Effects of Saccharomyces cerevisiae Fermentation on Off-Odour Reduction and Flavour Compounds in Pig Large Intestines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Saccharomyces cerevisiae
2.3. Samples Preparation
2.4. Determination of Colour
2.5. Determination of the pH Value
2.6. Determination of the TBARS Value
2.7. Determination of the TVB-N Value
2.8. Sensory Evaluation
2.9. SPME Processing and Volatile Substance Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Colours of the PLIs Under Different Treatments
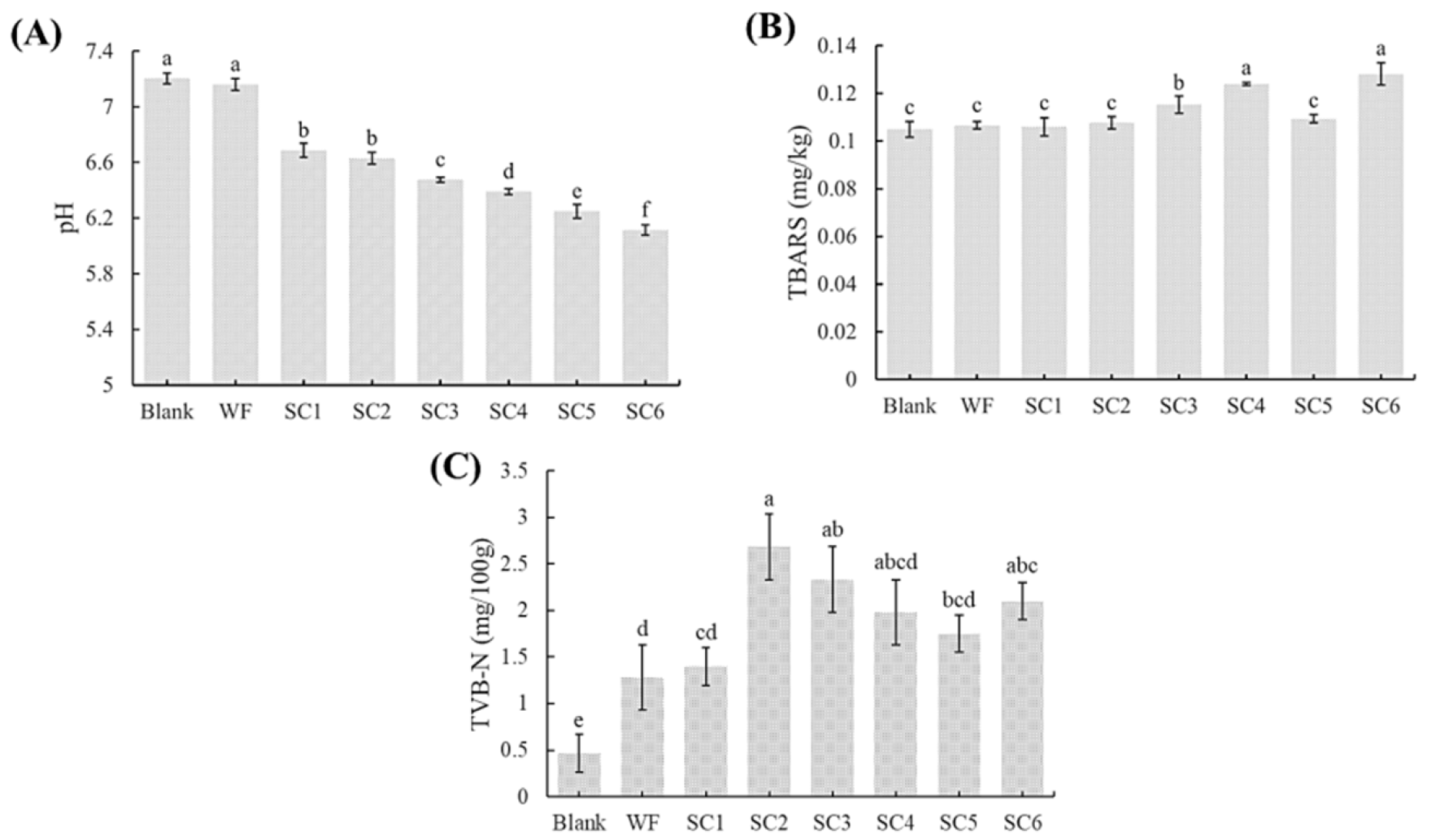
3.2. pH Values of PLIs Under Different Treatments
3.3. TBARS Values of PLIs Under Different Treatments
3.4. TVB-N Values of PLIs Under Different Treatments
3.5. Sensory Evaluation of PLIs Under Different Treatments
3.6. Flavouromics Analysis of PLIs Under Different Treatments
3.6.1. PCA
3.6.2. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
3.6.3. Volatile Compounds of PLIs Under Different Treatments
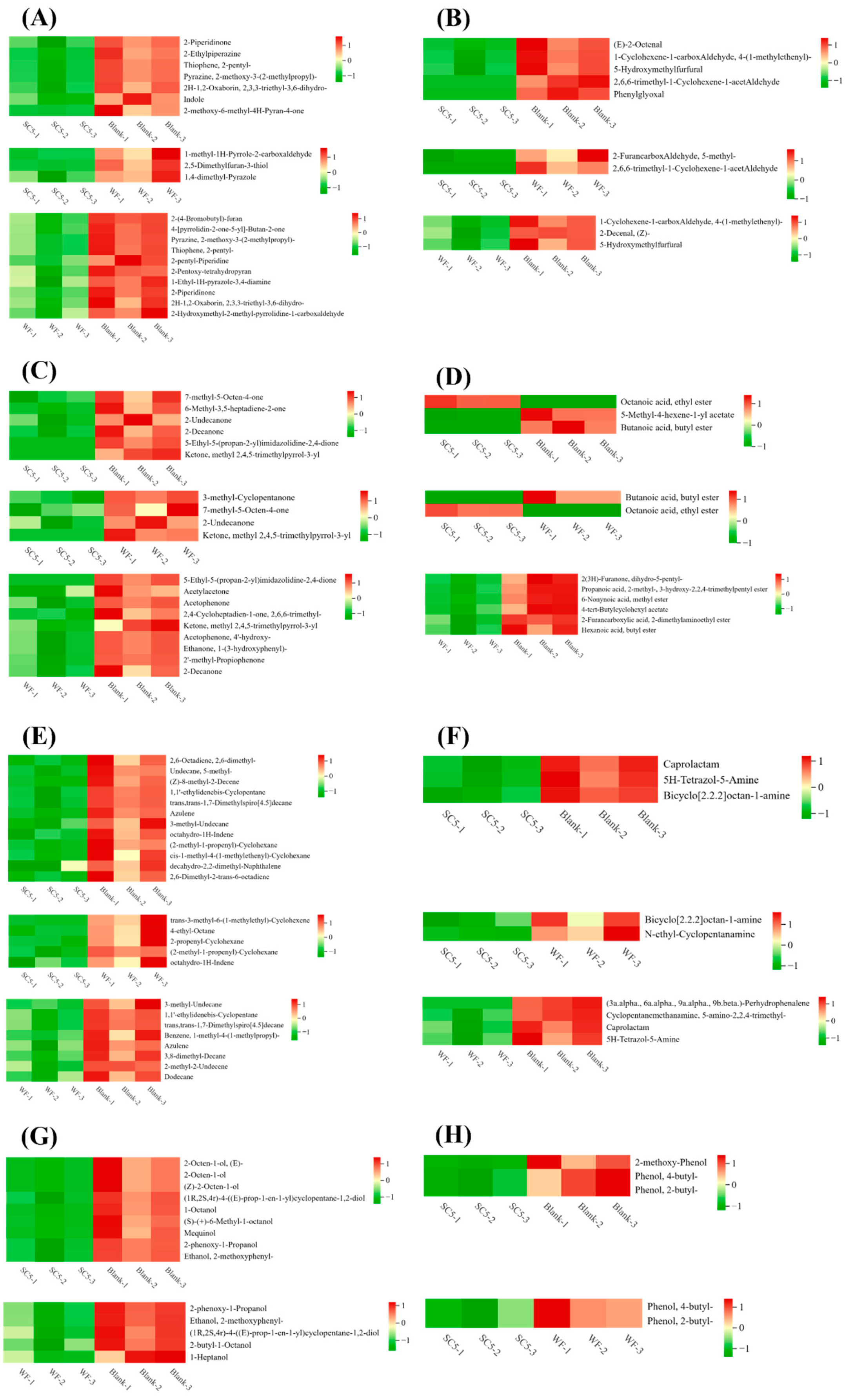
3.6.4. Identification and Analysis of Differentially Abundant Metabolites
Heterocyclic Compounds
Aldehydes
Ketones
Esters
Hydrocarbons
Amines
Alcohols
Phenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Zang, M.; Wang, S.; Zhang, Z.; Li, D.; Li, X.; Zhao, Y. Effects of defatting and cooking methods on pig large intestines volatile compounds by flavor omics and fatty acids. LWT 2023, 189, 115500. [Google Scholar] [CrossRef]
