Bergamot and Olive Extracts as Beer Ingredients: Impact on Cell Viability, Reactive Oxygen Species, and RNA Expression of Antioxidant Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Antioxidant Compounds
2.2.1. Total Phenolic Content (TP)
2.2.2. Total Flavonoid Content (TF)
2.2.3. Total Carbohydrates and Ascorbic Acid Detection
2.2.4. Polyphenolic Profile Determination
2.2.5. Validation Method for Polyphenols
2.3. Lyophilized Sample Preparation for Cell Culture Treatments
2.4. Cells and Culture Conditions
2.5. Cell Viability
2.6. Determination of Reactive Oxygen Species (ROS)
2.7. RNA Isolation Reverse Transcription and Quantitative PCR
2.8. Statistical Analysis
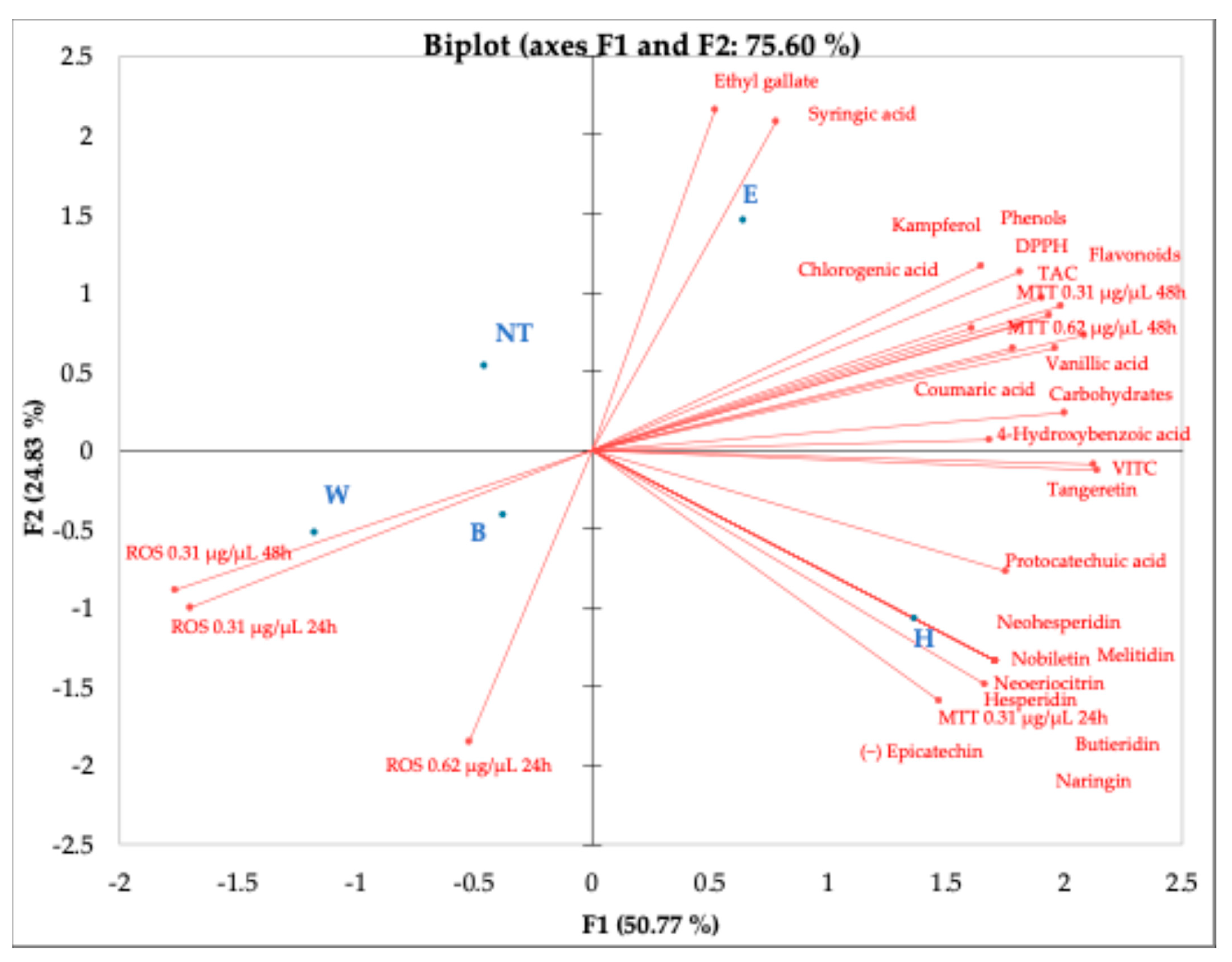
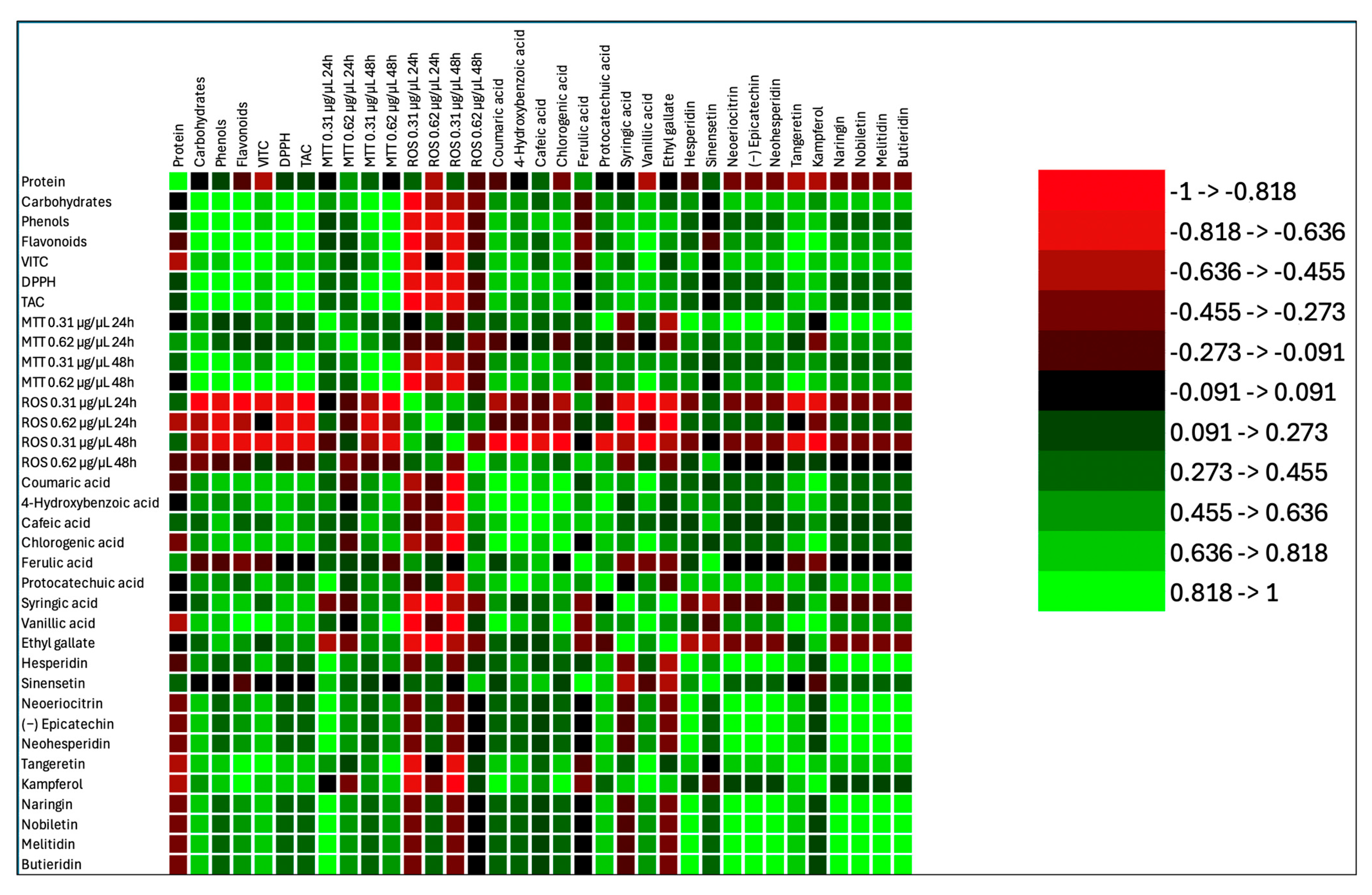
3. Results and Discussion
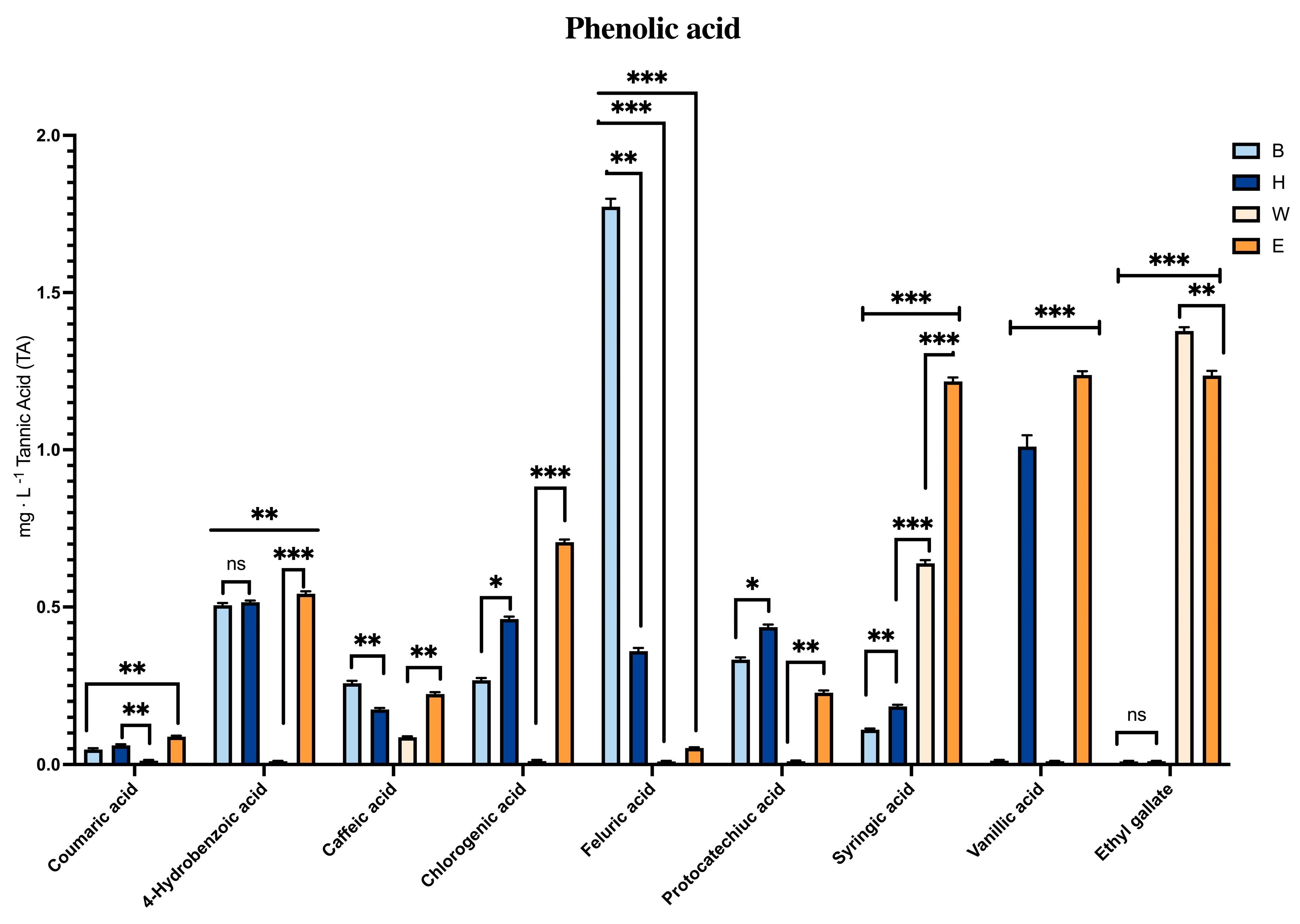
3.1. Effect of Antioxidant Compounds
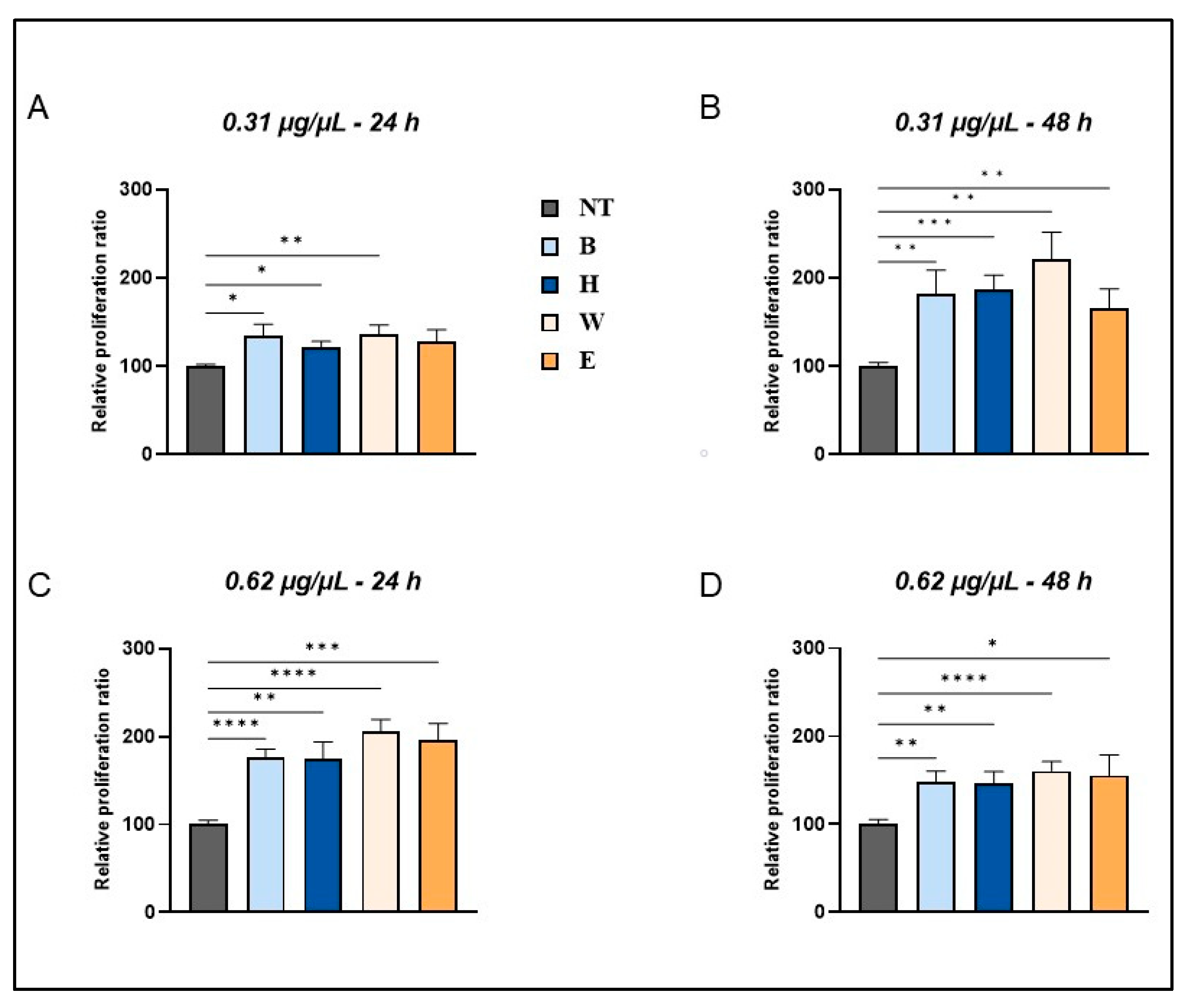
3.2. Effects of Lyophilized Beer Samples on Cell Viability
3.3. Effects of Lyophilized Beer Samples on Reactive Oxygen Species Level
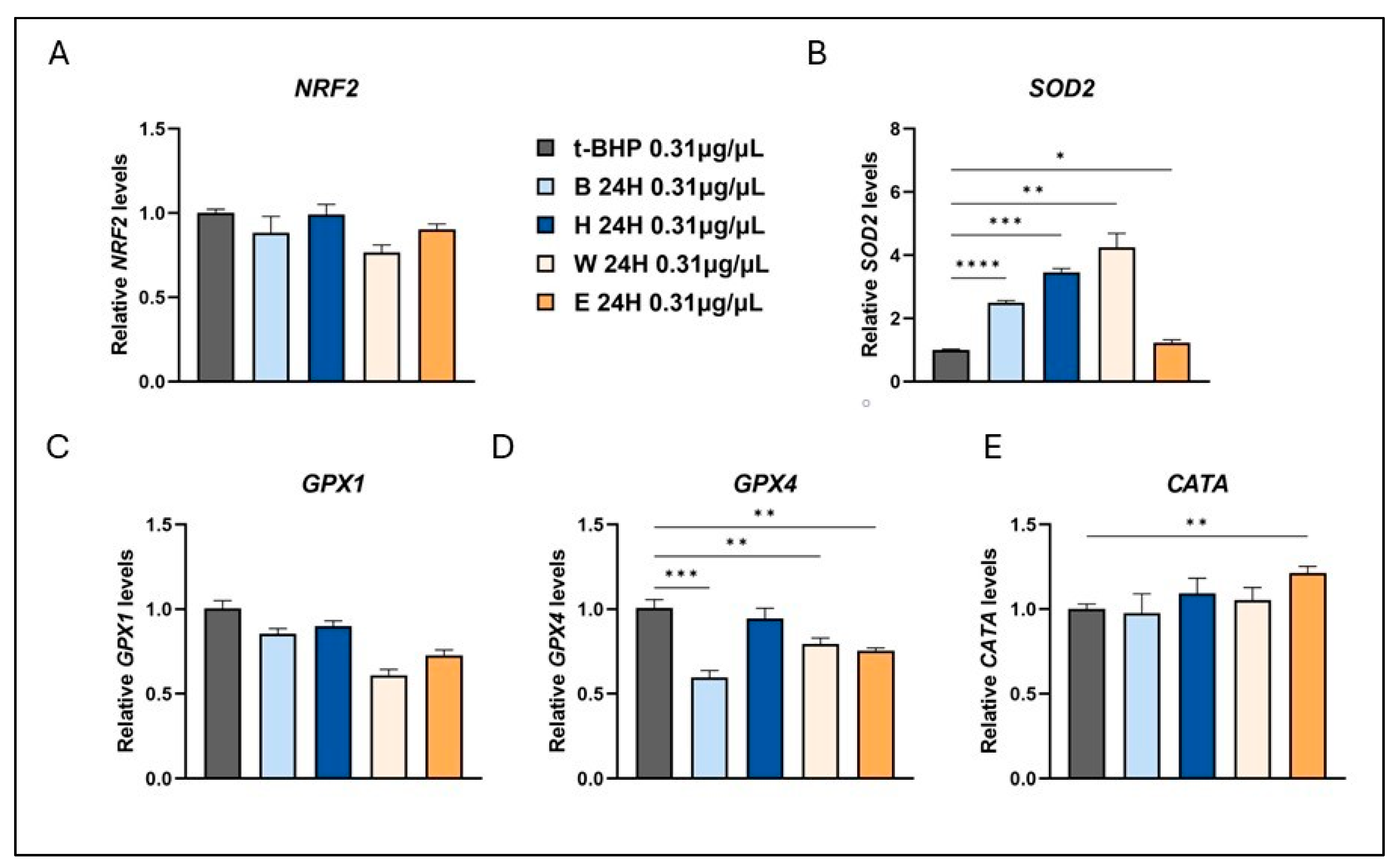
3.4. Effects of Lyophilized Beer Samples on RNA Levels of Antioxidant Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poelmans, E.; Swinnen, J.F.M. 1 A brief economic history of beer. In Oxford University Press eBooks; Oxford University Press: Oxford, UK, 2011; pp. 3–28. [Google Scholar] [CrossRef]
