Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Optimizing the Preparation Conditions of Liposomes
2.2.1. Preparation of Liposomes
2.2.2. Paeonol Encapsulation Efficiency
2.2.3. Experimental Design for Optimization of PAE-ONLs
2.3. Characterization
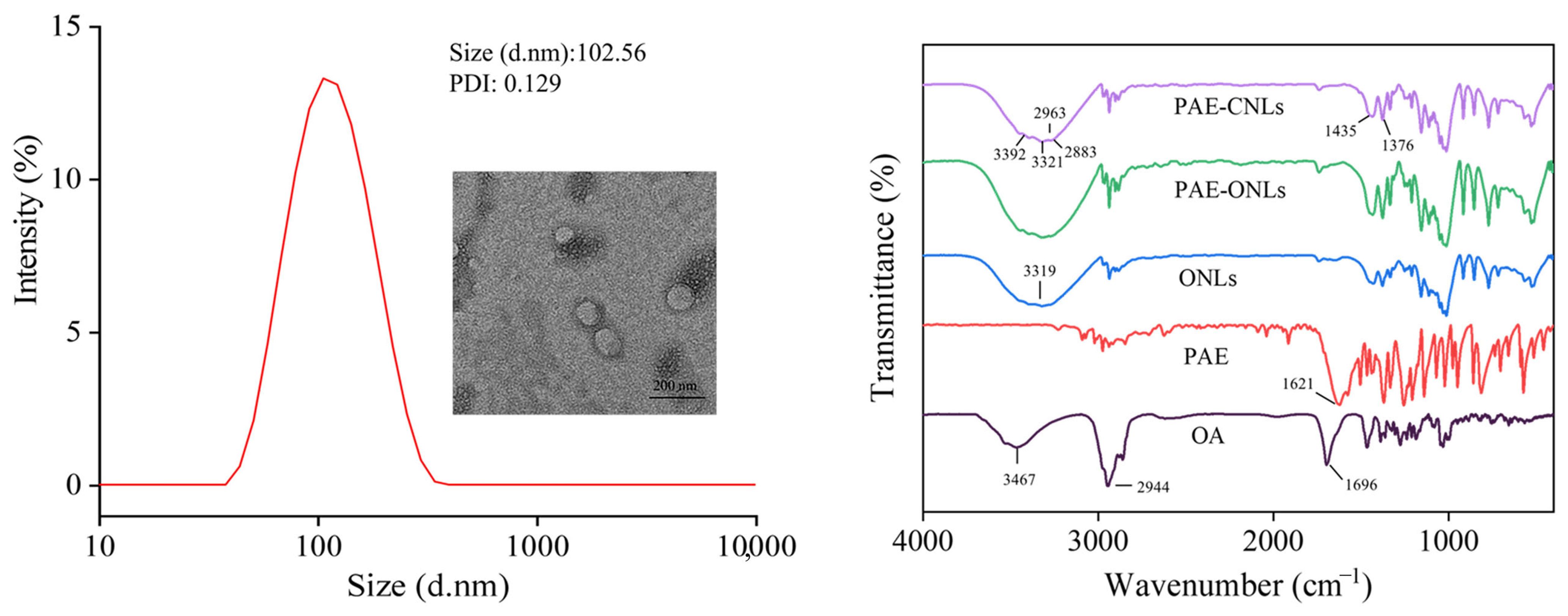
2.3.1. Particle Size Distribution and Zeta Potential
2.3.2. TEM Analysis
2.3.3. FTIR Analysis
2.4. Stability Studies
2.4.1. Storage Stability
2.4.2. Thermal Stability
2.4.3. Salt Stability
2.4.4. pH Stability
2.5. Comparison of Thermodynamic Stability and Hydrogen Bonding
2.6. Evaluation of Anti-Inflammatory Activity in Zebrafish
2.6.1. Feeding and Ovulation of Zebrafish
2.6.2. Anti-Inflammatory Activity of Liposomes
2.7. Inflammation in RAW 264.7 Macrophages Stimulated by LPS
2.7.1. Cell Culture
2.7.2. Measurement of Cell Viability
2.7.3. Determination of the Concentrations of Inflammatory Factors
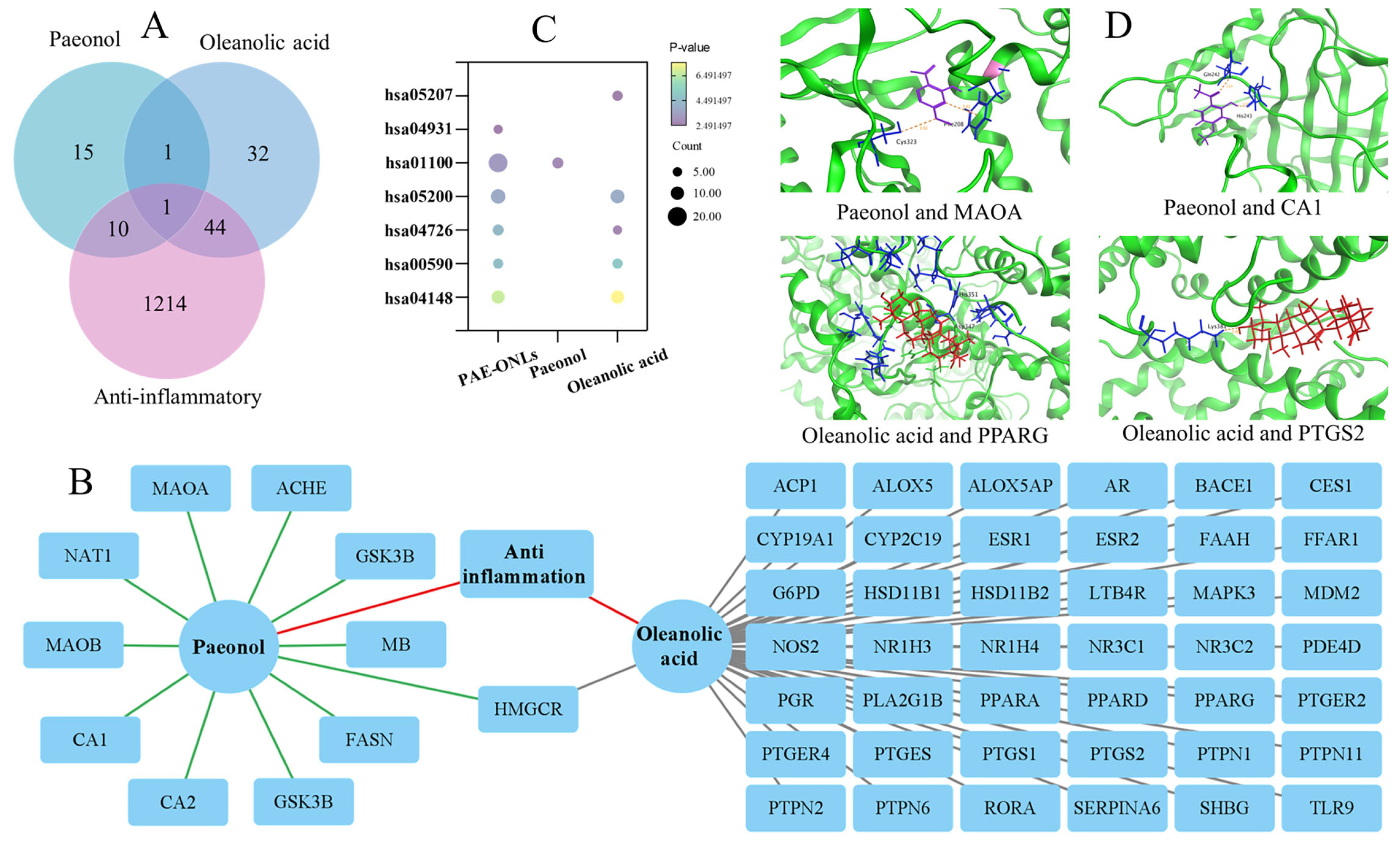
2.8. Synergistic Anti-Inflammatory Effects by Network Pharmacology-Based Investigation
2.9. Statistical Analyses
3. Results
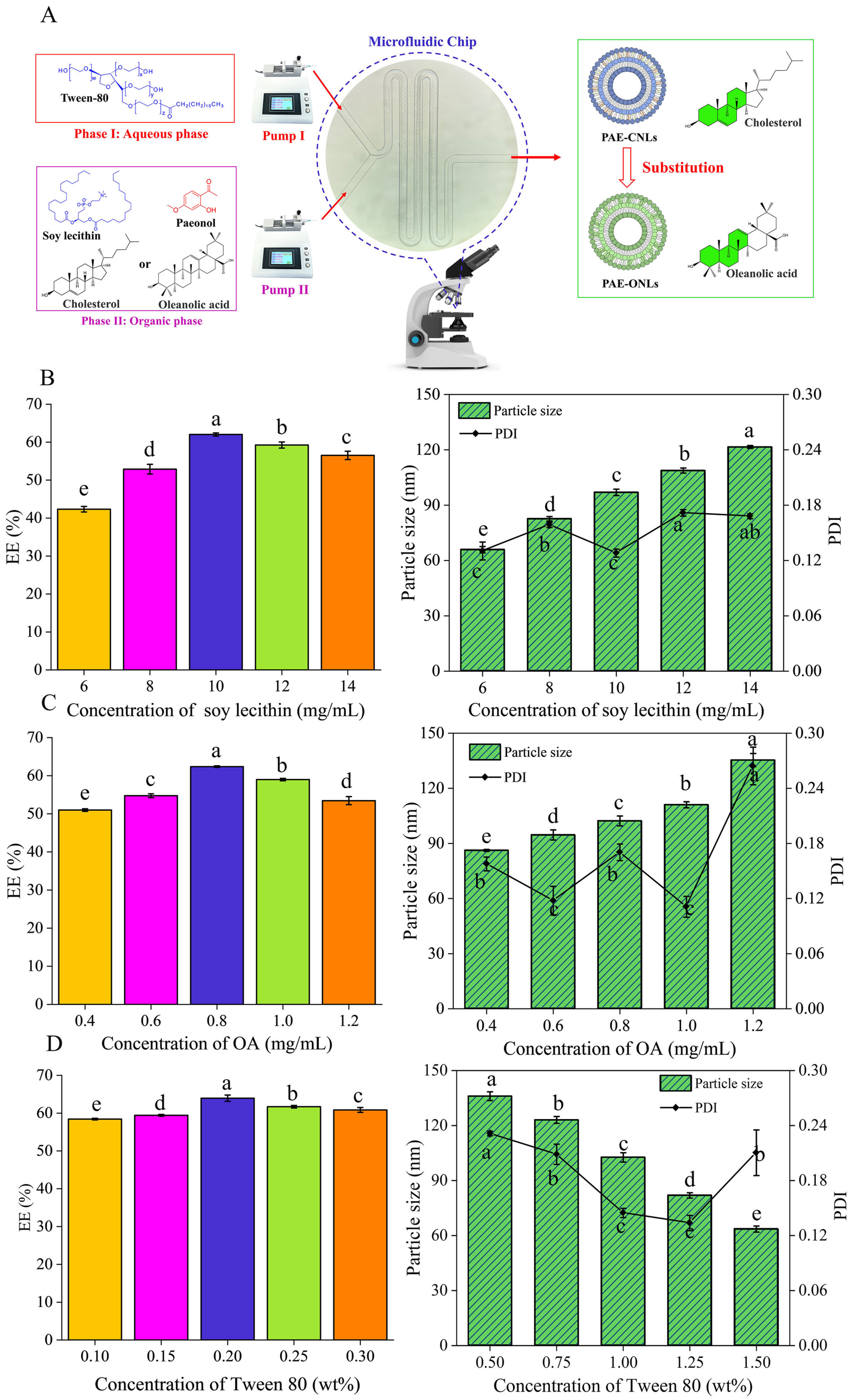
3.1. Single-Factor Experiments
3.1.1. Effect of Soybean Phosphatide Concentration on the EE of PAE-ONLs
3.1.2. Effect of Oleanolic Acid Concentrations on the EE of PAE-ONLs
3.1.3. Effect of Tween 80 Concentrations on the EE of PAE-ONLs
3.2. Optimization of PAE-ONLs by the Theoretical Response Surface Models
3.3. Characterization
3.4. Stability Studies
3.4.1. Storage Stability
3.4.2. Thermal Stability
3.4.3. Salt Stability
3.4.4. pH Stability
3.5. Comparison of Thermodynamic Stability and Hydrogen Bonding
3.6. Evaluation of Anti-Inflammatory Activity in Zebrafish
3.7. Inflammation in RAW 264.7 Macrophages Stimulated by LPS
3.7.1. Measurement of Cell Viability
3.7.2. PAE-ONLs Inhibit Inflammatory Factors in LPS-Stimulated RAW 264.7 Macrophages
