Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review
Abstract
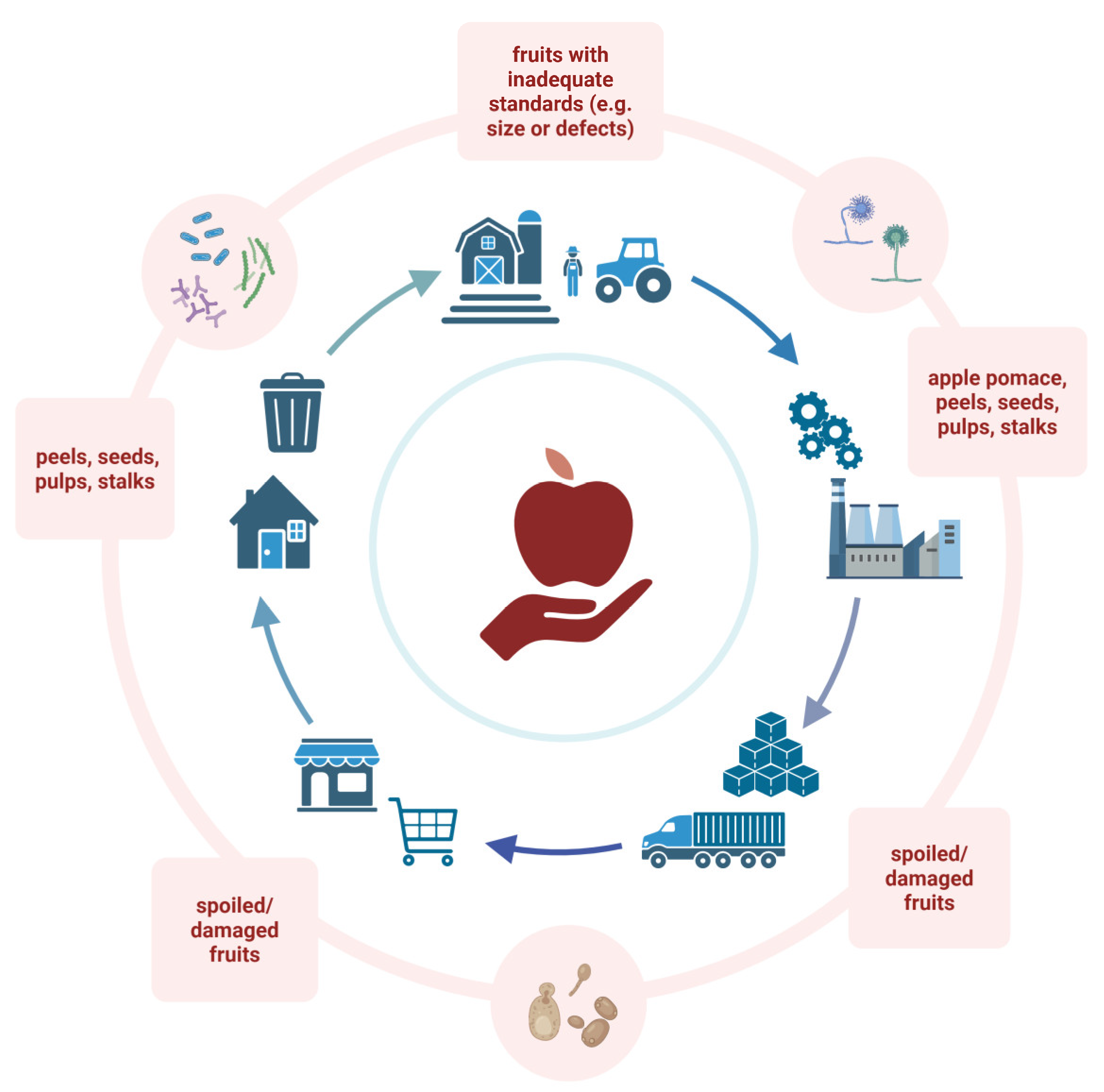
1. Introduction
2. Apple By-Products Composition
3. Valorization of Apple By-Products as a Source of Highly Valuable Materials
4. Microbial Diversity in the Generation of New Foods from Apple By-Products
4.1. Apple Juices
4.2. Apple Pomaces
4.3. Apple Peels
5. Apple By-Products to Support the Growth of Desired Microbes
6. Case Studies on the Synergic Use of Apple By-Products and Microbes to Promote the Design of Added-Value Foods
| Apple By-Product | Matrix Type | Species | Application | Reference |
|---|---|---|---|---|
| Apple fiber | Dairy products | Lacticaseibacillus casei | Incorporation into yoghurt Protective matrix for the probiotic strain | [178] |
| Freeze-dried apple pomace | Yoghurt starter culture | Improve the rheological properties Increase dietary fiber and phytochemical contents | [179] | |
| Apple pomace extract | Yoghurt starter culture | Enhance antioxidant and anti-inflammatory potential | [124] | |
| Freeze-dried apple pomace | Meat products | Autochthonous lactic bacteria | Increase in the brightness of baked meat | [132] |
| Freeze-dried apple pomace | Autochthonous Turkey sausage | Decrease pH, cooking loss, and yellowness Improve the total phenolic content | [133] | |
| Apple pomace | Autochthonous Buffalo meat sausage | Improve physicochemical and sensory properties | [194] | |
| Apple pomace powder | Autochthonous Goshtaba microbial resources (Traditional Indian meatballs) | Fat replacer: decreased fat content in the final product Improve sensory properties | [195] | |
| Apple by-products | Fermented beverages | Saccharomyces cerevisiae, Torulaspora delbrueckii | Increase in the level of volatile compounds | [180] |
| Apple pomace | Saccharomyces cerevisiae r.f. bayanus | Fermentation kinetics not affected Higher alcoholic level Increase in polyphenol compound level | [181] | |
| Apple pomace | Lacticaseibacillus rhamnosus and Lacticaseibacillus casei | Aromatizer in beer | [152] | |
| Apple pomace | Baking products | Weissella cibaria, Leuconostoc mesenteroides, Lactiplantibacillus plantarum, Saccharomyces cerevisiae, Hanseniaspora uvarum | Increase in total and insoluble dietary fibers Extend the shelf life of the wheat bread | [147] |
| Apple pomace | Autochthonous microbes | Increase fiber content and antioxidant properties of extruded snacks and baked scones | [148] | |
| Apple pomace | Production of gluten-free bread enriched with apple pomace Increase phenolic compound content | [149] |
7. Limitations Associated with the Consumption of Apple By-Products
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture, Foreign Agricultural Service. Data and Analysis. Available online: https://www.fas.usda.gov/data (accessed on 7 May 2025).
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kui, L.; Zhang, J.; Xie, Y.; Wang, L.; Yan, Y.; Wang, N.; Xu, J.; Li, C.; Wang, W.; et al. Improved hybrid de novo genome assembly of domesticated apple (Malus × domestica). GigaScience 2016, 5, s13742-016. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Kumar, S.; Das, A.; Kumar, R.; Bains, A.; Sridhar, K.; Duhan, J.S.; Sharma, M. Recovery of agricultural waste biomass: A path for circular bioeconomy. Sci. Total Environ. 2023, 870, 161904. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Avery, S.V.; Singleton, I. Chapter Two—Microbes associated with fresh produce: Sources, types and methods to reduce spoilage and contamination. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 29–82. [Google Scholar]
