Organic vs. Conventional Chestnuts (Castanea sativa Mill.): A Focus on Antioxidant Activity, Volatile Compounds, and Sensory Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals and Reagents
2.3. Morphological Features
2.4. Sensory Evaluation
2.5. Total Phenolic Compound (TPC) Content and Total Antioxidant Capacity (TAC) Determination
2.5.1. Extracts’ Preparation for Polyphenol Compounds and Antioxidant Activity Determination
2.5.2. Total Phenolic Compound (TPC) Content
2.5.3. Total Antioxidant Capacity (TAC) Determination
2.6. HS-SPME/GC-MS Analysis of Volatile Chemical Composition
2.7. Statistical Analysis
3. Results
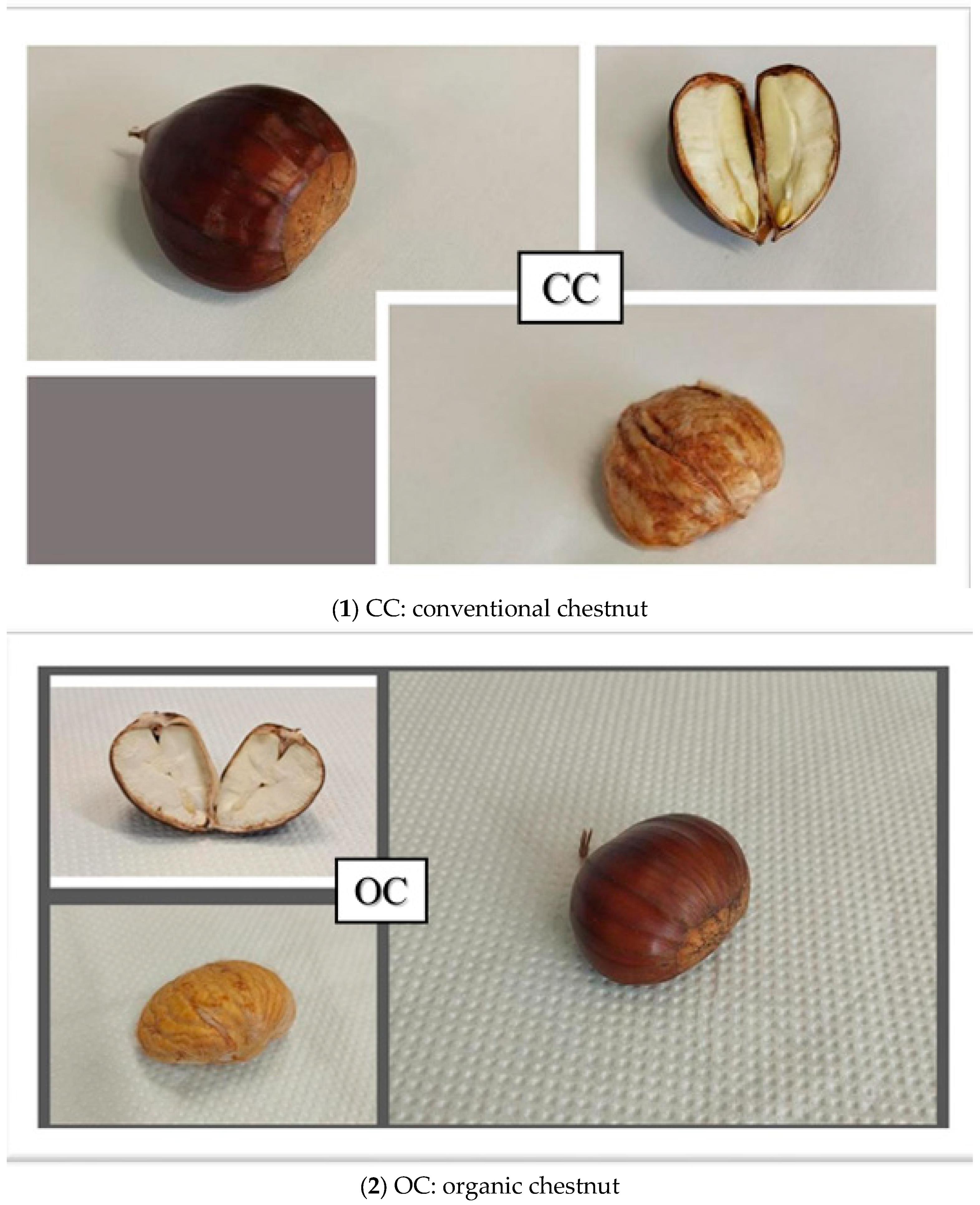
3.1. Morphological Features
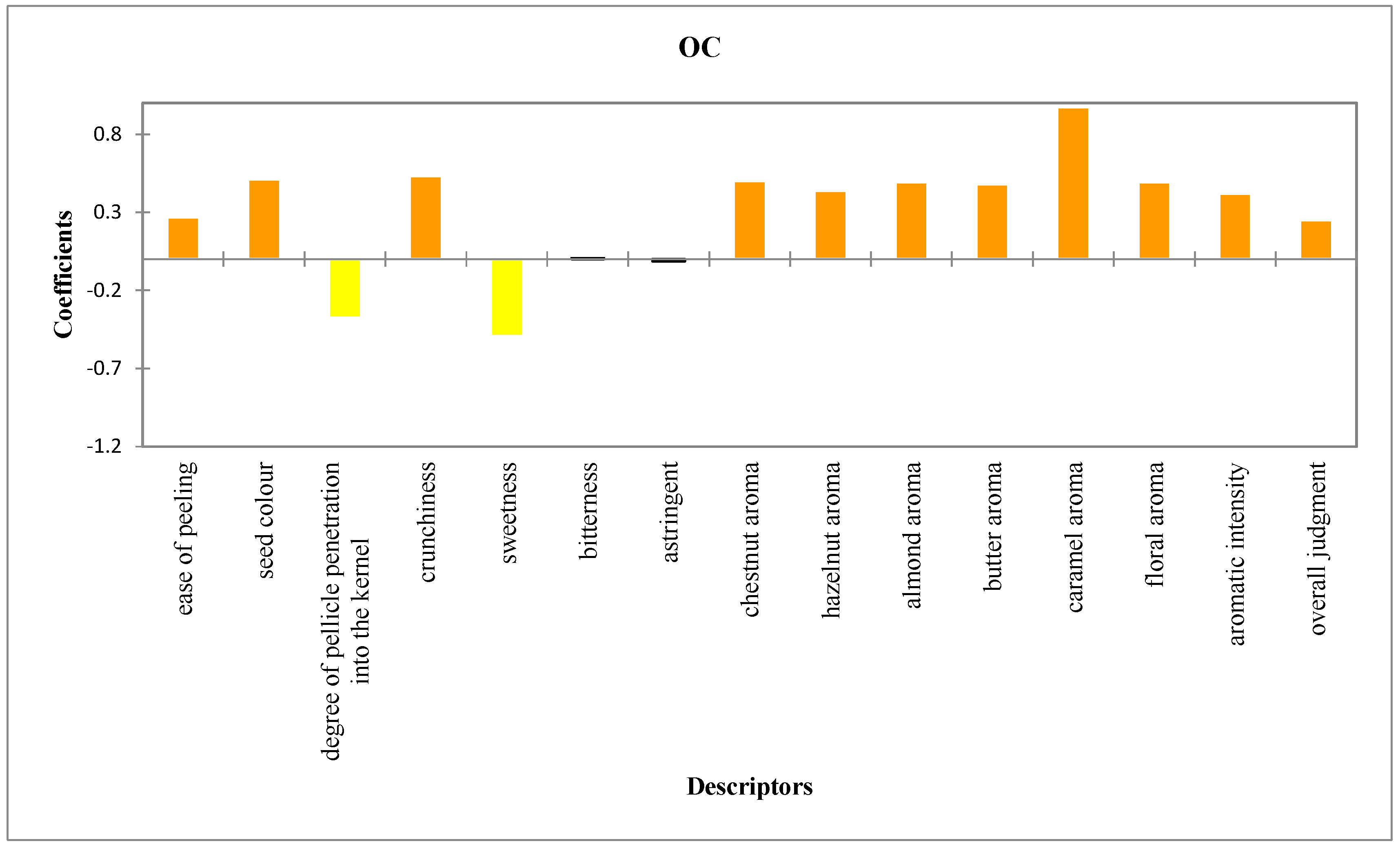