3.8. Network Pharmacology-Based Investigation of the Synergistic Anti-Inflammatory Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Huang, S.; Zhai, B.; Fan, Y.; Sun, J.; Cheng, J.; Zou, J.; Zhang, X.; Shi, Y.; Guo, D. Development of paeonol liposomes: Design, optimization, in vitro and in vivo evaluation. Int. J. Nanomed. 2022, 17, 5027–5046. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical liposomal delivery—Specific considerations of innovation and challenges. Biomater. Sci. 2023, 11, 62–75. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.-T.; Wang, J.-H.; Chen, M.-H.; Zhang, Z.-X.; Liu, Y.; Kong, L.; Li, X.-T.; Tang, L. Methotrexate-modified docetaxel liposome targeting with ginsenoside Rh2 as a membrane stabilizer for the treatment of ovarian cancer. J. Drug Deliv. Sci. Technol. 2024, 98, 105917. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, Y.; Chang, Y.; Xue, C.; Zhang, T. Preparation and properties of fucoxanthin-loaded liposomes stabilized by sea cucumber derived cholesterol sulfate instead of cholesterol. J. Biosci. Bioeng. 2023, 135, 160–166. [Google Scholar] [CrossRef]
- de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; Cassali, G.D.; de Paula Sabino, A.; Townsend, D.M.; Oliveira, M.C.; de Barros, A.L.B. Evaluation of acute toxicity and in vitro antitumor activity of a novel doxorubicin-loaded folate-coated pH-sensitive liposome. Biomed. Pharmacother. 2023, 165, 115280. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.; Gallardo, I.; Ruiz, L.; Alvarez, Y.; Cachofeiro, V.; Margolles, A.; Hernandez, M.; Nieto, M.L. Oleanolic acid ameliorates intestinal alterations associated with EAE. J. Neuroinflamm. 2020, 17, 363. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Z.; Zhang, J.; Jian, W.; Fu, Q. Antitumour activity of oleanolic acid: A systematic review and meta-analysis. Oncol. Lett. 2024, 28, 582. [Google Scholar] [CrossRef]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An update on pentacyclic triterpenoids ursolic and oleanolic acids and related derivatives as anticancer candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef]
- Liu, Q.-X.; Liu, X.; Yang, B.; Liu, T.-Q.; Yu, Q.; Ling, F.; Wang, G.-X. Evaluation of the antiviral activity of oleanolic acid against nervous necrosis virus. Fish Shellfish Immunol. 2024, 153, 109847. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Ko, Y.I.; Park, S.J.; Jin, I.J.; Kim, Y.M. Oleanolic acid and ursolic acid stabilize liposomal membranes. Lipids 1997, 32, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Y.; Li, Y.; Zheng, Y.; Chen, G.; Shi, Y. Investigation on the antitumor effects of paeonol against renal cell carcinoma based on network pharmacology and experimental validation. J. Ethnopharmacol. 2022, 285, 114857. [Google Scholar] [CrossRef]
- Ding, Y.; Dang, B.; Zhang, Y.; Hu, S.; Wang, Y.; Zhao, C.; Zhang, T.; Gao, Z. Paeonol attenuates Substance P-induced urticaria by inhibiting Src kinase phosphorylation in mast cells. Cell. Immunol. 2023, 388–389, 104728. [Google Scholar] [CrossRef]
- Qian, W.; Li, X.; Liu, Q.; Lu, J.; Wang, T.; Zhang, Q. Antifungal and antibiofilm efficacy of paeonol treatment against biofilms comprising Candida albicans and/or Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2022, 12, 884793. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Abdel-Latif, R.; Hafez, S.M.N.A.; Kandeel, M.; Abdel-Gaber, S.A. Paeonol protects against methotrexate hepatotoxicity by repressing oxidative stress, inflammation, and apoptosis—The role of drug efflux transporters. J. Pharm. 2022, 15, 1296. [Google Scholar] [CrossRef]
- Qi, J.-h.; Dong, F.-x.; Wang, X.-l. Exploring targets and signaling pathways of paeonol involved in relieving inflammation based on modern technology. Mol. Divers. 2021, 26, 1731–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.-S.; Zhang, Z.-H.; Wang, Z.; Wan, Y.-T.; Wu, F.-W.; Liu, J.-C.; Peng, J.-X.; Wang, H.-Y.; Hong, L. Paeonol repurposing for cancer therapy: From mechanism to clinical translation. Biomed. Pharmacother. 2023, 165, 115277. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020, 250, 117544. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W.G. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Chitturi, H.; Venuganti, V.V.K. Process optimization for preparation of curcumin and quercetin co-encapsulated liposomes using microfluidic device. Microfluid. Nanofluid. 2024, 28, 55. [Google Scholar] [CrossRef]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Synthesis and characterization of paclitaxel-loaded pegylated liposomes by the microfluidics method. Mol. Pharm. 