- Li, X.M.; Zang, M.W.; Li, D.; Wang, S.W.; Zhang, K.H.; Zhang, Z.Q.; Zhao, B.; Zhang, S.L.; Zhao, Y. Effect of boiled-water-cooking time on quality and characteristic of flavor components in pig large intestines. Int. J. Gastron. Food Sci. 2024, 35, 100899. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, Y.E.; Kim, C.H.; Min, J.S.; Yim, D.G.; Jo, C. Determining the Optimal Cooking Time for Cooking Loss, Shear Force, and Off-Odor Reduction of Pork Large Intestines. Food Sci. Anim. Resour. 2022, 42, 332–340. [Google Scholar] [CrossRef]
- Kohara, K.; Kadomoto, R.; Kozuka, H.; Sakamoto, K.; Hayata, Y. Deodorizing effect of coriander on the offensive odor of the porcine large intestine. Food Sci. Technol. Res. 2006, 12, 38–42. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Huang, Y.Z.; Wang, Z.M.; Cai, S.H.; Zhu, B.W.; Dong, X.P. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Park, S.-K.; Lee, J.-H.; Jo, D.-M.; Kang, M.-G.; Jang, Y.-M.; Cho, Y.-J.; Hong, D.-L.; Kim, Y.-M. Reduction of Trimethylamine by Saccharomyces cerevisiae Isolated from Fermented Food. Korean J. Fish. Aquat. Sci. 2019, 52, 121–126. [Google Scholar] [CrossRef]
- Gao, R.C.; Li, X.; Liu, H.J.; Cui, Y.X.; Wu, X.Y.; Jin, W.G.; Yuan, L. Optimization of removal of off-odor in mullet (Channa argus) head soup by yeast using response surface methodology and variations of volatile components during fermentation. J. Food Process Preserv. 2021, 45, e15920. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.N.; Goff, H.D.; Zhong, F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Xiong, Z.J.; Sun, D.W.; Pu, H.B.; Xie, A.G.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituric acid reactive substances (TSARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef]
- Fu, Z.X.; Li, Y.C.; Zhang, C.Y.; Chen, W.J.; Meng, F.B.; Liu, D.Y. Nontargeted metabolomics reveals dynamic changes in the quality of fresh yak meat during ice-temperature preservation. LWT 2024, 206, 116579. [Google Scholar] [CrossRef]