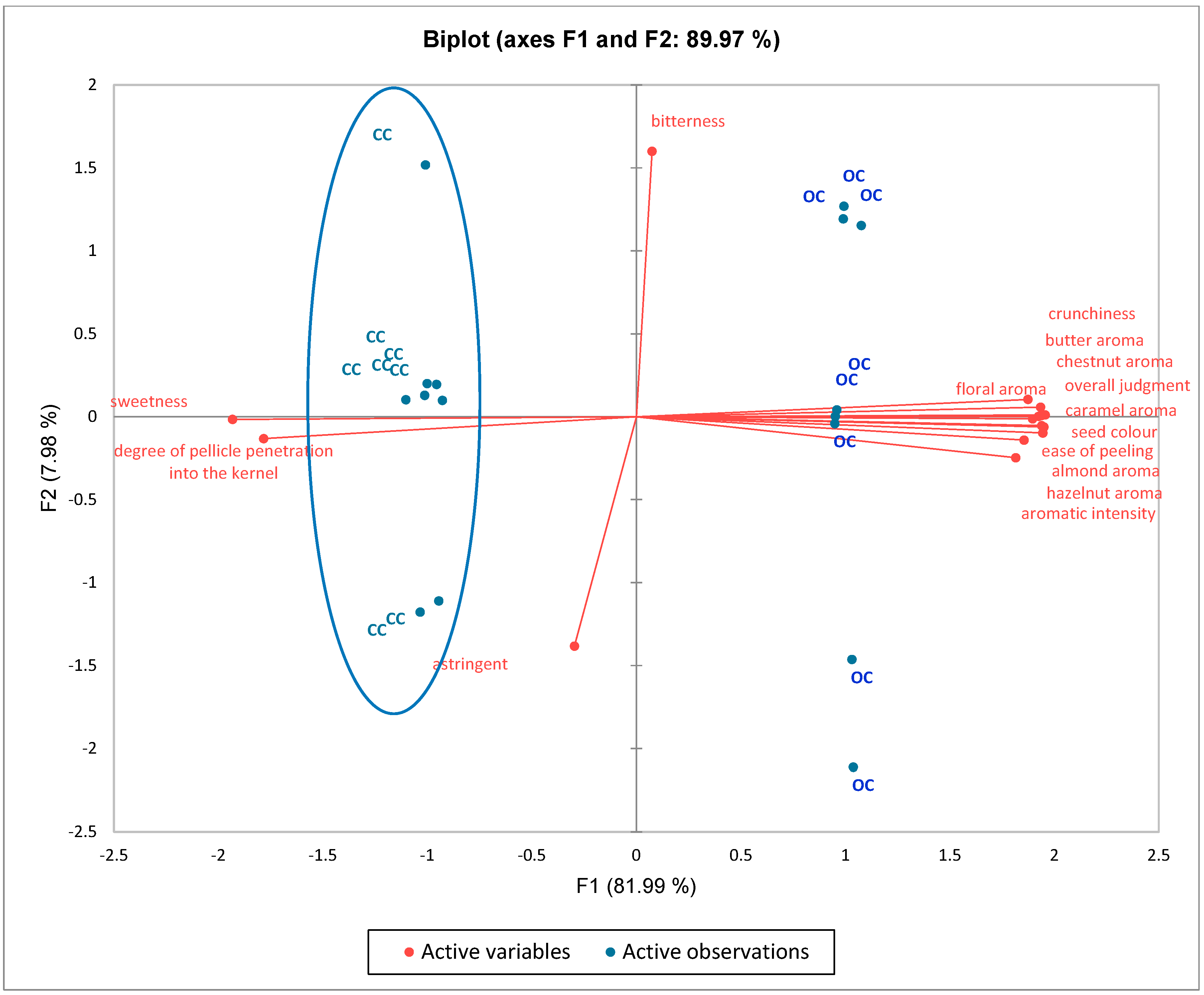
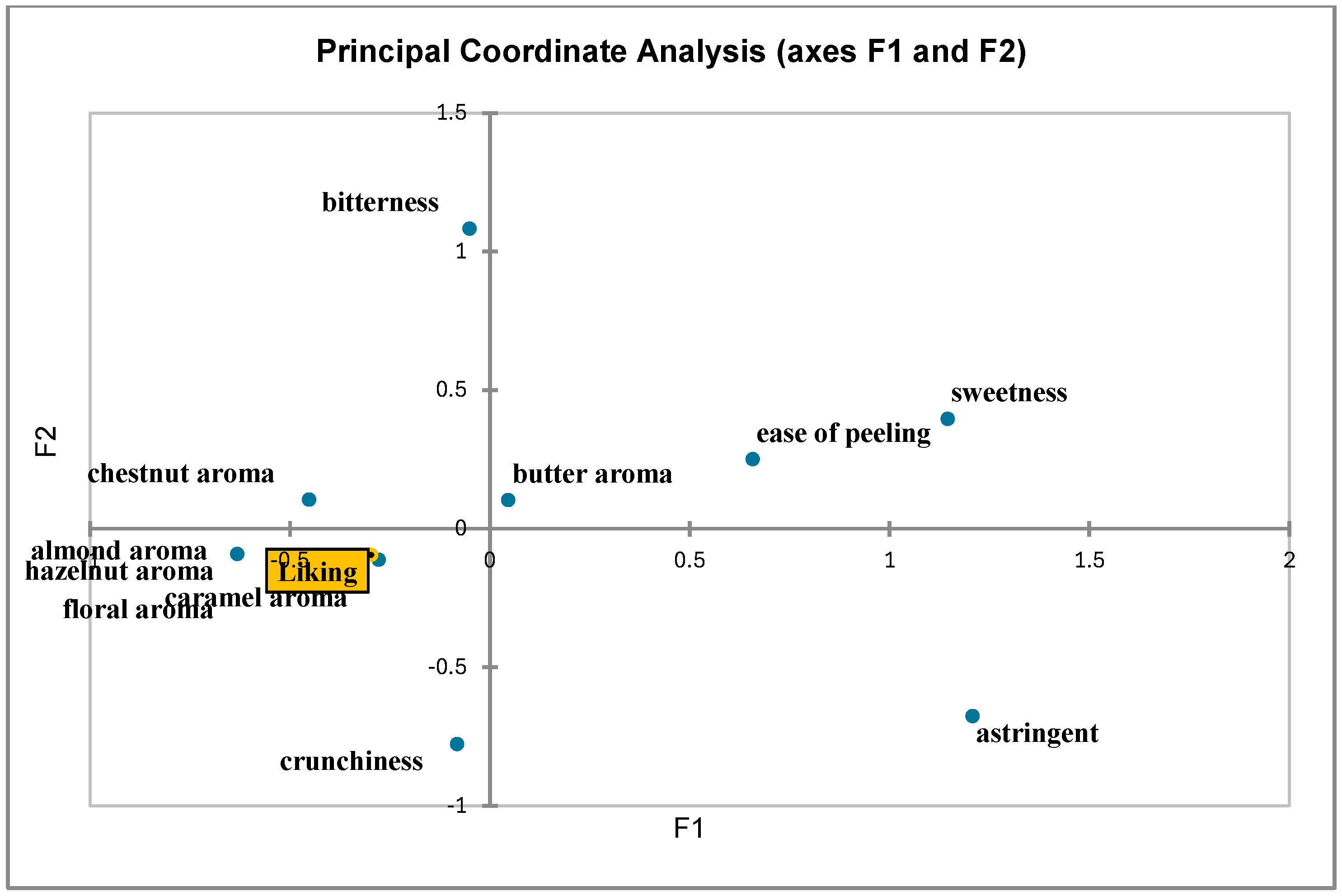
3.2. Sensory Evaluation
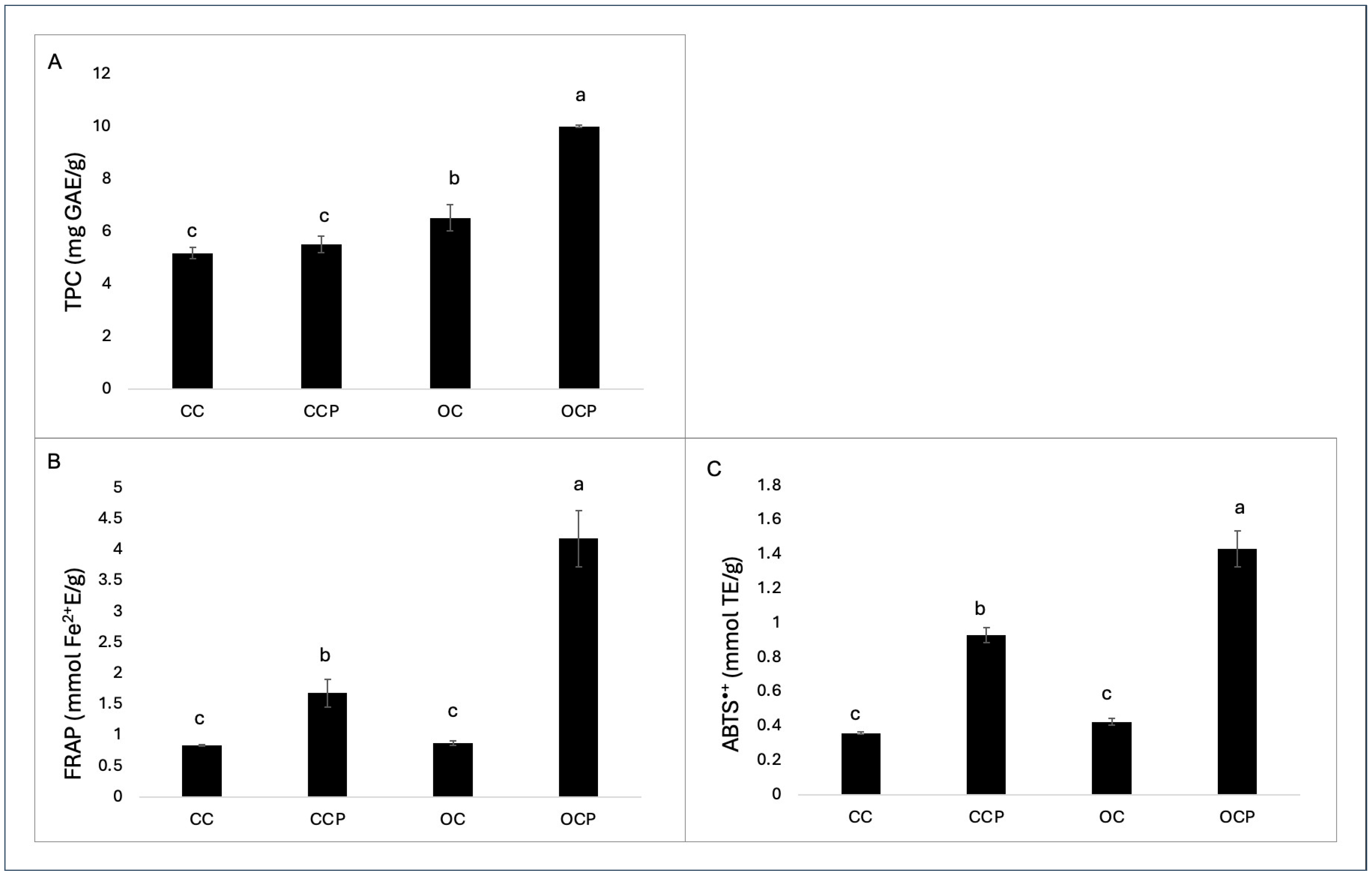
3.3. Total Phenolic Compounds (TPC) and Total Antioxidant Capacity (TAC)
3.4. Volatile Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Organic Farming in the EU, A Decade of Organic Growth. Agricultural Market Brief 20. 2023. Available online: https://agriculture.ec.europa.eu/document/download/df01a3c7-c0fb-48f1-8eca-ce452ea4b8c2_en?filename=agri-market-brief-20-organic-farming-eu_en.pdf (accessed on 2 June 2025).
- Bio in Cifre. SINAB Sistema d’informazione Nazionale sull’Agricoltura Biologica. 2023. Available online: https://www.sinab.it/sites/default/files/2023-07/BIO%20IN%20CIFRE%202023.pdf (accessed on 2 June 2025).