2023, 20, 6184–6196. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lian, J.; Xu, Z.; Ning, Y.; Shi, M.; Zhao, Z.; Zhang, Z. Chitosan-coated nanoliposomes for efficient delivery of betanin with enhanced stability and bioavailability. Food Hydrocoll. 2022, 132, 107871. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, T.; Zhao, Y.; Song, H.; Zhang, L.; Wu, X.; Lu, B. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, R.; Lyu, F.; Liu, M.; Gao, J.; Cao, G. Optimization of Preparation Conditions for Lysozyme Nanoliposomes Using Response Surface Methodology and Evaluation of Their Stability. Molecules 2016, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Liu, Q.; Gui, C.; Li, G.; Lei, Z. Investigation of Ionic Liquids as Extraction Solvents for Separating Bicyclic Aromatic S/N-Compounds from FCC Diesel. ACS Sustain. Chem. Eng. 2023, 11, 7573–7585. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, W.; Zhang, X.; Chen, X. Neocryptotanshinone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm. Sin. B 2015, 5, 323–329. [Google Scholar] [CrossRef]
- Luo, J.-F.; Yue, L.; Wu, T.-T.; Zhao, C.-L.; Ye, J.-H.; He, K.; Zou, J. Triterpenoid and coumarin isolated from Astilbe grandis with anti-inflammatory effects through inhibiting the NF-κB pathway in LPS-induced RAW264.7 cells. Molecules 2023, 28, 5731. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H. Reconsidering the Importance of the Association of Egg Consumption and Dietary Cholesterol With Cardiovascular Disease Risk. JAMA 2019, 321, 1055–1056. [Google Scholar] [CrossRef]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One stone four birds: A novel liposomal delivery system multi-functionalized with ginsenoside rh2 for tumor targeting therapy. Nano-Micro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral activities of oleanolic acid and its analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef]
