Scholar] [CrossRef]
- Allouche, R.; Hafeez, Z.; Papier, F.; Dary-Mourot, A.; Genay, M.; Miclo, L. In Vitro Anti-Inflammatory Activity of Peptides Obtained by Tryptic Shaving of Surface Proteins of Streptococcus thermophilus LMD-9. Foods 2022, 11, 1157. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, T.; Qu, X.; Hu, T.; Jiang, X.; Zhao, C. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. Int. J. Mol. Sci. 2016, 17, 1701. [Google Scholar] [CrossRef] [PubMed]
- Dandoy, D.; Fremaux, C.; de Frahan, M.H.; Horvath, P.; Boyaval, P.; Hols, P.; Fontaine, L. The Fast Milk Acidifying Phenotype of Streptococcus thermophilus can be Acquired by Natural Transformation of the Genomic Island Encoding the Cell-Envelope Proteinase PrtS. Microb. Cell Fact. 2011, 10 (Suppl. 1), S21. [Google Scholar] [CrossRef]
- Rodriguez-Serrano, G.M.; Garcia-Garibay, M.; Cruz-Guerrero, A.E.; Gomez-Ruiz, L.; Ayala-Nino, A.; Castaneda-Ovando, A.; Gonzalez-Olivares, L.G. Proteolytic System of Streptococcus thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.M.; Jardin, J.; Perrin, C.; Dary, A.; Miclo, L. Hydrolysis of Milk-Derived Bioactive Peptides by Cell-Associated Extracellular Peptidases of Streptococcus thermophilus. Appl. Microbiol. Biotechnol. 2013, 97, 9787–9799. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.M.; Lecomte, X.; Paris, C.; Galia, W.; Dary, A.; Miclo, L. New Insights into the Proteolytic System of Streptococcus thermophilus: Use of Isracidin to Characterize Cell-Associated Extracellular Peptidase Activities. J. Agric. Food Chem. 2015, 63, 7522–7531. [Google Scholar] [CrossRef] [PubMed]
- Galia, W.; Perrin, C.; Genay, M.; Dary, A. Variability and Molecular Typing of Streptococcus thermophilus Strains Displaying Different Proteolytic and Acidifying Properties. Int. Dairy J. 2009, 19, 89–95. [Google Scholar] [CrossRef]
- Delorme, C.; Bartholini, C.; Bolotine, A.; Ehrlich, S.D.; Renault, P. Emergence of a Cell Wall Protease in the Streptococcus thermophilus Population. Appl. Environ. Microbiol. 2010, 76, 451–460. [Google Scholar] [CrossRef]
- Chang, O.K.; Perrin, C.; Galia, W.; Saulnier, F.; Miclo, L.; Roux, E.; Driou, A.; Humbert, G.; Dary, A. Release of the Cell-Envelope Protease PrtS in the Growth Medium of Streptococcus thermophilus 4F44. Int. Dairy J. 2012, 23, 91–98. [Google Scholar] [CrossRef]
- Miclo, L.; Roux, E.; Genay, M.; Brusseaux, E.; Poirson, C.; Jameh, N.; Perrin, C.; Dary, A. Variability of Hydrolysis of Beta-, Alphas1-, and Alphas2-Caseins by 10 Strains of Streptococcus thermophilus and Resulting Bioactive Peptides. J. Agric. Food Chem. 2012, 60, 554–565. [Google Scholar] [CrossRef]
- Chang, O.K.; Roux, É.; Awussi, A.A.; Miclo, L.; Jardin, J.; Jameh, N.; Dary, A.; Humbert, G.; Perrin, C. Use of a Free Form of the Streptococcus thermophilus Cell Envelope Protease PrtS as a Tool to Produce Bioactive Peptides. Int. Dairy J. 2014, 38, 104–115. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of Producing Bioactive Peptides from Milk Proteins to Functionalize Fermented Milk Products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Beaubier, S.; Pineda-Vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the In Vitro Digestibility of Rapeseed Albumins Resistant to Gastrointestinal Proteolysis While Preserving the Functional Properties Using Enzymatic Hydrolysis. Food Chem. 2023, 407, 135132. [Google Scholar] [CrossRef] [PubMed]