- Tricase, C.; Lamonaca, E.; Ingrao, C.; Bacenetti, J.; Giudice, A.L. A comparative life cycle assessment between organic and conventional barley cultivation for sustainable agriculture pathways. J. Clean. Prod. 2018, 172, 3747–3759. [Google Scholar] [CrossRef]
- Coppola, G.; Costantini, M.; Fusi, A.; Ruiz-Garcia, L.; Bacenetti, J. Comparative life cycle assessment of conventional and organic hazelnuts production systems in Central Italy. Sci. Total Environ. 2022, 826, 154107. [Google Scholar] [CrossRef] [PubMed]
- Cernusca, M.M.; Aguilar, F.X.; Gold, M.A. Post-purchase evaluation of U.S. consumers’ preferences for chestnuts. Agrofor. Syst. 2012, 86, 355–364. [Google Scholar] [CrossRef]
- Yavuz, S.; Islam, A.; Tomkaz, T. Examination of modern and traditional applications in hazelnut production. Acta Hortic. 2018, 1226, 329–332. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and in vitro preliminary anti-inflammatory activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) burs, leaves, and chestnuts extracts and their metabolite profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Progress in evaluating chestnuts quality: A review of recent developments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- Li, R.; Sharma, A.K.; Zhu, J.; Zheng, B.; Xiao, G.; Chen, L. Nutritional biology of chestnuts: A perspective review. Food Chem. 2022, 395, 133575. [Google Scholar] [CrossRef]
- Delgado, T.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Chapter 6: Bioactive Compounds of Chestnuts as Health Promoters. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part II; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 6, pp. 132–154. ISBN 978-1-68108-244-8. [Google Scholar]
- Ciucure, C.T.; Geana, E.I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and Nutritional Profile Composition in Fruits of Different Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Romania. Separations 2022, 9, 66. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Costantini, L.; Garzoli, S.; Merendino, N.; Massantini, R. Characterizing the antioxidant activity and sensory profile of chestnuts (Castanea sativa Mill.) grown in the Cimini Mountains of central Italy. J. Agric. Food Res. 2024, 16, 101113. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Massantini, R.; Corona, P. Better selection of chestnut cultivars: Experimenting the sensory characterization of the Marrone chestnuts compared to the “Chataigne” chestnuts. Eur. Food Res. Technol. 2024, 250, 961–969. [Google Scholar] [CrossRef]
- Poljak, I.; Vahcic, N.; Gacic, M.; Idžojtic, M. Morphology and chemical composition of fruits of the traditional Croatian chestnut variety ‘Lovran Marron’. Food Technol. Biotechnol. 2016, 54, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Corona, P.; Frangipane, M.T.; Moscetti, R.; Lo Feudo, G.; Castellotti, T.; Massantini, R. Chestnut Cultivar Identification through the Data Fusion of Sensory Quality and FT-NIR Spectral Data. Foods 2021, 10, 2575. [Google Scholar] [CrossRef] [PubMed]
- Mohsenin, N.N. Physical Properties of Plant and Animal Material; Gordon and Breach Science Publishers Inc.: New York, NY, USA, 1980. [Google Scholar]
- ISO 8589; Sensory Analysis. General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- UNI EN ISO 13299; Sensory Analysis. Methodology. General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- Frangipane, M.T.; Garzoli, S.; De Vita, D.; Massantini, R.; Corona, P. Identifying the sensory profile and fatty acid composition for quality valorization of Marrone chestnut cultivars. Eur. Food Res. Technol. 2024, 250, 2837–2847. [Google Scholar] [CrossRef]
- Warmund, M.R.; Elmore, J.R.; Adhikari, K.; McGraw, S. Descriptive sensory analysis and free sugar contents of chestnut cultivars grown in North America. J. Sci. Food Agric. 2011, 91, 1940–1945. [Google Scholar] [CrossRef]
- Serdar, U.; Bounous, G.; Ertürk, U.; Akyuz, B.; Fulbright, D.W. Evaluation of the descriptive characteristics of chestnut. Acta Hortic. 2018, 1220, 35–44. [Google Scholar] [CrossRef]