- Gu, Z.; Lin, S.; Yan, W.; Chen, D.; Zeng, Z.; Chen, L.; Li, Y.; He, B. Enhanced water solubility and anti-tumor activity of oleanolic acid through chemical structure modification. Int. J. Mol. Sci. 2022, 23, 13291. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, M.; Jia, H.; Zhang, K.; Liu, Y.; Cheng, M.; Xiao, W. A review of structural modification and biological activities of oleanolic acid. Chin. J. Nat. Med. 2024, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.-S.; Yoo, E.-S.; Kim, S.-H.; Lee, J.-H.; Han, S.-H.; Jung, S.-H.; Jung, G.-H.; Jung, J.-Y. Anticancer effects of oleanolic acid on human melanoma cells. Chem. Biol. Interact. 2021, 347, 109619. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef]
- Liu, P.; Shen, J.; Cao, J.; Jiang, W. p-Coumaric acid-loaded nanoliposomes: Optimization, characterization, antimicrobial properties and preservation effects on fresh pod pepper fruit. Food Chem. 2024, 435, 137672. [Google Scholar] [CrossRef]
- Lu, J.; Zamaratskaia, G.; Langton, M.; Röhnisch, H.E.; Karkehabadi, S. Minimizing anti-nutritional factors in wet protein extraction from Swedish faba beans through the application of response surface methodology. Food Chem. 2024, 460, 140700. [Google Scholar] [CrossRef]
- Jaradat, E.; Meziane, A.; Lamprou, D.A. Paclitaxel-loaded elastic liposomes synthesised by microfluidics technique for enhance transdermal delivery. Drug Deliv. Transl. Res. 2025, 15, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]
- Yang, C.; Gong, L.; Li, X.; Li, W.; Meng, X.; Liu, B. Carboxymethyl chitosan coated alpha-linolenic acid nanoliposomes: Preparation, stability and release in vitro and in vivo. Food Chem. 2023, 404, 134526. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, Z.; Wang, L.; Lei, F.; Wang, Z.; Ma, X.; Gong, Z.; Wang, J.; He, J.; Wang, D. Preparation of paeonol ethosomes by microfluidic technology combined with gaussians and evaluation of biological activity by zebrafish. ACS Omega 2024, 9, 44425–44435. [Google Scholar] [CrossRef] [PubMed]
- Thabassum Akhtar Iqbal, S.; Tirupathi Pichiah, P.B.; Raja, S.; Arunachalam, S. Paeonol Reverses Adriamycin Induced Cardiac Pathological Remodeling through Notch1 Signaling Reactivation in H9c2 Cells and Adult Zebrafish Heart. Chem. Res. Toxicol. 2019, 33, 312–323. [Google Scholar] [CrossRef]
