Physicochemical Properties and Biological Activities of Polysaccharides from Dendrobium officinale Leaves in Response to Different Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
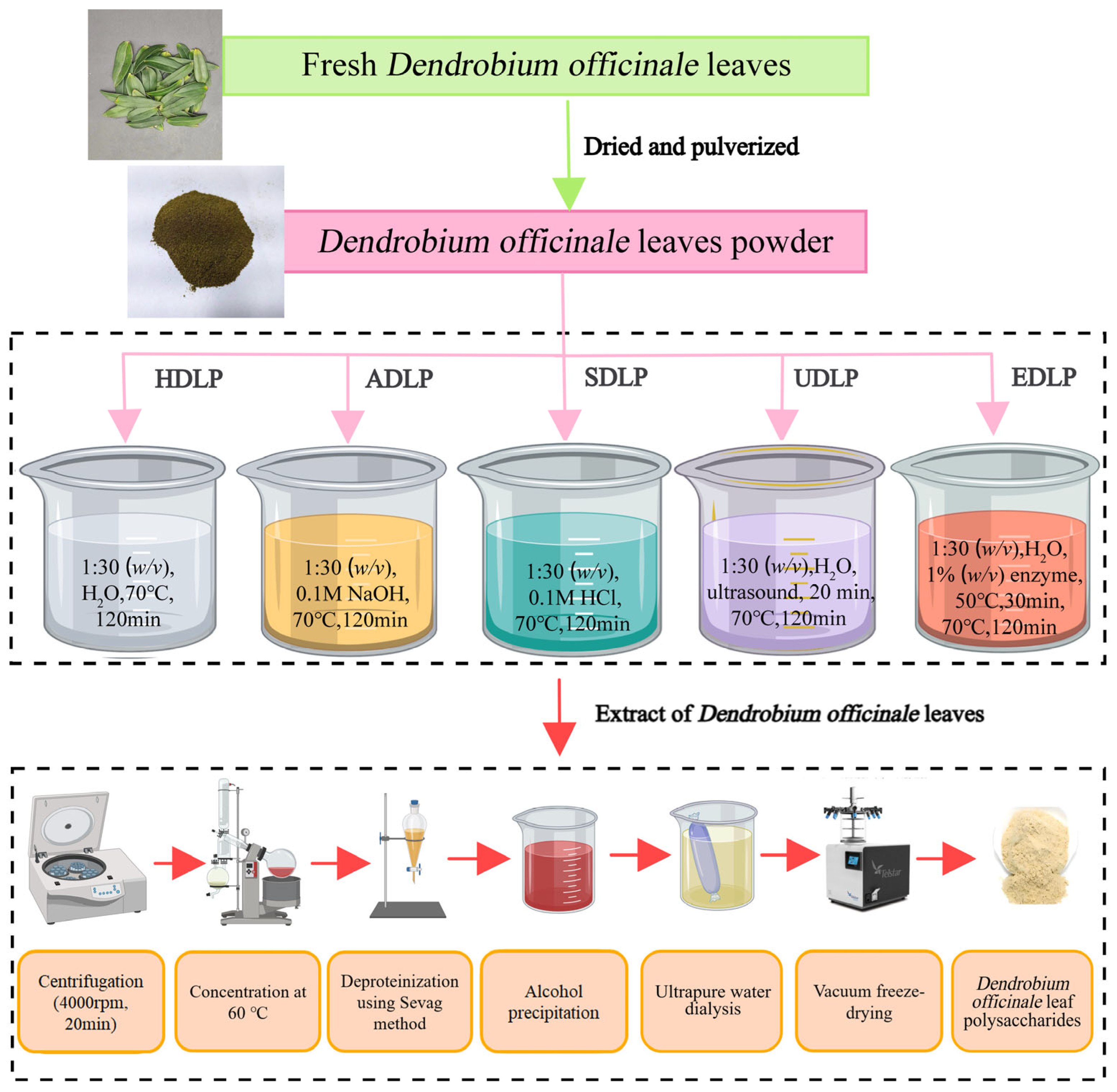
2.2. Extraction of Polysaccharides
2.2.1. Hot Water Extraction
2.2.2. Ultrasound-Assisted Extraction
2.2.3. Acid and Alkali Extraction
2.2.4. Enzyme-Assisted Extraction
2.3. Physicochemical Characteristics Analyses
2.3.1. Chemical Composition
2.3.2. Molecular Weight Determination
2.3.3. Monosaccharide Composition Analysis
2.3.4. Congo Red Test
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Fourier Infrared (FT-IR) Spectral Scanning
2.4. Adsorption Capacity Determination
2.4.1. Cholesterol Adsorption Capacity (CAC)
2.4.2. Nitrite Adsorption Capacity (NAC)
2.5. Antioxidant Activity Analysis
2.6. Immunostimulatory Activity Analysis
2.6.1. Cell Culture
2.6.2. Cell Viability Assay
2.6.3. Phagocytosis
2.6.4. NO, TNF-α, IL-1β and IL-6 Concentrations Determination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield and Chemical Composition
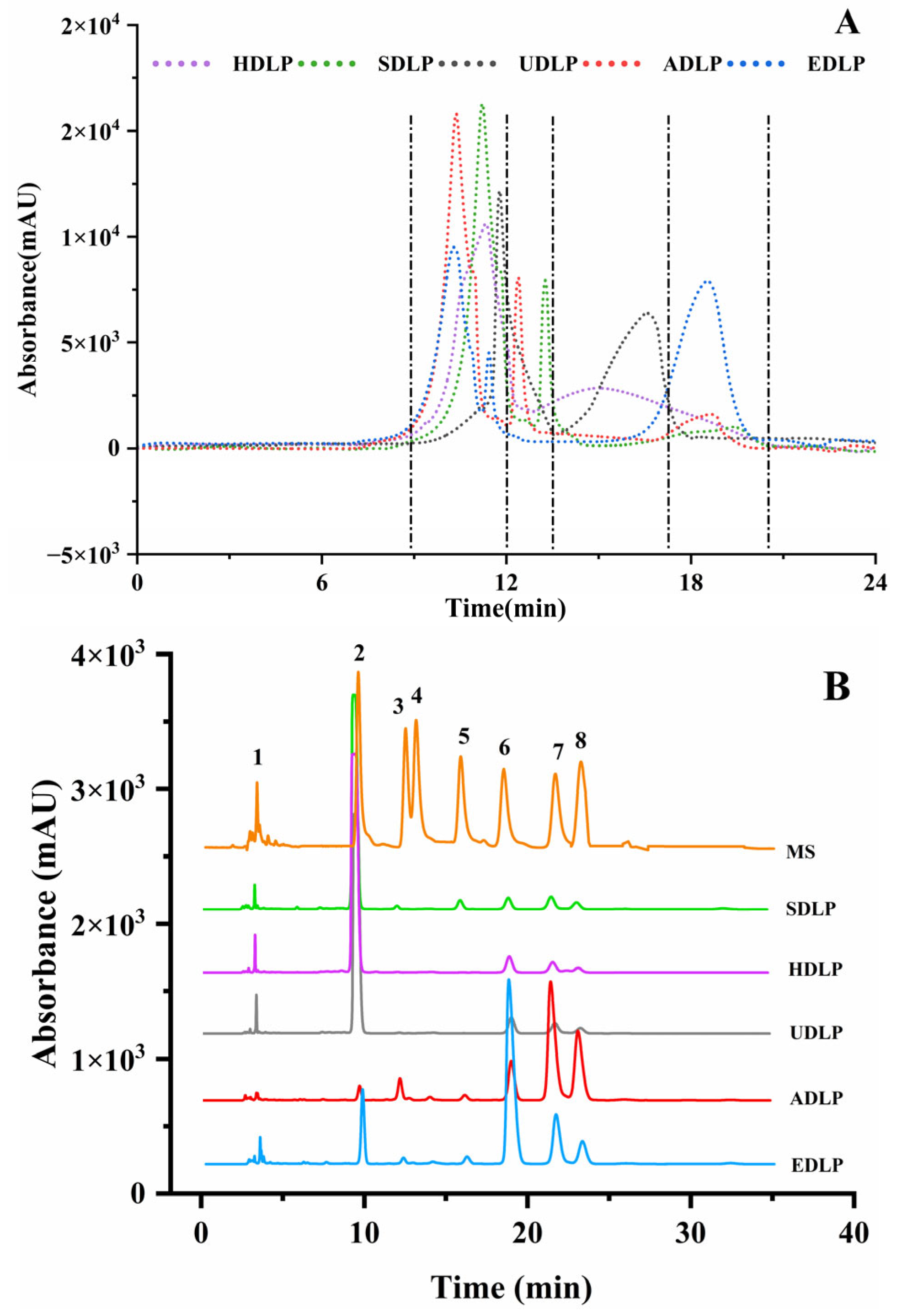
3.2. Molecular Weight
3.3. Monosaccharide Composition
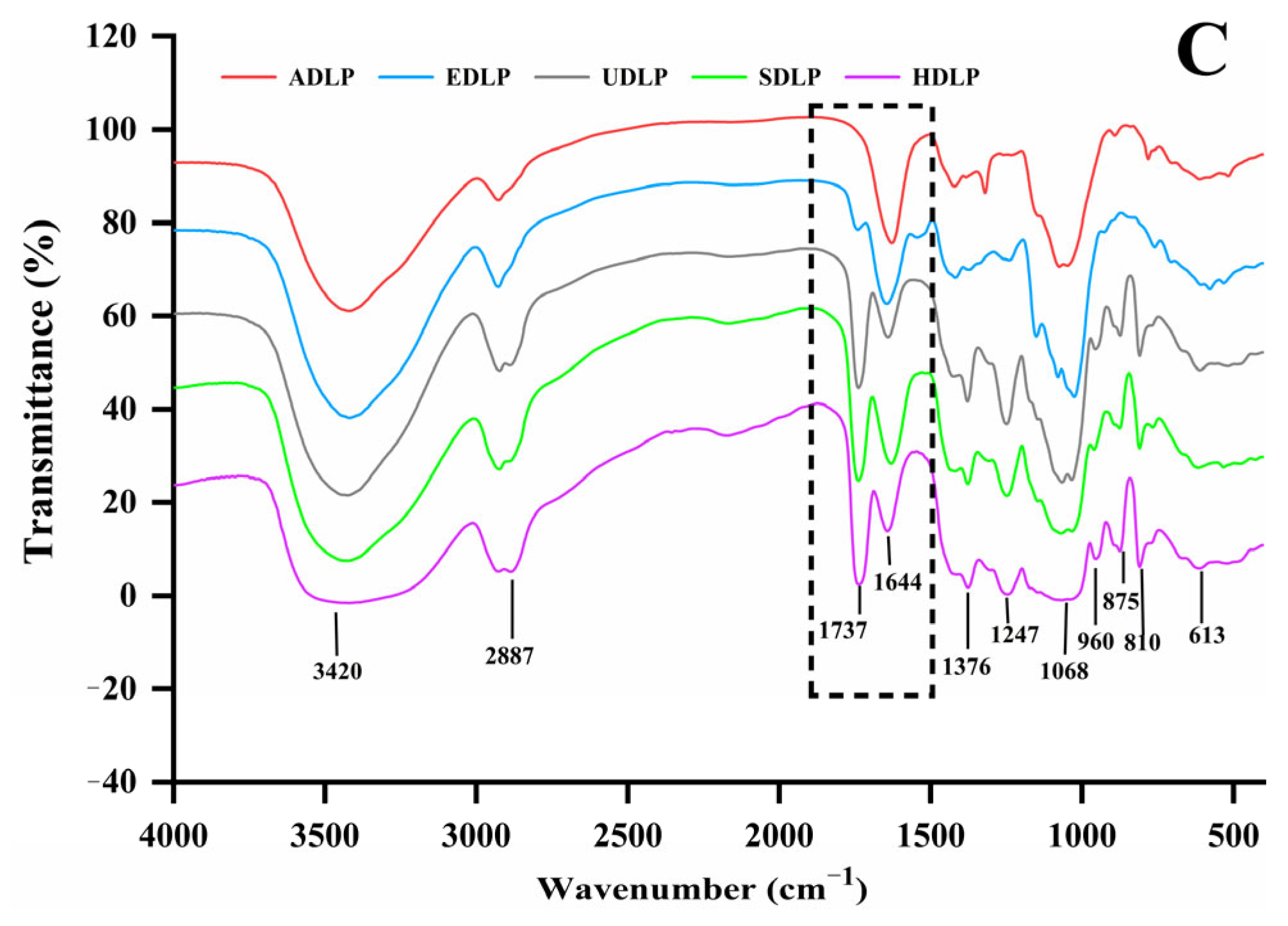
3.4. FT-IR
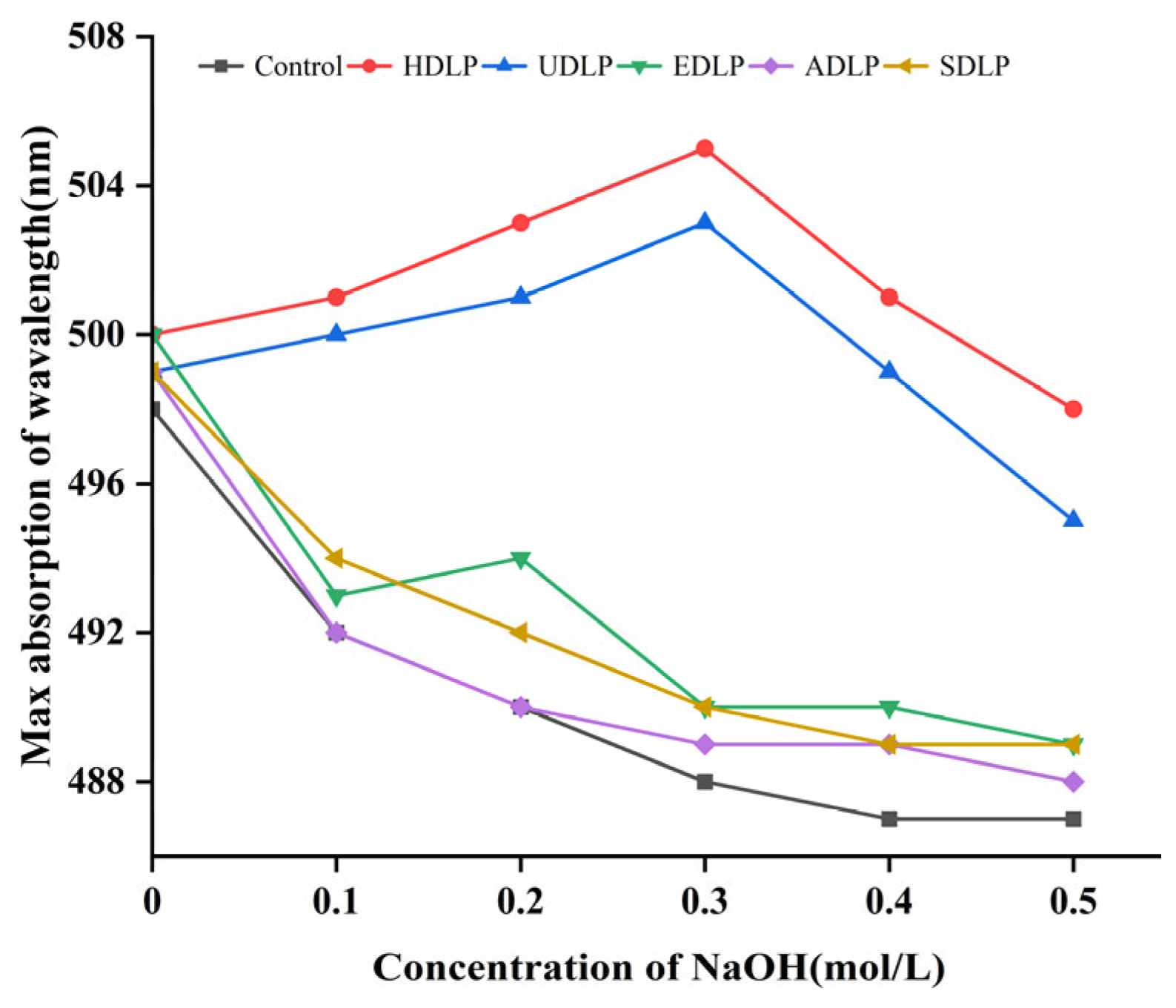
3.5. Congo Red Test
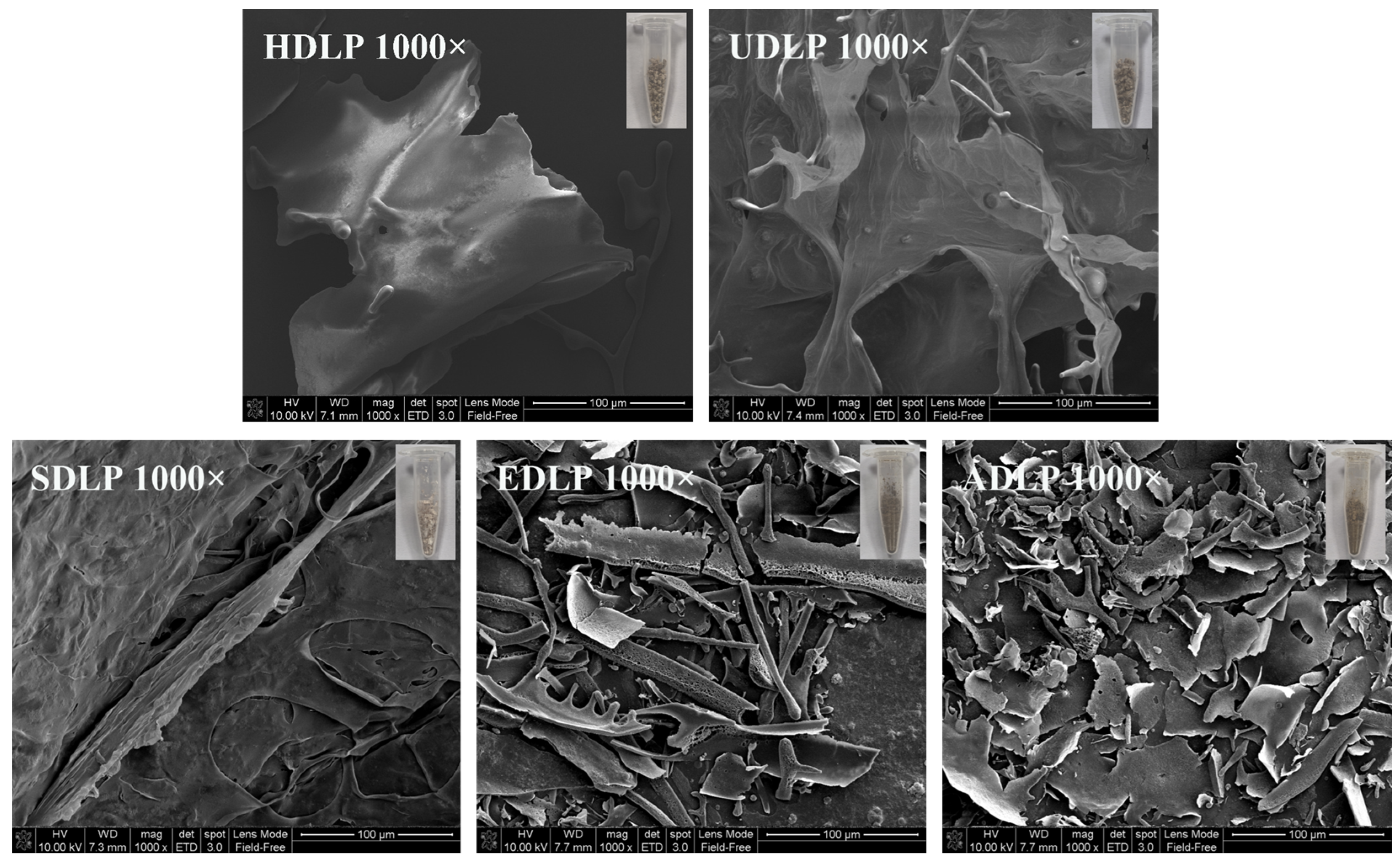
3.6. SEM Analysis
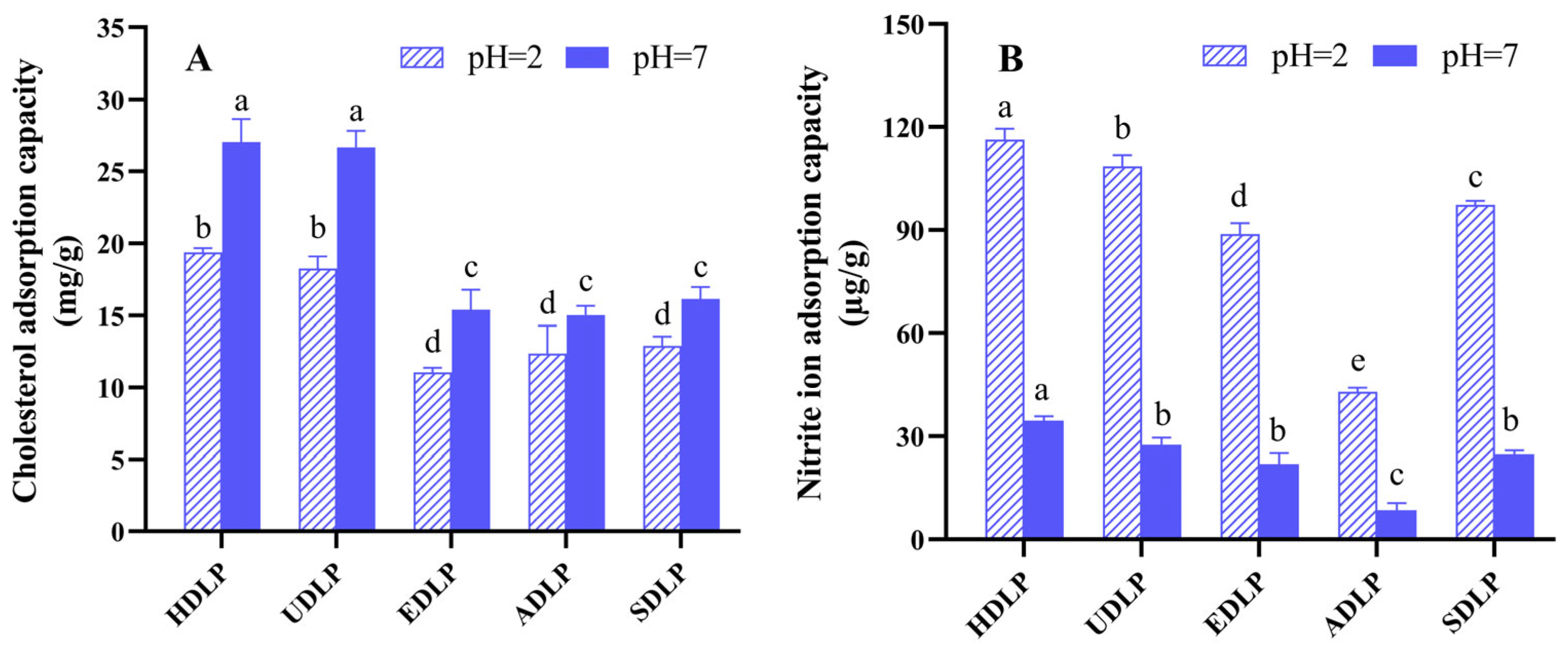
3.7. In Vitro Adsorption Capacity
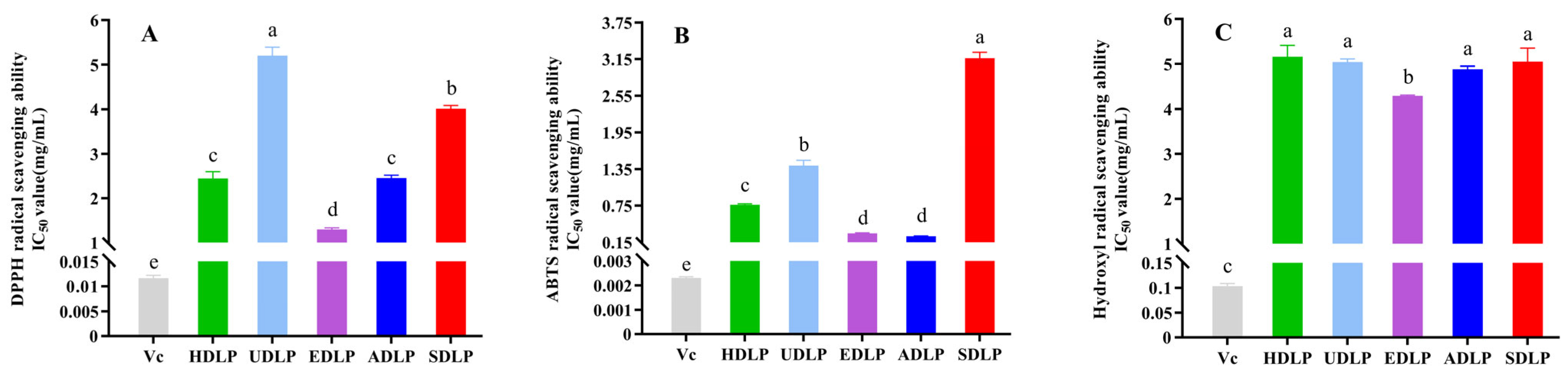
3.8. In Vitro Antioxidant Capacity
3.9. Immunostimulatory Capacity
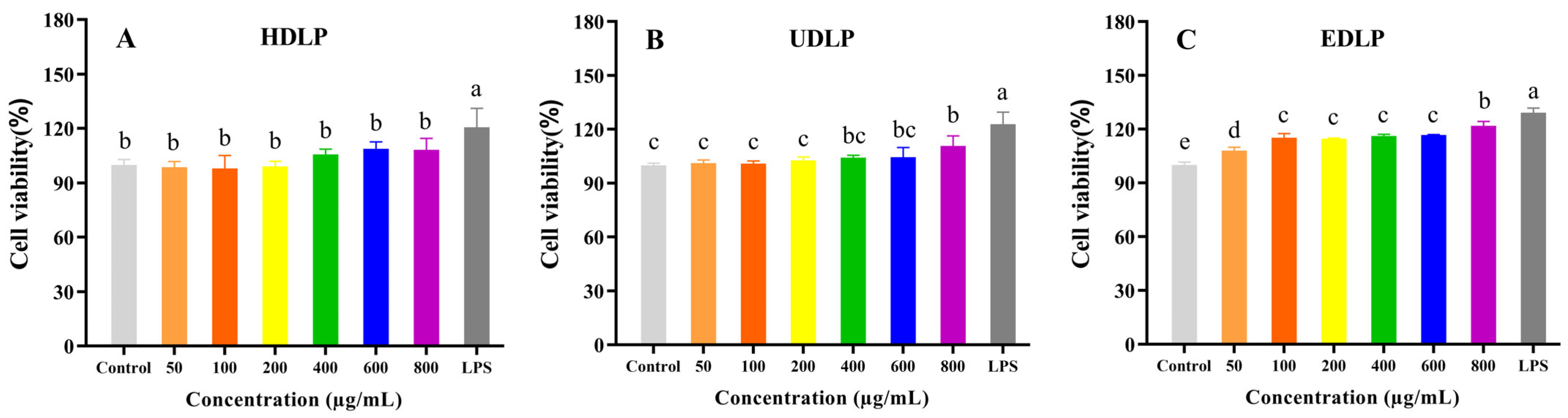
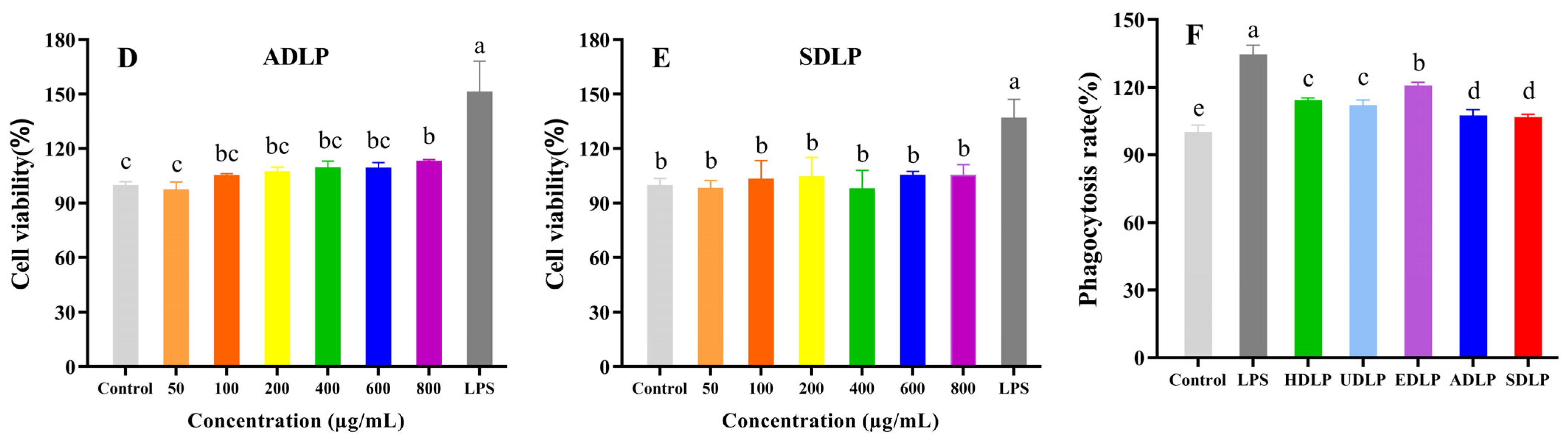
3.9.1. Cell Proliferation Activity and Phagocytosis