- Shi, S.Y.; Wen, J.P.; Geng, H.; Zhan, X.B.; Liu, Y.X. Physicochemical properties, structural properties and gels 3D printing properties of wheat starch. Int. J. Biol. Macromol. 2024, 261, 129885. [Google Scholar] [CrossRef] [PubMed]
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Olayanju, A. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control 2020, 110, 106963. [Google Scholar] [CrossRef]
- Aung, T.; Eun, J.B. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Wang, S.; Zhou, Y.A.; Nie, Q.; Zhou, C.Y.; Ning, J.W.; Ren, C.P.; Tang, C.; Zhang, J.M. Effects of Saccharomyces cerevisiae and Kluyveromyces marxianus on the Physicochemical, Microbial, and Flavor Changes of Sauce Meat during Storage. Foods 2024, 13, 396. [Google Scholar] [CrossRef]
- Lin, H.X.; Zhao, S.S.; Han, X.Y.; Guan, W.Q.; Liu, B.; Chen, A.Q.; Sun, Y.S.; Wang, J.Y. Effect of static magnetic field extended supercooling preservation on beef quality. Food Chem. 2022, 370, 131264. [Google Scholar] [CrossRef]
- Liu, Y.L.; Cao, Y.T.; Woldemariam, K.Y.; Zhong, S.J.; Yu, Q.L.; Wang, J. Antioxidant effect of yeast on lipid oxidation in salami sausage. Front. Microbiol. 2023, 13, 1113848. [Google Scholar] [CrossRef]
- Cao, Y.J.; Hao, R.; Guo, Z.L.; Han, L.; Yu, Q.L.; Zhang, W. Combined effects of superchilling and natural extracts on beef preservation quality. LWT 2022, 153, 112520. [Google Scholar] [CrossRef]
- Bekhit, A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- GB/T 9959.2-2008; Fresh and Frozen Pork Lean Cuts. National Standards of the People’s Republic of China: Beijing, China, 2008.