- Santos, M.J.; Pinto, T.; Mota, J.; Correia, E.; Vilela, A. Influence of the cooking system, chemical composition, and α-amylase activity on the sensory profile of chestnut cultivars—Longal and judia—And their consequence on consumer’s acceptability. Int. J. Gastron. Food Sci. 2023, 34, 100799. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Booth, M.; Fu, M.; Peterson, D.G. Consumer acceptance and sensory perception of roasted American European hybrid hazelnuts. J. Food Sci. 2024, 89, 4440–4449. [Google Scholar] [CrossRef]
- Costantini, L.; Lukšič, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Mujic, I.; Zivkovic, J.; Savic, V.; Alibabic, V.; Staver, M.; Jug, T.; Franic, M.; Damijanic, K. Analysis of volatile compounds in chestnut using solid phase microextraction coupled with GC-MS. Acta Hortic. 2018, 1220, 203–208. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability; Chestnut (Castanea sativa Mill.). TG/124/4; International Union for the Protection of New Varieties of Plants: Geneve, Switzerland, 2017. [Google Scholar]
- Poljak, I.; Vahcic, N.; Vidaković, A.; Tumpa, K.; Žarkovic, I.; Idžojtic, M. Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics. Agronomy 2021, 11, 516. [Google Scholar] [CrossRef]
- Contador, L.; Robles, B.; Shinya, P.; Medel, M.; Pinto, C.; Reginato, G.; Infante, R. Characterization of texture attributes of raw almond using a trained sensory panel. Fruits 2015, 70, 231–237. [Google Scholar] [CrossRef]
- Riondato, I.; Akyüz, B.; Beccaro, G.; Casey, J.; Conedera, M.; Coulié, J.; Diamandis, S.; Gomes-Laranjo, J.; Nishio, S.; Ramos-Cabrer, A. Cultivars list and breeding. In The Chestnut Handbook: Crop and Forest Management; Beccaro, G., Alma, G., Beccaro, A., Bounous, G., Gomes-Laranjo, J., Eds.; Taylor & Francis Group, LLC: Abingdon, UK, 2020; Chapter 4; pp. 52–117. [Google Scholar]
- Xu, K.; Jiang, K.; Yang, A.; Zhang, Z.; Lin, Z.; Wang, T.; Xu, L.; Meng, F.; Wang, B. Sensory and chemical characterization of chestnuts processed in different methods using instrumental analyses and the Check-all-that-apply method. Arab. J. Chem. 2024, 17, 105876. [Google Scholar] [CrossRef]
- Karaosmanoğlu, H. Lipid characteristics, bioactive properties, and mineral content in hazelnut grown under different cultivation systems. J. Food Process. Preserv. 2022, 46, e16717. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative study on phytochemical profiles and antioxidant capacities of chestnuts produced in different geographic area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Suna, S.; Avşar, B.; Koçer, S.; Çopur, Ö.U. Effects of different pretreatments on the physicochemical characteristics and quality criteria of chestnut (Castanea sativa Mill.) pickle: A new value-added product. J. Food Process. Preseretion 2021, 45, e15669. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, W.; Dang, Z.; Ma, R.; Yang, F. Chestnut pellicle as a new potential source of antioxidant activity, inhibition on cooking oil oxidation, and identification of phenolic compounds by HPLC–ESI–MS/MS. Eur. Food Res. Technol. 2023, 249, 1589–1598. [Google Scholar] [CrossRef]
- Carbonaro, M.; Mattera, M.; Nicoli, S.; Bergamo, P.; Cappelloni, M. Modulation of Antioxidant Compounds in Organic vs. Conventional Fruit (Peach, Prunus persica L., and Pear, Pyrus communis L.). J. Agric. Food Chem. 2002, 50, 5458–5462. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, J.; Ma, N.; Sun, Y.; Wang, J.; Liu, J.M.; Zhang, Y.; Lu, Y.; Wang, S. Comparative analysis of quality, structural, and flavor alterations in chestnuts (Castanea mollissima Blume) subjected to different thermal processing techniques. Food Chem. 2025, 474, 143149. [Google Scholar] [CrossRef]
| Descriptors | Sensory Attribute Definitions | Standards and Reference Materials |
|---|---|---|
| ease of peeling | the ease of peeling the shell and pellicle away from the nut | different levels of adherence of shell/pellicle to the nut (a value of 0 corresponds to hard, while 10 corresponds to easy) |
| seed color | the external color of the seed, after removing the pellicle | seed color with a degree of darkness (value 0 corresponds to a light colour seed, while value 10 corresponds to dark) |