- Paiva, R.a.M.; Mutz, Y.S.; Conte-Junior, C.A. A review on the obtaining of functional beers by addition of Non-Cereal adjuncts rich in antioxidant compounds. Antioxidants 2021, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Živković, A.; Krstanović, V.; Mastanjević, K. Functional Beer—A review on possibilities. Beverages 2020, 6, 51. [Google Scholar] [CrossRef]
- Muscolo, A.; Marra, F.; Salafia, F.; Andronaco, P.; Di Sanzo, R.; Carabetta, S.; Russo, M. Bergamot and olive extracts as beer ingredients: Their influence on nutraceutical and sensory properties. Eur. Food Res. Technol. 2022, 248, 2067–2077. [Google Scholar] [CrossRef]
- Maruca, N.G.; Laghetti, N.G.; Mafrica, N.R.; Turiano, N.D.; Hammer, N.K. The Fascinating History of Bergamot (Citrus bergamia Risso & Poiteau), The Exclusive Essence of Calabria: A review. J. Environ. Sci. Eng. —A 2017, 6, 22–30. [Google Scholar] [CrossRef][Green Version]
- Gattuso, A.; Mafrica, R.; Cannavò, S.; Mafrica, D.; De Bruno, A.; Poiana, M. Quality evaluation of bergamot juice produced in different areas of Calabria region. Foods 2024, 13, 2080. [Google Scholar] [CrossRef]
- Adorisio, S.; Muscari, I.; Fierabracci, A.; Thuy, T.T.; Marchetti, M.C.; Ayroldi, E.; Delfino, D.V. Biological effects of bergamot and its potential therapeutic use as an anti-inflammatory, antioxidant, and anticancer agent. Pharm. Biol. 2023, 61, 639–646. [Google Scholar] [CrossRef]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Foods 2015, 20, 10–19. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Com-pound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Nardini, M. An Overview of Bioactive Phenolic Molecules and Antioxidant Properties of Beer: Emerging Trends. Molecules 2023, 28, 3221. [Google Scholar] [CrossRef]