- Kang, C.D.; Zhang, Y.Y.; Zhang, M.Y.; Qi, J.; Zhao, W.T.; Gu, J.; Guo, W.P.; Li, Y.Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Heredia, J.Z.; Wagner, M.; Jofré, F.C.; Savio, M.; Azcarate, S.M.; Camiña, J.M. An overview on multi-elemental profile integrated with chemometrics for food quality assessment: Toward new challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 8173–8193. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Yu, Y.M.; Saleh, A.S.M.; Sun, X.X.; Wang, Z.Y.; Lu, Y.; Zhang, D.Q.; Zhang, C.J. Exploring the interaction between myofibrillar proteins and pyrazine compounds: Based on molecular docking, molecular dynamics simulation, and multi-spectroscopy techniques. Int. J. Biol. Macromol. 2023, 253, 126844. [Google Scholar] [CrossRef]
- Mottram, D.S.; Edwards, R.A. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agric. 1983, 34, 517–522. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Chen, G.H.; Liu, X.; Ouyang, Y.; Yu, W.; Xiong, G.Q.; Wang, L.; Wang, C.; Qiao, Y. Influence of thermal processing on sensory profile of crayfish meat. Int. J. Gastron. Food Sci. 2024, 37, 100992. [Google Scholar] [CrossRef]
- Yu, X.P.; Chen, L.; Sheng, L.; Tong, Q.Y. Volatile Compounds Analysis and Off-Flavors Removing of Porcupine Liver. Food Sci. Technol. Res. 2016, 22, 283–289. [Google Scholar] [CrossRef]
- Squires, E.J.; Bone, C.; Cameron, J. Pork Production with Entire Males: Directions for Control of Boar Taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef]
- Zhang, J.L.; Hu, Y.W.; Wang, S.J.; Liu, Y.B.; Li, L.; Gao, M.X. Non-targeted metabolomics analyze dough fermented by S. cerevisiae and L. plantarum to reveal the formation of flavor substances of bread. LWT 2023, 176, 114538. [Google Scholar] [CrossRef]
- Wang, R.; Yang, C.; Song, H.L. Key meat flavour compounds formation mechanism in a glutathione-xylose Maillard reaction. Food Chem. 2012, 131, 280–285. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.C.; Zhao, M.Y.; Hou, L.; Liang, J.J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef]
- Qiu, L.Q.; Zhang, M.; Chang, L. Effects of lactic acid bacteria fermentation on the phytochemicals content, taste and aroma of blended edible rose and shiitake beverage. Food Chem. 2023, 405, 134722. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.H.; Zeng, M.Y.; Xu, X.X. Research progress of fishy odor in aquatic products: From substance identification, formation mechanism, to elimination pathway. Food Res. Int. 2024, 178, 113914. [Google Scholar] [CrossRef]