- De la Peña-Armada, R.; Mateos-Aparicio, I. Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules 2022, 27, 6937. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Cui, S.; Yu, Q.; Ma, R. Anaerobic co-digestion of animal manures with corn stover or apple pulp for enhanced biogas production. Renew. Energy 2018, 118, 335–342. [Google Scholar] [CrossRef]
- Maslovarić, M.D.; Vukmirović, Đ.; Pezo, L.; Čolović, R.; Jovanović, R.; Spasevski, N.; Tolimir, N. Influence of apple pomace inclusion on the process of animal feed pelleting. Food Addit. Contam. Part A 2017, 34, 1353–1363. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Haider, M.W.; Abbas, S.M.; Saeed, M.A.; Farooq, U.; Waseem, M.; Adil, M.; Javed, M.R.; Haq, I.U.; Osei Tutu, C. Environmental and Nutritional Value of Fruit and Vegetable Peels as Animal Feed: A Comprehensive Review. Anim. Res. One Health 2025, 3, 149–164. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; de la Torre-Ramírez, J.M.; Martínez-López, S.; Ayuso-García, L.M.; Dellapina, G.; Poli, G.; Verardo, V.; Gómez-Caravaca, A.M. Green Extraction of Phenolic Compounds from Artichoke By-Products: Pilot-Scale Comparison of Ultrasound, Microwave, and Combined Methods with Pectinase Pre-Treatment. Antioxidants 2025, 14, 423. [Google Scholar] [CrossRef]
- Nour, V. Increasing the Content of Bioactive Compounds in Apple Juice Through Direct Ultrasound-Assisted Extraction from Bilberry Pomace. Foods 2024, 13, 4144. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Han, K.N.; Meral, H.; Demirdöven, A. Recovery of carotenoids as bioactive compounds from peach pomace by an eco-friendly ultrasound-assisted enzymatic extraction. J. Food Sci. Technol. 2024, 61, 2354–2366. [Google Scholar] [CrossRef]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef]
- Alvi, T.; Asif, Z.; Khan, M.K.I. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique-A review. Food Biosci. 2022, 46, 101580. [Google Scholar] [CrossRef]
- Sarker, M.S.; Alam, M.M.; Jiao, C.; Shuqi, W.; Xiaohui, L.; Ali, N.; Mallasiy, L.O.; Alshehri, A.A. Maximizing polyphenol yield: Ultrasound-assisted extraction and antimicrobial potential of mango peel. Prep. Biochem. Biotechnol. 2025, 55, 349–358. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza Mesquita, L.M.; Strieder, M.M.; Contieri, L.S.; Pizani, R.S.; Sanches, V.L.; Viganó, J.; Bezerra, R.M.N.; Rostagno, M.A. Eco-friendly and high-performance extraction of flavonoids from lemon peel wastes by applying ultrasound-assisted extraction and eutectic solvents. Sustain. Chem. Pharm. 2024, 39, 101558. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and mechanisms of dietary polyphenols affected by non-thermal processing technology in fruits and vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Ampese, L.C.; Ziero, H.D.D.; Sganzerla, W.G.; Forster-Carneiro, T. Apple pomace biorefinery: Integrated approaches for the production of bioenergy, biochemicals, and value-added products—An updated review. J. Environ. Chem. Eng. 2022, 10, 108358. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies—A review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.; et al. Path2Green: Introducing 12 green extraction principles and a novel metric for assessing sustainability in biomass valorization. Green Chem. 2024, 26, 10087–10106. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef]