3.9.2. NO, TNF-α, IL-6, and IL-β Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, R.; Liao, J.; Sun, Y.; Wang, Y.; Li, P.; Du, B. A review of advances in the extraction, structural characterization, gel properties, and biological activity mechanisms of Dendrobium officinale polysaccharides. Int. J. Biol. Macromol. 2025, 311, 143756. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Bao, H.; Wang, Y.; Wang, F.; Jiang, Q.; Li, H.; Ding, Y.; Zhu, C. Variations in cold resistance and contents of bioactive compounds among Dendrobium officinale Kimura et Migo strains. Foods 2024, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Zeng, D.; Yu, Z.; Teixeira da Silva, J.A.; Duan, J.; He, C.; Zhang, J. Transcriptomic and metabolomic analyses reveal the main metabolites in Dendrobium officinale leaves during the harvesting period. Plant Physiol. Biochem. 2022, 190, 24–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S. Dendrobium officinale leaves as a new antioxidant source. J. Funct. Foods 2017, 37, 400–415. [Google Scholar] [CrossRef]
- Fang, J.; Lin, Y.; Xie, H.; Farag, M.A.; Feng, S.; Li, J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem. X 2022, 13, 100207. [Google Scholar] [CrossRef]
- Liu, B.; Shang, Z.; Li, Q.; Zha, X.; Wu, D.; Yu, N.; Han, L.; Peng, D.; Luo, J. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020, 143, 651–664. [Google Scholar] [CrossRef]
- Qin, L.; Chen, S.; Xie, L.; Yu, Q.; Chen, Y.; Xie, J. Recent advances in Mung bean polysaccharides: Extraction, physicochemical properties and biological activities. Process Biochem. 2022, 121, 248–256. [Google Scholar] [CrossRef]
- Gong, T.; Liu, S.; Wang, H.; Zhang, M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- Xiong, G.; Ma, L.; Zhang, H.; Li, Y.; Zou, W.; Wang, X.; Xu, Q.; Xiong, J.; Hu, Y.; Wang, X. Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022, 194, 484–498. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/microwave-assisted extraction. LWT—Food Sci. Technol. 2022, 154, 112446. [Google Scholar] [CrossRef]