- Boulay, M.; Al Haddad, M.; Rul, F. Streptococcus thermophilus Growth in Soya Milk: Sucrose Consumption, Nitrogen Metabolism, Soya Protein Hydrolysis and Role of the Cell-Wall Protease PrtS. Int. J. Food Microbiol. 2020, 335, 108903. [Google Scholar] [CrossRef] [PubMed]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Defaix, C.; Kapel, R.; Galet, O. A Protein Isolate and Process for the Production Thereof. France Patent WO2019096862A1, 12 June 2019. [Google Scholar]
- Terzaghi, B.E.; Sandine, W.E. Improved Medium for Lactic Streptococci and Their Bacteriophages. Appl. Microbiol. 1975, 29, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Gault, S.; Jaworek, M.W.; Winter, R.; Cockell, C.S. High Pressures Increase α-Chymotrypsin Enzyme Activity Under Perchlorate Stress. Commun. Biol. 2020, 3, 550. [Google Scholar] [CrossRef]
- Beaubier, S.; Framboisier, X.; Ioannou, I.; Galet, O.; Kapel, R. Simultaneous Quantification of the Degree of Hydrolysis, Protein Conversion Rate and Mean Molar Weight of Peptides Released in the Course of Enzymatic Proteolysis. J. Chromatogr. B 2019, 1105, 1–9. [Google Scholar] [CrossRef]
- Sadat, L.; Cakir-Kiefer, C.; N’Negue, M.-A.; Gaillard, J.-L.; Girardet, J.-M.; Miclo, L. Isolation and Identification of Antioxidative Peptides from Bovine α-Lactalbumin. Int. Dairy J. 2011, 21, 214–221. [Google Scholar] [CrossRef]
- Galia, W.; Jameh, N.; Perrin, C.; Genay, M.; Dary-Mourot, A. Acquisition of PrtS in Streptococcus thermophilus is Not Enough in Certain Strains to Achieve Rapid Milk Acidification. Dairy Sci. Technol. 2016, 96, 623–636. [Google Scholar] [CrossRef]
- Beaubier, S.; Framboisier, X.; Fournier, F.; Galet, O.; Kapel, R. A New Approach for Modelling and Optimizing Batch Enzymatic Proteolysis. Chem. Eng. J. 2021, 405, 126871. [Google Scholar] [CrossRef]
- Chabanon, G.; Chevalot, I.; Framboisier, X.; Chenu, S.; Marc, I. Hydrolysis of Rapeseed Protein Isolates: Kinetics, Characterization and Functional Properties of Hydrolysates. Process Biochem. 2007, 42, 1419–1428. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Sato, H.H. Biologically Active Peptides: Processes for Their Generation, Purification and Identification and Applications as Natural Additives in the Food and Pharmaceutical Industries. Food Res. Int. 2015, 74, 185–198. [Google Scholar] [CrossRef]
- Beaubier, S.; Albe-Slabi, S.; Aymes, A.; Bianeis, M.; Galet, O.; Kapel, R. A Rational Approach for the Production of Highly Soluble and Functional Sunflower Protein Hydrolysates. Foods 2021, 10, 664. [Google Scholar] [CrossRef]
- Vorob’ev, M.M.; Paskonova, E.A.; Vitt, S.V.; Belikov, V.M. Kinetic Description of Proteolysis. Part 2. Substrate Regulation of Peptide Bond Demasking and Hydrolysis. Liquid Chromatography of Hydrolyzates. Nahrung 1986, 30, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Butré, C.I.; Sforza, S.; Wierenga, P.A.; Gruppen, H. Determination of the Influence of the pH of Hydrolysis on Enzyme Selectivity of Bacillus licheniformis Protease Towards Whey Protein Isolate. Int. Dairy J. 2015, 44, 44–53. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Liu, C.; Li, J. The Degradation, Antioxidant and Antimutagenic Activity of the Mucilage Polysaccharide from Dioscorea opposita. Carbohydr. Polym. 2016, 150, 227–231. [Google Scholar] [CrossRef]