- Kim, I.J.; Park, S.; Kyoung, H.; Song, M.; Kim, S.R. Microbial valorization of fruit processing waste: Opportunities, challenges, and strategies. Curr. Opin. Food Sci. 2024, 56, 101147. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Cirat, R.; Benmechernene, Z.; Cunedioğlu, H.; Rutigliano, M.; Scauro, A.; Abderrahmani, K.; Mebrouk, K.; Capozzi, V.; Spano, G.; la Gatta, B.; et al. Cross-Over Application of Algerian Dairy Lactic Acid Bacteria for the Design of Plant-Based Products: Characterization of Weissella cibaria and Lactiplantibacillus plantarum for the Formulation of Quinoa-Based Beverage. Microorganisms 2024, 12, 2042. [Google Scholar] [CrossRef]
- Cirat, R.; Capozzi, V.; Benmechernene, Z.; Spano, G.; Grieco, F.; Fragasso, M. LAB Antagonistic Activities and Their Significance in Food Biotechnology: Molecular Mechanisms, Food Targets, and Other Related Traits of Interest. Fermentation 2024, 10, 222. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-based biocontrol solutions for fruits and vegetables: Recent insight, patents, and innovative trends. Recent Pat. Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Tartaglia, J.; Accotto, G.P.; Beato, M.S.; Bernini, V.; Bevivino, A.; Boniotti, M.B.; Budroni, M.; Buzzini, P.; Carrara, S.; et al. Treasures of Italian Microbial Culture Collections: An Overview of Preserved Biological Resources, Offered Services and Know-How, and Management. Sustainability 2024, 16, 3777. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Bimbo, F. Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation 2021, 7, 54. [Google Scholar] [CrossRef]
- Selmi, H.; Rocchetti, M.T.; Capozzi, V.; Semedo-Lemsaddek, T.; Fiocco, D.; Spano, G.; Abidi, F. Lactiplantibacillus plantarum from Unexplored Tunisian Ecological Niches: Antimicrobial Potential, Probiotic and Food Applications. Microorganisms 2023, 11, 2679. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging Postharvest Technologies to Enhance the Shelf-Life of Fruit and Vegetables: An Overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action. Int. J. Mol. Sci. 2021, 22, 12076. [Google Scholar] [CrossRef]
- Adesemoye, E.T.; Sanni, A.I.; Spano, G.; Capozzi, V.; Fragasso, M. Lactic Acid Bacteria Diversity in Fermented Foods as Potential Bio-Resources Contributing to Alleviate Malnutrition in Developing Countries: Nigeria as a Case Study. Fermentation 2025, 11, 103. [Google Scholar] [CrossRef]
- He, L.; Yan, Y.; Wu, M.; Ke, L. Advances in the Quality Improvement of Fruit Wines: A Review. Horticulturae 2024, 10, 93. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Capozzi, V.; Tufariello, M.; Berbegal, C.; Fragasso, M.; De Simone, N.; Spano, G.; Russo, P.; Venerito, P.; Bozzo, F.; Grieco, F. Microbial Resources and Sparkling Wine Differentiation: State of the Arts. Fermentation 2022, 8, 275. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H. Solid-state fermentation with Zygomycetes fungi as a tool for biofortification of apple pomace with γ-linolenic acid, carotenoid pigments and phenolic antioxidants. Food Res. Int. 2023, 173 Pt 2, 113448. [Google Scholar] [CrossRef]