- Nardini, M.; Foddai, M.S. Phenolics profile and antioxidant activity of special beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Tarko, T.; Krankowski, F.; Duda-Chodak, A. The Impact of Compounds Extracted from Wood on the Quality of Alcoholic Beverages. Molecules 2023, 28, 620. [Google Scholar] [CrossRef] [PubMed]
- Maria, G.A.; Riccardo, N. Citrus bergamia, Risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 2020, 522, 522–532. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Bullo, M.; Lamuela-Raventos, R.; Salas-Salvado, J. Mediterranean diet and oxidation: Nuts and olive oil as important sources of fat and antioxidants. Curr. Top. Med. Chem. 2011, 11, 1797–1810. [Google Scholar] [CrossRef]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Jaouni, S.K.A.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- De Bruno, A.; Zappia, A.; Piscopo, A.; Poiana, M. Qualitative evaluation of fermented olives grown in Southern Italy (cvs. Carolea, Grossa of Gerace and Nocellara Messinese). Emir. J. Food Agric. 2019, 587, 587–596. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Muscolo, A.; Calderaro, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Soil salinity improves nutritional and health promoting compounds in three varieties of lentil (Lens culinaris Med.). FoodBioscience 2020, 35, 100571. [Google Scholar] [CrossRef]
- Papalia, T.; Barreca, D.; Panuccio, M. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef]