- Tao, X.; Hu, X.; Wu, T.; Zhou, D.; Yang, D.; Li, X.; Fu, Y.; Zheng, F.; Yue, H.; Dai, Y. Characterization and screening of anti-melanogenesis and anti-photoaging activity of different enzyme-assisted polysaccharide extracts from Portulaca oleracea L. Phytomedicine 2023, 116, 154879. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from traditional chinese medicines: Extraction, purification, modification, and biological activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Jia, R.; Ou, Z.; Li, Z.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and α-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, S.; Wang, Z.; Zhang, G. Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Biochem. Eng. J. 2022, 177, 108227. [Google Scholar] [CrossRef]
- Zhong, C.; Tian, W.; Chen, H.; Yang, Y.; Xu, Y.; Chen, Y.; Chen, P.; Zhu, S.; Li, P.; Du, B. Structural characterization and immunoregulatory activity of polysaccharides from Dendrobium officinale leaves. J. Food Biochem. 2022, 46, e14023. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.; Xiang, X.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.; et al. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, L.; Ruan, Y.; Wen, C.; Ge, M.; Qian, Y.; Ma, B. Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods. Food Sci. Hum. Wellness 2023, 12, 130–139. [Google Scholar] [CrossRef]
- Song, Y.; Han, A.; Park, S.; Cho, C.; Rhee, Y.; Hong, H. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, K.; Wei, S.; Xiang, X.; Ding, Y.; Li, H.; Zhao, L.; Qin, W.; Gan, R.; Wu, D. Comparison of structural characteristics and bioactivities of polysaccharides from loquat leaves prepared by different drying techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Q.; Lin, S.; Liu, W.; Du, G.; Zhao, L.; Zhang, Q.; Lin, D.R.; Liu, Y.T.; Qin, W.; et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars. Int. J. Biol. Macromol. 2019, 135, 274–281. [Google Scholar] [CrossRef]
- Geng, X.; Guo, D.; Wu, B.; Wang, W.; Zhang, D.; Hou, S.; Bau, T.; Lei, J.; Xu, L.; Cheng, Y.; et al. Effects of different extraction methods on the physico-chemical characteristics and biological activities of polysaccharides from Clitocybe squamulosa. Int. J. Biol. Macromol. 2024, 259, 129234. [Google Scholar] [CrossRef]
- Yuan, H.; Dong, L.; Zhang, Z.; He, Y.; Ma, X. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis. Food Sci. Hum. Wellness 2022, 11, 1010–1017. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Liu, Y.; Qin, Z.; Liu, H.; Ma, Y.; Wang, X. Isolation and structural characterization of cell wall polysaccharides from sesame kernel. LWT—Food Sci. Technol. 2022, 163, 113574. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Ma, H.; Ma, Y.; Yan, J. Physicochemical and functional characteristics of polysaccharides from okra extracted by using ultrasound at different frequencies. Food Chem. 2021, 361, 130138. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 10549. [Google Scholar] [CrossRef]
- Cai, G.; Dong, H.; Liu, S.; Wu, W.; Yang, H. Comparative evaluation of the physiochemical properties, and antioxidant and hypoglycemic activities of Dendrobium officinale leaves processed using different drying techniques. Antioxidants 2023, 12, 1911. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y.; Li, W.; Qiu, Y.; Hua, C.; Zhang, Y.; Guo, Z.; Xie, Z. Immunomodulatory activity and active mechanisms of a low molecular polysaccharide isolated from Lanzhou lily bulbs in RAW264.7 macrophages. J. Funct. Foods 2022, 92, 105071. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wen, C.; Zhou, J.; Gu, J.; Duan, Y.; Zhang, H.; Ren, X.; Ma, H. Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L. Int. J. Biol. Macromol. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κB signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef]
- Tang, Y.; He, X.; Liu, G.; Wei, Z.; Sheng, J.; Sun, J.; Li, C.; Xin, M.; Li, L.; Yi, P. Effects of different extraction methods on the structural, antioxidant and hypoglycemic properties of red pitaya stem polysaccharide. Food Chem. 2023, 405, 134804. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liu, Y.; Zhao, M.; Wang, J. Ultrasound assisted extraction of polysaccharides from Lentinus edodes and its anti-hepatitis B activity in vitro. Int. J. Biol. Macromol. 2018, 107, 2217–2223. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Wang, H.; Huang, F.; Wang, L. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef]
- Li, J.; Tao, W.; Zhou, W.; Xing, J.; Luo, M.; Lu, S.; Yang, Y. Dendrobium officinale leaf polysaccharide has a dual effect of alleviating the syndromes of immunosuppressed mice and modulating immune system of normal mice. J. Funct. Foods 2024, 113, 105974. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from Sargassum pallidum. Carbohydr. Polym. 2020, 239, 116230. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, X.; Xue, Z.; Wang, Y.; Lu, Y.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int. J. Biol. Macromol. 2020, 163, 1640–1648. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Cao, Y.; Huang, J.; Lin, H.; Zhao, T.; Liu, L.; Shen, P.; McClements, D.J.; Chen, J.; et al. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: Comparison of hot water and ultrasound-assisted extraction methods. Food Chem. 2023, 401, 134156. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Song, T.; Xu, Z.; Wang, M. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem. 2024, 445, 138752. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Rahman, A.; Li, J.; Wei, C.; Chen, J.; Linhardt, R.J.; Ye, X.; Chen, S. Extraction methods affect the structure of Goji (Lycium barbarum) polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Pan, C.; Yang, X.; Chen, S.; Qi, B.; Huang, H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 182, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, H.; Han, C.; Zeng, S.; Xu, X.; Lu, D.; He, H. Extraction, characterization and antioxidant activity of polysaccharide from Pouteria campechiana seed. Carbohydr. Polym. 2020, 229, 115409. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, X.; Li, T.; Song, M.; Wang, S.; Wen, T.; Zhu, Z. Structural characterization of polysaccharides from Dictyophora rubrovolvata mycelium and their immunostimulatory activity in RAW264.7 cells. Process Biochem. 2024, 139, 22–32. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jia, S.; Huang, L.; Wang, Z.; Li, C.; Xie, M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef]
- Zeng, M.; Kong, Q.; Liu, F.; Chen, J.; Sang, H. The Anticancer activity of Lycium barbarum polysaccharide by inhibiting autophagy in human skin squamous cell carcinoma cells in vitro and in vivo. Int. J. Polym. Sci. 2019, 2019, 5065920. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Effects of the molecular weight and protein and sulfate content of Chlorella ellipsoidea polysaccharides on their immunomodulatory activity. Int. J. Biol. Macromol. 2018, 107, 70–77. [Google Scholar] [CrossRef]