- Nedele, A.K.; Gross, S.; Rigling, M.; Zhang, Y.Y. Reduction of green off-flavor compounds: Comparison of key odorants during fermentation of soy drink with Lycoperdon pyriforme. Food Chem. 2021, 334, 127591. [Google Scholar] [CrossRef]
- Vermeulen, N.; Czerny, M.; Gänzle, M.G.; Schieberle, P.; Vogel, R.F. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 2007, 45, 78–87. [Google Scholar] [CrossRef]
- Xiang, L.B.; Zhu, W.Y.; Jiang, B.; Chen, J.J.; Zhou, L.; Zhong, F. Volatile compounds analysis and biodegradation strategy of beany flavor in pea protein. Food Chem. 2023, 402, 134275. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.Q.; Yu, H.Y.; Chen, Z.Y.; Tian, H.X. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juiceInfluence of 4 lactic acid bacteria. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.R.; Zhao, Q.Y.; Lei, Y.S.; Gao, C.X.; Yuan, Q.Y.; Sun, X.Y.; Liu, X.B.; Ma, T.T. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef]
- Zhang, L.X.; Qin, Z.H.; Zhang, L.; Jiang, Y.J.; Zhu, J.L. Dynamic changes of quality and flavor characterization of Zhejiang rosy vinegar during fermentation and aging based on untargeted metabolomics. Food Chem. 2023, 404, 134702. [Google Scholar] [CrossRef]
- Yen, P.P.L.; Pratap-Singh, A. Vacuum microwave dehydration decreases volatile concentration and soluble protein content of pea (Pisum sativum L.) protein. J. Sci. Food Agric. 2021, 101, 167–178. [Google Scholar] [CrossRef]
- Wei, R.; Jiang, B.; Chen, J.J.; Xiang, L.B.; Liu, X.Y. Removal of fishy flavor in kelp (Laminaria japonica) by natural antioxidant soaking combined with microbial fermentation. Food Biosci. 2024, 60, 104212. [Google Scholar] [CrossRef]
- Yu, M.G.; Wang, B.S.; Wang, Y.; Tang, Y.; Liu, C.; Song, H.L.; Hou, B.C.; Li, B.L.; Zhao, W. Odor profile characterization and variety identification of brown lactobacillus beverage based on untargeted metabolomics. J. Food Compos. Anal. 2023, 120, 105293. [Google Scholar] [CrossRef]