- Pacelli, C.; De Rasmo, D.; Signorile, A.; Grattagliano, I.; Di Tullio, G.; D’Orazio, A.; Nico, B.; Pietro Comi, G.; Ronchi, D.; Ferranini, E.; et al. Mitochondrial defect and PGC-1α dysfunction in parkin-associated familial Parkinson’s disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Gaballo, A.; Signorile, A.; Ferretta, A.; Tanzarella, P.; Pacelli, C.; Di Paola, M.; Cocco, T.; Maffia, M. Resveratrol Modulation of protein expression in parkin-Mutant human skin fibroblasts: A proteomic approach. Oxidative Med. Cell. Longev. 2017, 2017, 2198243. [Google Scholar] [CrossRef] [PubMed]
- Tanzarella, P.; Ferretta, A.; Barile, S.N.; Ancona, M.; De Rasmo, D.; Signorile, A.; Papa, S.; Capitanio, N.; Pacelli, C.; Cocco, T. Increased levels of CAMP by the Calcium-Dependent activation of soluble adenylyl cyclase in Parkin-Mutant fibroblasts. Cells 2019, 8, 250. [Google Scholar] [CrossRef]
- Van De Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Matrella, M.L.; Valletti, A.; Marra, F.; Mallamaci, C.; Cocco, T.; Muscolo, A. Phytochemicals from Red Onion, Grown with Eco-Sustainable Fertilizers, Protect Mammalian Cells from Oxidative Stress, Increasing Their Viability. Molecules 2022, 27, 6365. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging properties of Plant-Derived natural biomolecule Para-Coumaric acid in the prevention of oxidative Stress-Induced diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Roy, A.J.; Prince, P.S.M. Preventive effects of p-coumaric acid on cardiac hypertrophy and alterations in electrocardiogram, lipids, and lipoproteins in experimentally induced myocardial infarcted rats. Food Chem. Toxicol. 2013, 60, 348–354. [Google Scholar] [CrossRef]