- Tian, W.; Xiao, N.; Yang, Y.; Xiao, J.; Zeng, R.; Xie, L.; Qiu, Z.; Li, P.; Du, B. Structure, antioxidant and immunomodulatory activity of a polysaccharide extracted from Sacha inchi seeds. Int. J. Biol. Macromol. 2020, 162, 116–126. [Google Scholar] [CrossRef]
- He, B.; Zheng, Q.; Guo, L.; Huang, J.; Yun, F.; Huang, S.; Lin, J. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020, 145, 11–20. [Google Scholar] [CrossRef]

| HDLP | UDLP | SDLP | ADLP | EDLP | |
|---|---|---|---|---|---|
| Extraction yield (%) | 4.76 ± 0.14 c | 5.22 ± 0.19 bc | 5.40 ± 0.20 b | 3.20 ± 0.23 d | 6.31 ± 0.39 a |
| Chemical composition (%, w/w) | |||||
| Polysaccharide | 74.20 ± 4.45 a | 75.11 ± 0.99 a | 69.36 ± 1.20 b | 55.37 ± 1.57 c | 52.10 ± 2.75 c |
| Protein | 9.17 ± 0.14 b | 9.48 ± 0.45 b | 2.65 ± 0.10 d | 8.56 ± 0.42 c | 11.02 ± 0.51 a |
| Monosaccharide composition (%, mol) | |||||
| Mannose | 83.72 ± 0.90 a | 83.31 ± 0.89 a | 75.78 ± 1.22 b | 2.08 ± 0.02 d | 7.47 ± 0.03 c |
| Rhamnose | 0.23 ± 0.01 d | 0.25 ± 0.04 d | 1.45 ± 0.08 b | 6.48 ± 0.19 a | 1.24 ± 0.05 c |
| Glucosamine | 0.53 ± 0.01 b | 0.39 ± 0.24 b | 0.44 ± 0.02 b | 0.88 ± 0.19 a | 0.59 ± 0.06 b |
| Glucuronic acid | 0.24 ± 0.00 c | 0.23 ± 0.00 c | 3.56 ± 0.16 a | 1.49 ± 0.09 b | 1.50 ± 0.08 b |
| Galacturonic acid | 9.83 ± 0.40 c | 10.07 ± 0.04 c | 10.02 ± 0.67 c | 18.21 ± 0.03 b | 71.37 ± 0.17 a |
| Glucose | 1.28 ± 0.46 d | 1.32 ± 0.45 d | 3.09 ± 0.76 c | 41.93 ± 0.32 a | 10.84 ± 0.14 b |
| Galactose | 4.16 ± 0.75 d | 4.19 ± 0.30 d | 5.66 ± 0.03 b | 28.94 ± 0.26 a | 7.00 ± 0.02 c |