- Paniagua-García, A.I.; Garita-Cambronero, J.; González-Rojo, S.; Díez-Antolínez, R. Optimization of lactic acid production from apple and tomato pomaces by thermotolerant bacteria. J. Environ. Manag. 2024, 366, 121806. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Rudziak, A.; Kieliszek, M. Optimization of propionic acid production in apple pomace extract with Propionibacterium freudenreichii. Prep. Biochem. Biotechnol. 2019, 49, 974–986. [Google Scholar] [CrossRef]
- Vlad, C.C.; Păcularu-Burada, B.; Vasile, A.M.; Milea, Ș.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Upgrading the Functional Potential of Apple Pomace in Value-Added Ingredients with Probiotics. Antioxidants 2022, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Kuvvet, C.; Uzuner, S.; Cekmecelioglu, D. Improvement of Pectinase Production by Co-culture of Bacillus spp. Using Apple Pomace as a Carbon Source. Waste Biomass Valorization 2019, 10, 1241–1249. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable extraction of bioactive compound from apple pomace through lactic acid bacteria (LAB) fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef]
- Navarro, M.E.; Brizuela, N.S.; Flores, N.E.; Morales, M.; Semorile, L.C.; Valdes La Hens, D.; Caballero, A.C.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E. Preservation of Malolactic Starters of Lactiplantibacillus plantarum Strains Obtained by Solid-State Fermentation on Apple Pomace. Beverages 2024, 10, 52. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, L.; Zhang, M.; Deng, Y.; Suo, W.; Zhang, H.; Wang, C.; Li, H. Bioconversion of Apple Pomace into Microbial Protein Feed Based on Extrusion Pretreatment. Appl. Biochem. Biotechnol. 2022, 194, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Chen, Y.; Sagada, G.; Xu, B.; Chao, W.; Zou, F.; Ng, W.K.; Sun, Y.; Wang, L.; Zhong, Z.; Shao, Q. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2020, 51, 1000–1011. [Google Scholar] [CrossRef]
- Mota, M.; Martins, M.J.; Policarpo, G.; Sprey, L.; Pastaneira, M.; Almeida, P.; Maurício, A.; Rosa, C.; Faria, J.; Martins, M.B.; et al. Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala. Horticulturae 2022, 8, 713. [Google Scholar] [CrossRef]
- Piagentini, A.M.; Pirovani, M.E. Total Phenolics Content, Antioxidant Capacity, Physicochemical Attributes, and Browning Susceptibility of Different Apple Cultivars for Minimal Processing. Int. J. Fruit Sci. 2017, 17, 102–116. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J. Influence of apple peel powder addition on the physico-chemical characteristics and nutritional quality of bread wheat cookies. Food Sci. Technol. Int. 2020, 26, 574–582. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Hajiabbas, M.; Hamidi, M.; Nie, L.; Megalizzi, V.; Musonge, P.; Dodi, G.; Shavandi, A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules 2023, 28, 675. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Kalhoro, D.H.; Yin, Y. Identification of nutritional composition and antioxidant activities of fruit peels as a potential source of nutraceuticals. Front. Nutr. 2023, 9, 1065698. [Google Scholar] [CrossRef]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Rodríguez Madrera, R.; Suárez Valles, B. Characterization of apple seeds and their oils from the cider-making industry. Eur. Food Res. Technol. 2018, 244, 1821–1827. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Akpabio, U.D.; Akpakpan, A.E.; Enin, G.N. Evaluation of proximate compositions and mineral elements in the star apple peel, pulp and seed. Magnesium (Mg) 2012, 6, 29–49. [Google Scholar]