- Tehami, W.; Nani, A.; Khan, N.A.; Hichami, A. New insights into the anticancer effects of P-Coumaric acid: Focus on colorectal cancer. Dose-Response 2023, 21, 15593258221150704. [Google Scholar] [CrossRef]
- Ziółkiewicz, A.; Kasprzak-Drozd, K.; Rusinek, R.; Markut-Miotła, E.; Oniszczuk, A. The influence of polyphenols on atherosclerosis development. Int. J. Mol. Sci. 2023, 24, 7146. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on its Anti-Obesity Property. Curr. Mol. Pharmacol. 2019, 13, 7–16. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients 2023, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Muddu, K.; Jayaprakash, N.K.; Asha, S.; Sravan, K. Biological Potential of Ethyl Gallate. Res. J. Biotechnol. 2023, 19, 122–125. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Valle-Jiménez, X.; Brunauer, R.; Alavez, S. Vanillic Acid Improves Stress Resistance and Substantially Extends Life Span in Caenorhabditis elegans. J. Gerontol. Ser. A 2023, 78, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Okoduwa, S.I.R.; Abdulwaliyu, I.; Igiri, B.E.; Arekemase, S.O.; Okoduwa, U.J.; Itiat, J.F.; Egbule, M.N.; Mustapha, R.A. Multi-therapeutic potential of flavonoids as an essential component in nutraceuticals for the treatment and management of human diseases. Phytomedicine Plus 2024, 4, 100558. [Google Scholar] [CrossRef]