| Molecular weight (Da) | |||||
| Peak (Ι) | 1.80 ± 0.15 × 106 | 6.56 ± 0.13 × 105 | 1.47 ± 0.03 × 106 | 2.74 ± 0.14 × 106 | 2.55 ± 0.02 × 106 |
| Area (%) | 67.37 ± 3.14 | 10.59 ± 0.96 | 64.29 ± 2.60 | 69.91 ± 3.55 | 41.65 ± 0.27 |
| Peak (II) | 9.02 ± 0.67 × 104 | 1.94 ± 0.14 × 104 | 9.85 ± 0.76 × 104 | 5.01 ± 0.22 × 105 | 1.04 ± 0.02 × 106 |
| Area (%) | 32.63 ± 3.14 | 89.41 ± 0.96 | 34.94 ± 1.38 | 12.75 ± 0.50 | 5.77 ± 0.08 |
| Peak (III) | ND | ND | 0.48 ± 0.16 × 103 | 4.1 ± 0.29 × 103 | 3.31 ± 0.03 × 103 |
| Area (%) | ND | ND | 0.39 ± 0.01 | 17.35 ± 4.05 | 52.58 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cai, G.; Chen, H.; Zhou, H.; Qu, H.; Yang, H. Physicochemical Properties and Biological Activities of Polysaccharides from Dendrobium officinale Leaves in Response to Different Extraction Methods. Foods 2025, 14, 2029. https://doi.org/10.3390/foods14122029
Chen Y, Cai G, Chen H, Zhou H, Qu H, Yang H. Physicochemical Properties and Biological Activities of Polysaccharides from Dendrobium officinale Leaves in Response to Different Extraction Methods. Foods. 2025; 14(12):2029. https://doi.org/10.3390/foods14122029
Chicago/Turabian StyleChen, Yang, Gonglin Cai, Hufu Chen, Huabin Zhou, Hang Qu, and Hailong Yang. 2025. "Physicochemical Properties and Biological Activities of Polysaccharides from Dendrobium officinale Leaves in Response to Different Extraction Methods" Foods 14, no. 12: 2029. https://doi.org/10.3390/foods14122029
APA StyleChen, Y., Cai, G., Chen, H., Zhou, H., Qu, H., & Yang, H. (2025). Physicochemical Properties and Biological Activities of Polysaccharides from Dendrobium officinale Leaves in Response to Different Extraction Methods. Foods, 14(12), 2029. https://doi.org/10.3390/foods14122029