- Bogatyreva, N.S.; Finkelstein, A.V.; Galzitskaya, O.V. Trend of Amino Acid Composition of Proteins of Different Taxa. J. Bioinform. Comput. Biol. 2006, 4, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Kapel, R.; Niquet-Léridon, C.; Tessier, F.; Mession, J.-L.; Buffière, C.; Hafnaoui, N.; Migné, C.; Houinsou-Houssou, B.; Riaublanc, A.; Solé, V.; et al. True Ileal Amino Acid Digestibility and Digestible Amino Acid Scores (DIAASs) of the Cruciferin and Napin Fractions of Rapeseed: Impact of Processing. Food Chem. 2025, 474, 143161. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models, 1st ed.; Springer: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar] [CrossRef]
- Byun, E.B.; Kim, W.S.; Sung, N.Y.; Byun, E.H. Epigallocatechin-3-Gallate Regulates Anti-Inflammatory Action Through 67-kDa Laminin Receptor-Mediated Tollip Signaling Induction in Lipopolysaccharide-Stimulated Human Intestinal Epithelial Cells. Cell Physiol. Biochem. 2018, 46, 2072–2081. [Google Scholar] [CrossRef]
- Shimizu, M. Interaction Between Food Substances and the Intestinal Epithelium. Biosci. Biotechnol. Biochem. 2010, 74, 232–241. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.; Xu, F.; Yuan, Q.; Ju, X.; Wang, L. Anti-Inflammatory and Transepithelial Transport Activities of Rapeseed (Brassica napus) Napin-Derived Dipeptide Thr-Leu in Caco-2 and RAW264.7 Cocultures. J. Agric. Food Chem. 2023, 71, 8437–8447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, H.; Liu, X. A Novel Fermented Rapeseed Meal, Inoculated with Selected Protease-Assisting Screened B. subtilis YY-4 and L. Plantarum 6026, Showed High Availability and Strong Antioxidant and Immunomodulation Potential Capacity. Foods 2022, 11, 2118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Wang, R.; Hao, X.; Duan, Y.; Meng, Z.; An, X.; Qi, J. Dietary Fermented Soybean Meal Inclusion Improves Growth Performance and Ileal Barrier Function of the Weaned Piglets Challenged by Enterotoxigenic Escherichia Coli K88. Anim. Feed Sci. Technol. 2020, 268, 114596. [Google Scholar] [CrossRef]
- Luti, S.; Mazzoli, L.; Ramazzotti, M.; Galli, V.; Venturi, M.; Marino, G.; Lehmann, M.; Guerrini, S.; Granchi, L.; Paoli, P.; et al. Antioxidant and Anti-Inflammatory Properties of Sourdoughs Containing Selected Lactobacilli Strains are Retained in Breads. Food Chem. 2020, 322, 126710. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, Z.; Beaubier, S.; Aymes, A.; Christophe, S.; Akbar, S.; Kapel, R.; Miclo, L. Study on Rapeseed Albumin Hydrolysis by PrtS Protease from Streptococcus thermophilus and Bioactivity Characterization of Resulting Hydrolysates. Foods 2025, 14, 2235. https://doi.org/10.3390/foods14132235
Hafeez Z, Beaubier S, Aymes A, Christophe S, Akbar S, Kapel R, Miclo L. Study on Rapeseed Albumin Hydrolysis by PrtS Protease from Streptococcus thermophilus and Bioactivity Characterization of Resulting Hydrolysates. Foods. 2025; 14(13):2235. https://doi.org/10.3390/foods14132235
Chicago/Turabian StyleHafeez, Zeeshan, Sophie Beaubier, Arnaud Aymes, Ségolène Christophe, Samina Akbar, Romain Kapel, and Laurent Miclo. 2025. "Study on Rapeseed Albumin Hydrolysis by PrtS Protease from Streptococcus thermophilus and Bioactivity Characterization of Resulting Hydrolysates" Foods 14, no. 13: 2235. https://doi.org/10.3390/foods14132235
APA StyleHafeez, Z., Beaubier, S., Aymes, A., Christophe, S., Akbar, S., Kapel, R., & Miclo, L. (2025). Study on Rapeseed Albumin Hydrolysis by PrtS Protease from Streptococcus thermophilus and Bioactivity Characterization of Resulting Hydrolysates. Foods, 14(13), 2235. https://doi.org/10.3390/foods14132235