- Leopoldini, M.; Malaj, N.; Toscano, M.; Sindona, G.; Russo, N. On the inhibitor effects of bergamot juice flavonoids binding to the 3-hydroxy-3-methylglutaryl-coa reductase (HMGR) enzyme. J. Agric. Food Chem. 2010, 58, 10768–10773. [Google Scholar] [CrossRef]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. Defining the Cholesterol Lowering Mechanism of Bergamot (Citrus bergamia) Extract in HepG2 and Caco-2 Cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef]
- Ebadi, P.; Fazeli, M. Evaluation of the potential in vitro effects of propolis and honey on wound healing in human dermal fibroblast cells. South Afr. J. Bot. 2021, 137, 414–422. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research Strategies in the Study of the Pro-oxidant Nature of Polyphenol Nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef]
- Goyal, S.; Thirumal, D.; Singh, S.; Kumar, D.; Singh, I.; Kumar, G.; Sindhu, R.K. Basics of Antioxidants and Their Importance. In Antioxidants: Nature’s Defense Against Disease, 1st ed.; Sindhu, R.K., Singh, I., Arockia Babu, M., Eds.; Wiley: New York, NY, USA, 2024; Chapter 1. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A Critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospíšil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, J.W.; Banerjee, S.; Bae, H.; Friggeri, A.; Lazarowski, E.R.; Abraham, E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010, 285, 33154–33164. [Google Scholar] [CrossRef] [PubMed]