| degree of pellicle penetration into the kernel | the degree of penetration of the seed coat into the embryo | a value of 0 corresponds to no penetration, while 10 corresponds to strong penetration (visible > 2.0 mm) |
| crunchiness | the amount of noise generated when the sample is chewed at a fast rate with the back teeth | 0 corresponds to a dried apple piece, while 10 corresponds to a fresh celery piece |
| astringency | the sensation of drying, drawing-up, or puckering of any of the mouth surfaces | diluted tannic acid solution (0.06–2 mg/mL) |
| sweetness | the basic taste associated with sugar (sucrose) | diluted sucrose solution (0.5–6 g/L) |
| bitterness | the basic taste associated with caffeine | diluted caffeine solution (0.03–0.2 g/L) |
| chestnut aroma | intensity of aroma of chestnut products | taste of chestnut |
| hazelnut aroma | sweet, oily, somewhat woody aromatics associated with hazelnuts | taste of hazelnut |
| almond aroma | sweet cherry pit-like nutty aromatics associated with almonds | taste of almond |
| butter aroma | aromatics commonly associated with natural, slightly salted butter | taste of butter |
| caramel aroma | aromatics associated with caramel | taste of caramel |
| floral aroma | aromatics associated with flowers | taste of floral |
| walnut aroma | aromatics associated with walnuts | taste of walnut |
| citrus aroma | aromatics associated with citrus fruits | taste of citrus |
| wood/musk | aromatics associated with wood or musk | taste of woody |
| aromatic intensity | the characteristic flavor of the chestnut at the seed break | aromatics commonly associated with chestnut |
| Samples | Weight (g) | Length (mm) | Width (mm) | Thickness (mm) | Geometric Mean Diameter (mm) | Arithmetic Mean Diameter (mm) | Surface Area (mm2) | Sphericity (%) | Volume (m3) |
|---|---|---|---|---|---|---|---|---|---|
| OC | 19.57 ± 0.18 a | 40.37 ± 0.22 a | 32.32 ± 0.20 a | 23.32 ± 0.19 a | 31.11 ± 0.18 a | 32.00 ± 0.18 a | 3040.34 ± 36.37 a | 0.77 ± 0.01 a | 15,921.26 ± 284.60 a |
| CC | 19.25 ± 0.20 b | 40.02 ± 0.24 b | 32.05 ± 0.21 b | 23.00 ± 0.18 b | 30.79 ± 0.18 b | 31.69 ± 0.19 b | 2977.64 ± 36.32 b | 0.76 ± 0.01 a | 15,430.80 ± 284.58 b |
| Pr > F(Model) | 0.001 | 0.012 | 0.042 | 0.002 | 0.001 | 0.001 | 0.001 | 0.274 | 0.001 |
| Significant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Sensory Attributes | Organic Chesnut (OC) | Conventional Chestnut (CC) |
|---|---|---|
| ease of peeling | 5.54 ± 0.28 a | 5.00 ± 0.20 b |
| seed color | 6.00 ± 0.53 a | 4.98 ± 0.50 b |
| degree of pellicle penetration into the kernel | 0.00 ± 0.00 a | 0.75 ± 0.42 b |
| crunchiness | 9.03 ± 0.57 a | 7.96 ± 0.51 b |
| sweetness | 6.98 ± 0.48 a | 7.96 ± 0.50 b |
| bitterness | 0.94 ± 0.11 a | 0.93 ± 0.10 b |
| astringency | 0.93 ± 0.09 a | 0.95 ± 0.10 b |
| chestnut aroma | 9.00 ± 0.52 a | 8.00 ± 0.50 b |
| hazelnut aroma | 5.88 ± 0.47 a | 5.00 ± 0.45 b |
| almond aroma | 5.00 ± 0.51 a | 4.01 ± 0.50 b |
| butter aroma | 3.96 ± 0.50 a | 3.00 ± 0.46 b |
| caramel aroma | 1.95 ± 0.98 a | 0.00 ± 0.00 b |
| floral aroma | 5.95 ± 0.51 a | 4.96 ± 0.41 b |
| aromatic intensity | 8.81 ± 0.47 a | 7.98 ± 0.40 b |
| overall judgment | 8.98 ± 0.26 a | 8.48 ± 0.23 b |
| Attributes | Floral Aroma | Astringency | Caramel Aroma | Sweetness | Hazelnut Aroma | Almond Aroma | Bitterness | Ease of Peeling | Butter Aroma | Aromatic Intensity | Crunchiness | Overall Judgment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| floral aroma | 1 | 0.200 | 0.111 | 0.048 | 0.111 | 0.333 | 0.207 | 0.022 | 0.005 | 0.022 | 0.005 | 0.048 |
| astringency | 0.200 | 1 | 0.200 | 0.086 | 0.022 | 0.000 | 0.190 | 0.004 | 0.052 | 0.004 | 0.009 | 0.086 |
| caramel aroma | 0.111 | 0.200 | 1 | 0.429 | 0.111 | 0.333 | 0.105 | 0.200 | 0.048 | 0.022 | 0.403 | 0.429 |
| sweetness | 0.048 | 0.086 | 0.429 | 1 | 0.429 | 0.571 | 0.045 | 0.086 | 0.020 | 0.086 | 0.002 | 0.020 |
| hazelnut aroma | 0.111 | 0.022 | 0.111 | 0.429 | 1 | 0.333 | 0.105 | 0.022 | 0.132 | 0.200 | 0.005 | 0.048 |