- Lee, W.; Yang, E.-J.; Ku, S.-K.; Song, K.-S.; Bae, J.-S. Anti-inflammatory effects of oleanolic acid on lps-induced inflammation in vitro and in vivo. Inflammation 2012, 36, 94–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, H.; Huang, S.; Li, Y.; Long, J.; Han, Y.; Lin, Q.; Gong, T.; Sun, X.; et al. Replacing cholesterol with asiatic acid to prolong circulation and enhance anti-metastatic effects of non-PEGylated liposomes. J. Control. Release 2024, 366, 585–595. [Google Scholar] [CrossRef]
- Guo, W.; Huang, J.; Wang, N.; Tan, H.-Y.; Cheung, F.; Chen, F.; Feng, Y. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Shim, R.S.; Shah, F.H.; Eom, Y.S.; Salman, S.; Kim, S.J. Discovery of natural inhibitors for osteoarthritis targeting inflammatory pathway with pharmacoinformatics and molecular docking. Adv. Tradit. Med. 2025, 25, 211–219. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Wang, L.; Li, J.; Wu, W.; Wang, Q. Network pharmacology and experimental validation methods to reveal the active compounds and hub targets of Curculigo orchioides Gaertn in rheumatoid arthritis. J. Orthop. Surg. Res. 2023, 18, 861. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, H.; Luan, J.; Tian, M.; Zhang, X.; Sohail, A.; Liang, D.; Liu, J.; Tao, F.; Wang, Z.; et al. Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology. Foods 2025, 14, 2030. https://doi.org/10.3390/foods14122030
Ma X, Zhang H, Luan J, Tian M, Zhang X, Sohail A, Liang D, Liu J, Tao F, Wang Z, et al. Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology. Foods. 2025; 14(12):2030. https://doi.org/10.3390/foods14122030
Chicago/Turabian StyleMa, Xianzheng, Hui Zhang, Jinkai Luan, Mingfa Tian, Xiuxin Zhang, Ammara Sohail, Dong Liang, Jiguo Liu, Fuzhan Tao, Zheng Wang, and et al. 2025. "Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology" Foods 14, no. 12: 2030. https://doi.org/10.3390/foods14122030
APA StyleMa, X., Zhang, H., Luan, J., Tian, M., Zhang, X., Sohail, A., Liang, D., Liu, J., Tao, F., Wang, Z., & Wang, D. (2025). Study of the Stability and Anti-Inflammatory Activity of Paeonol–Oleanolic Acid Liposomes by Microfluidic Technology. Foods, 14(12), 2030. https://doi.org/10.3390/foods14122030