- Auciello, F.R.; Ross, F.A.; Ikematsu, N.; Hardie, D.G. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Lett. 2014, 588, 3361–3366. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Expermental Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]



| Compounds | Rt | ±SD | RSD% | Recovery (%) |
|---|---|---|---|---|
| Coumaric acid | 12.396 | 0.008 | 0.496 | 102.1 |
| 4-Hydroxybenzoic acid | 4.060 | 0.014 | 1.310 | 103.8 |
| Caffeic acid | 7.751 | 0.011 | 0.601 | 102.0 |
| Ethyl gallate | 9.836 | 0.020 | 1.396 | 102.7 |
| Ferulic acid | 11.323 | 0.007 | 0.258 | 101.9 |
| Kampferol | 18.551 | 0.014 | 0.767 | 100.8 |
| Protocatechuic acid | 2.157 | 0.009 | 0.769 | 102.3 |
| Syringic acid | 8.996 | 0.008 | 0.283 | 97.3 |
| Vanillic acid | 7.344 | 0.015 | 1.172 | 95.9 |
| Chlorogenic acid | 8.637 | 0.015 | 0.837 | 95.9 |
| Naringin | 14.125 | 0.010 | 0.358 | 93.2 |
| Hesperidin | 14.531 | 0.007 | 0.321 | 95.8 |
| Sinensetin | 21.488 | 0.006 | 0.385 | 108.2 |
| Neoeriocitrin | 12.986 | 0.011 | 0.772 | 95.1 |
| (-)Epicatechin | 9.880 | 0.013 | 0.868 | 93.9 |
| Neohesperidin | 14.925 | 0.042 | 0.604 | 100.8 |
| Tangeretin | 23.290 | 0.019 | 0.964 | 104.4 |
| Melitidin | 11.422 | 0.009 | 0.655 | 102.8 |
| Nobiletin | 22.432 | 0.006 | 0.077 | 99.7 |
| Butieridin | 11.611 | 0.011 | 0.101 | 99.6 |
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| NRF2 | 5′-AGCCCAGCACATCCAGTCA-3′ | 5′-TGTGGGCAACCTGGGAGTAG-3′ |
| SOD2 | 5′-CTGGACAAACCTCAGCCCT-3′ | 5′-CTGATTTGGACAAGCAGCAA-3′ |