| almond aroma | 0.333 | 0.000 | 0.333 | 0.571 | 0.333 | 1 | 0.456 | 0.267 | 0.254 | 0.000 | 0.000 | 0.000 |
| bitterness | 0.207 | 0.190 | 0.105 | 0.045 | 0.105 | 0.456 | 1 | 0.527 | 0.219 | 0.190 | 0.159 | 0.045 |
| ease of peeling | 0.022 | 0.004 | 0.200 | 0.086 | 0.022 | 0.267 | 0.527 | 1 | 0.560 | 0.360 | 0.224 | 0.086 |
| butter aroma | 0.005 | 0.052 | 0.048 | 0.020 | 0.132 | 0.254 | 0.219 | 0.560 | 1 | 0.306 | 0.002 | 0.020 |
| aromatic intensity | 0.022 | 0.004 | 0.022 | 0.086 | 0.200 | 0.000 | 0.190 | 0.360 | 0.306 | 1 | 0.288 | 0.238 |
| crunchiness | 0.005 | 0.009 | 0.403 | 0.002 | 0.005 | 0.000 | 0.159 | 0.224 | 0.002 | 0.288 | 1 | 0.770 |
| overall judgment | 0.048 | 0.086 | 0.429 | 0.020 | 0.048 | 0.000 | 0.045 | 0.086 | 0.020 | 0.238 | 0.770 | 1 |
| N° | Component 1 | LRI 2 | LRI 3 | OCP (%Area) | OC (%Area) | CCP (%Area) | CC (%Area) |
|---|---|---|---|---|---|---|---|
| 1 | ethanol | 430 | 436 | 30.5 ± 1.2 a | 22.2 ± 1.3 b | 25.5 ± 1.4 c,b | 18.2 ± 0.9 d |
| 2 | 2-propanol | 475 | 481 | tr | 2.2 ± 0.06 a | - | 0.5 ± 0.02 b |
| 3 | acetone | 505 | 500 | 3.5 ± 0.08 a | 0.8 ± 0.02 b | 2.7 ± 0.05 c | 2.0 ± 0.05 d,c |
| 4 | 2-pentanol | 660 | 664 | 0.1 ± 0.01 a | 1.1 ± 0.06 b | tr | 0.5 ± 0.02 c |
| 5 | 2-pentanone | 690 | 684 | 1.0 ± 0.03 a | 1.5 ± 0.05 b,c | tr | 0.2 ± 0.02 d |
| 6 | 3-methyl-1-butanol | 722 | 719 | 1.2 ± 0.05 a | 3.3 ± 0.06 b | 0.5 ± 0.03 c | 0.9 ± 0.04 d |
| 7 | 2-methyl-1-butanol | 746 | 744 | 1.8 ± 0.04 a | 5.1 ± 0.05 b | 1.1 ± 0.04 c | 1.9 ± 0.04 d,a |
| 8 | 1-hexanol | 800 | 801 | 10.5 ± 0.09 a | 7.1 ± 0.10 b | 9.9 ± 0.14 c,a | 5.5 ± 1.60 d |
| 9 | 2-heptanone | 865 | 873 | 8.1 ± 0.20 a | 3.2 ± 0.04 b | 9.6 ± 0.15 c | 5.1 ± 0.05 d |
| 10 | benzaldehyde | 918 | 923 | 0.8 ± 0.04 a | 0.2 ± 0.02 b | 1.1 ± 0.04 c | 0.5 ± 0.02 d,b |
| 11 | 1-octen-3-ol | 975 | 980 | tr | 0.5 ± 0.02 a | - | 0.1 ± 0.01 b |
| 12 | 3-octanol | 993 | 997 | 1.2 ± 0.05 a | 5.5 ± 0.06 b | 1.5 ± 0.04 c,a | 4.0 ± 0.06 d |
| 13 | limonene | 1018 | 1026 | 2.3 ± 0.05 a | 1.1 ± 0.03 b | 1.8 ± 0.05 c | 0.6 ± 0.03 d |
| 14 | 1-octanol | 1075 | 1080 | 25.0 ± 2.0 a | 20.2 ± 2.5 b | 27.8 ± 1.8 c | 22.1 ± 2.1 d |
| 15 | 2-nonanone | 1089 | 1091 | 0.8 ± 0.03 a | tr | 0.1 ± 0.01 b | - |
| 16 | (E)-2-hexenal | 1200 | 1205 | 1.2 ± 0.07 a | 1.1 ± 0.05 a | 0.9 ± 0.03 b | 0.2 ± 0.12 c |
| 17 | decanal | 1207 | 1217 | 0.9 ± 0.05 a | 0.2 ± 0.02 b | - | - |
| 18 | 1-pentanol | 1230 | 1233 | 0.1 ± 0.01 a | 0.5 ± 0.02 b | - | - |
| 19 | octanal | 1285 | 1278 | 0.5 ± 0.03 a | 0.2 ± 0.02 b | 3.9 ± 0.04 c | 10.6 ± 0.09 d |
| 20 | 2-heptanol | 1333 | 1325 | tr | 1.8 ± 0.05 a | 1.7 ± 0.07 a | 3.9 ± 0.09 b |
| 21 | nonanal | 1387 | 1390 | 0.8 ± 0.03 a | 5.5 ± 0.07 b | 2.7 ± 0.05 c | 7.5 ± 0.06 d |
| 22 | acetic acid | 1420 | 1427 | 9.2 ± 0.08 a | 15.2 ± 0.2 b | 7.8 ± 0.05 c | 15.3 ± 0.1 d,b |
| SUM | 99.5 | 98.5 | 98.6 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frangipane, M.T.; Costantini, L.; Garzoli, S.; Merendino, N.; Massantini, R.; Corona, P. Organic vs. Conventional Chestnuts (Castanea sativa Mill.): A Focus on Antioxidant Activity, Volatile Compounds, and Sensory Profile. Foods 2025, 14, 2013. https://doi.org/10.3390/foods14122013
Frangipane MT, Costantini L, Garzoli S, Merendino N, Massantini R, Corona P. Organic vs. Conventional Chestnuts (Castanea sativa Mill.): A Focus on Antioxidant Activity, Volatile Compounds, and Sensory Profile. Foods. 2025; 14(12):2013. https://doi.org/10.3390/foods14122013
Chicago/Turabian StyleFrangipane, Maria Teresa, Lara Costantini, Stefania Garzoli, Nicolò Merendino, Riccardo Massantini, and Piermaria Corona. 2025. "Organic vs. Conventional Chestnuts (Castanea sativa Mill.): A Focus on Antioxidant Activity, Volatile Compounds, and Sensory Profile" Foods 14, no. 12: 2013. https://doi.org/10.3390/foods14122013
APA StyleFrangipane, M. T., Costantini, L., Garzoli, S., Merendino, N., Massantini, R., & Corona, P. (2025). Organic vs. Conventional Chestnuts (Castanea sativa Mill.): A Focus on Antioxidant Activity, Volatile Compounds, and Sensory Profile. Foods, 14(12), 2013. https://doi.org/10.3390/foods14122013