- Niu, Y.W.; Chen, X.M.; Xiao, Z.B.; Ma, N.; Zhu, J.C. Characterization of aroma-active compounds in three Chinese Moutai liquors by gas chromatography-olfactometry, gas chromatography-mass spectrometry and sensory evaluation. Nat. Prod. Res. 2017, 31, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.J.; Yang, C.; Yang, Y.D.; Peng, B.Z. Analysis of the Formation of Characteristic Aroma Compounds by Amino Acid Metabolic Pathways during Fermentation with Saccharomyces cerevisiae. Molecules 2023, 28, 3100. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, D.Y.; Zhou, G.H.; Xu, X.L. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, J.M.; Zhang, K.; Qi, Y.J.; Ma, W.; Wang, Z.B.; Ente, M.; Li, K. Analysis of volatiles from feces of released Przewalski’s horse (Equus przewalskii) in Gasterophilus pecorum (Diptera: Gasterophilidae) spawning habitat. Sci. Rep. 2021, 11, 15671. [Google Scholar] [CrossRef]
- Han, B.; Tang, Y.Y.; Xie, Y.D.; Liu, H.Y.; Zhou, H.; Wu, S.H.; Zhan, J.C.; Huang, W.D.; You, Y.L. The characteristics of histamine and tyramine degradation of Saccharomyces cerevisiae HL10 and HL17 and their application in wine fermentation. Food Microbiol. 2025, 131, 104804. [Google Scholar] [CrossRef]
- Huang, D.; Zhong, Y.; Liu, Y.L.; Song, Y.Y.; Zhao, X.X.; Qin, Y. Reducing higher alcohols by integrating indigenous Saccharomyces cerevisiae, nitrogen compensation, and chaptalization methods during fermentation of kiwifruit wine. LWT 2023, 184, 115059. [Google Scholar] [CrossRef]
- Guymon, J.F.; Crowell, E.A.; Ingraham, J.L. Formation of n-propyl alcohol by saccharomyces cerevisiae. Arch. Biochem. Biophys. 1961, 95, 163–168. [Google Scholar] [CrossRef]
- Yu, A.N.; Sun, B.G. Flavour substances of Chinese traditional smoke-cured bacon. Food Chem. 2005, 89, 227–233. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhu, B.Y.; Liang, K.; Zhang, Y.S.; Song, J.; Tu, L.N.; Zheng, Y.; Wang, M. Identification of phenols and their formation network during the brewing process of Shanxi aged vinegar. Food Chem. 2025, 470, 142635. [Google Scholar] [CrossRef]
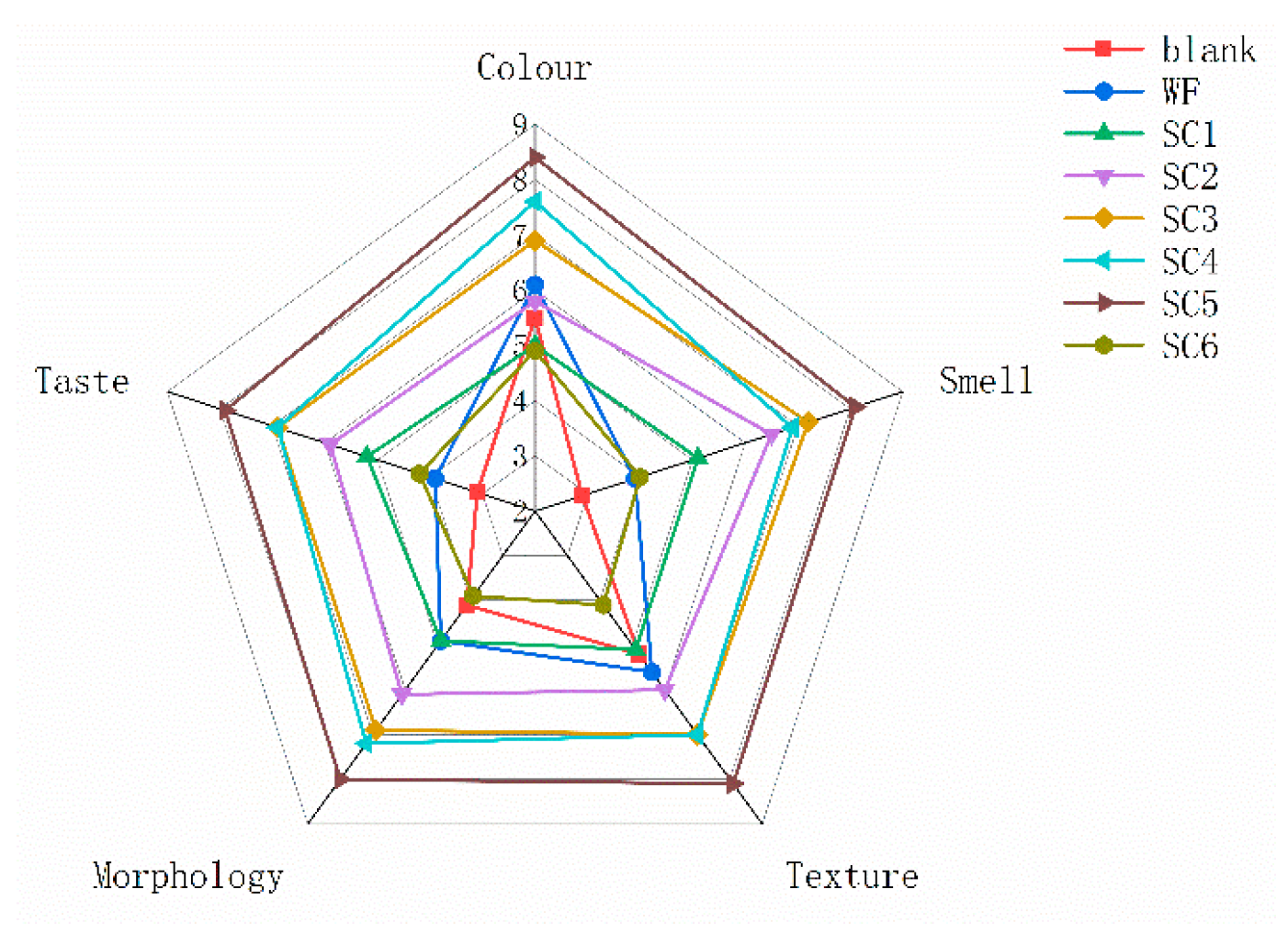



| Items | Standards of Grading | Score |
|---|---|---|
| Colour | The colour is uniform with lustre | 8~10 |
| The colour is too dark or too light with slight lustre | 5~7 | |
| The colour is uneven with mottled and poor gloss | 1~4 | |
| Smell | The smell is mellow and lacks an off-odour smell, along with a slight wine aroma | 8~10 |
| The smell is moderate with slightly off-odours of the PLIs, along with a light wine aroma | 5~7 | |
| The smell has obvious off-odours of the PLIs without a wine aroma | 1~4 | |
| Texture | Elasticity is excellent, and the texture is layered | 8~10 |