| GPX1 | 5′-AGAACGCCAAGAAGCAGAAGA-3′ | 5′-CATGAAGTTGGGCTCGAACC-3′ |
| GPX4 | 5′-AACTACACTCAGCTGCTGC-3′ | 5′-GCAGGTCTTCTCTCATCACC-3′ |
| CATA | 5′-TGGAAAGAAGACTCCCATCG-3′ | 5′-CCAGAGATCCCAGACCATGT-3′ |
| GAPDH | 5′-CAACTTTGGTATCGTGGAAGGAC-3′ | 5′-ACAGTCTTCTGGGTGGCAGTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matrella, M.L.; Amenta, B.; Canino, F.; Maffia, A.; Cocco, T.; Russo, M.; Muscolo, A. Bergamot and Olive Extracts as Beer Ingredients: Impact on Cell Viability, Reactive Oxygen Species, and RNA Expression of Antioxidant Enzymes. Foods 2025, 14, 2012. https://doi.org/10.3390/foods14122012
Matrella ML, Amenta B, Canino F, Maffia A, Cocco T, Russo M, Muscolo A. Bergamot and Olive Extracts as Beer Ingredients: Impact on Cell Viability, Reactive Oxygen Species, and RNA Expression of Antioxidant Enzymes. Foods. 2025; 14(12):2012. https://doi.org/10.3390/foods14122012
Chicago/Turabian StyleMatrella, Maria Laura, Bruna Amenta, Francesco Canino, Angela Maffia, Tiziana Cocco, Mariateresa Russo, and Adele Muscolo. 2025. "Bergamot and Olive Extracts as Beer Ingredients: Impact on Cell Viability, Reactive Oxygen Species, and RNA Expression of Antioxidant Enzymes" Foods 14, no. 12: 2012. https://doi.org/10.3390/foods14122012
APA StyleMatrella, M. L., Amenta, B., Canino, F., Maffia, A., Cocco, T., Russo, M., & Muscolo, A. (2025). Bergamot and Olive Extracts as Beer Ingredients: Impact on Cell Viability, Reactive Oxygen Species, and RNA Expression of Antioxidant Enzymes. Foods, 14(12), 2012. https://doi.org/10.3390/foods14122012