| Elasticity is moderate, and the texture is good | 5~7 | |
| The texture is very hard and inelastic | 1~4 | |
| Morphology | The structure of the tissue morphology is complete and is not sticky | 8~10 |
| The structure of the tissue morphology is relatively complete and is slightly sticky | 5~7 | |
| The structure of the tissue morphology is loose and has a sticky surface | 1~4 | |
| Taste | The taste is coordinated, and the flavour of the PLIs is well combined with the fermentation flavour | 8~10 |
| The taste is general, and the integration of the flavour of the PLIs with the flavour of the fermentation is insufficient | 5~7 | |
| The taste is poor, and the flavour of the PLIs and the flavour of the fermentation are not integrated | 1~4 |
| Samples | L* | a* | b* |
|---|---|---|---|
| Blank | 72.24 ± 0.95 ab | 2.09 ± 0.19 b | 11.25 ± 0.54 cd |
| WF | 74.30 ± 2.05 a | 2.53 ± 1.63 b | 10.77 ± 0.90 d |
| SC1 | 68.26 ± 3.92 b | 4.64 ± 0.21 a | 12.15 ± 0.03 bc |
| SC2 | 70.76 ± 1.96 ab | 4.39 ± 0.10 a | 12.53 ± 0.38 b |
| SC3 | 72.40 ± 2.12 ab | 4.56 ± 0.11 a | 12.98 ± 0.65 ab |
| SC4 | 73.21 ± 3.89 a | 5.03 ± 0.31 a | 12.54 ± 0.65 b |
| SC5 | 74.55 ± 1.25 a | 5.04 ± 0.10 a | 13.97 ± 0.53 a |
| SC6 | 70.32 ± 1.39 ab | 4.51 ± 0.92 a | 13.09 ± 0.36 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-X.; Li, Y.-C.; Cao, Z.; Li, X.; Zhang, L.; Meng, F.-B.; Zhou, Y.-H. Effects of Saccharomyces cerevisiae Fermentation on Off-Odour Reduction and Flavour Compounds in Pig Large Intestines. Foods 2025, 14, 2204. https://doi.org/10.3390/foods14132204
Liang Y-X, Li Y-C, Cao Z, Li X, Zhang L, Meng F-B, Zhou Y-H. Effects of Saccharomyces cerevisiae Fermentation on Off-Odour Reduction and Flavour Compounds in Pig Large Intestines. Foods. 2025; 14(13):2204. https://doi.org/10.3390/foods14132204
Chicago/Turabian StyleLiang, Ye-Xing, Yun-Cheng Li, Zheng Cao, Xue Li, Ling Zhang, Fan-Bing Meng, and Yong-Hua Zhou. 2025. "Effects of Saccharomyces cerevisiae Fermentation on Off-Odour Reduction and Flavour Compounds in Pig Large Intestines" Foods 14, no. 13: 2204. https://doi.org/10.3390/foods14132204
APA StyleLiang, Y.-X., Li, Y.-C., Cao, Z., Li, X., Zhang, L., Meng, F.-B., & Zhou, Y.-H. (2025). Effects of Saccharomyces cerevisiae Fermentation on Off-Odour Reduction and Flavour Compounds in Pig Large Intestines. Foods, 14(13), 2204. https://doi.org/10.3390/foods14132204