- Drogoudi, P.D.; Michailidis, Z.; Pantelidis, G. Peel and flesh antioxidant content and harvest quality characteristics of seven apple cultivars. Sci. Hortic. 2008, 115, 149–153. [Google Scholar] [CrossRef]
- Manrich, A. Apple industry: Wastes and possibilities. Int. J. Agric. Res. Environ. Sci. 2024, 5, 1–10. [Google Scholar]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Naseem, Z. Effect of microwave-assisted vacuum and hot air oven drying methods on quality characteristics of apple pomace powder. Food Prod. Process. Nutr. 2023, 5, 26. [Google Scholar] [CrossRef]
- Salari, S.; Ferreira, J.; Lima, A.; Sousa, I. Effects of Particle Size on Physicochemical and Nutritional Properties and Antioxidant Activity of Apple and Carrot Pomaces. Foods 2024, 13, 710. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, A.R.S. USDA Food and Nutrient Database for Dietary Studies. 2020, U.S. Department of Agriculture: Food Surveys Research Group Home Page. Available online: https://fdc.nal.usda.gov/ (accessed on 11 May 2025).
- The New Zealand Institute for Plant & Food Research Limited and Ministry of Health. New Zealand Food Composition Database 2024. Available online: https://www.foodcomposition.co.nz/search (accessed on 11 May 2025).
- Henríquez, C.; Speisky, H.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Simpson, R.; Almonacid, S. Development of an Ingredient Containing Apple Peel, as a Source of Polyphenols and Dietary Fiber. J. Food Sci. 2010, 75, 172–181. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Sachini, R.; Steffens, C.A.; Martin, M.S.D.; Schveitzer, B.; Fenili, C.L.; Petri, J.L. Mineral contents in the skin and flesh of fruits of apple cultivars. Rev. Bras. Frutic. 2020, 42, 572. [Google Scholar] [CrossRef]
- Yu, X.; Van De Voort, F.R.; Li, Z.; Yue, T. Proximate composition of the apple seed and characterization of its oil. Int. J. Food Eng. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A review on nutritional features, chemical composition, traditional and medicinal value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kot, A.M.; Kieliszek, M.; Bonin, S. Use of Propionibacterium freudenreichii T82 Strain for Effective Biosynthesis of Propionic Acid and Trehalose in a Medium with Apple Pomace Extract and Potato Wastewater. Molecules 2021, 26, 3965. [Google Scholar] [CrossRef]
- Sobczak, P.; Nadulski, R.; Kobus, Z.; Zawiślak, K. Technology for Apple Pomace Utilization within a Sustainable Development Policy Framework. Sustainability 2022, 14, 5470. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Delporte, C.; Alimoradi, H.; Podstawczyk, D.; Nie, L.; Bernaerts, K.V.; Shavandi, A. Kinetic modelling of the solid–liquid extraction process of polyphenolic compounds from apple pomace: Influence of solvent composition and temperature. Bioresour. Bioprocess. 2021, 8, 114. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT—Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kim, S.S.; Mitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early-to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Esatbeyoglu, T.; Zhang, B.; Sheri, V.; Dhumal, S.; Rais, N.; Al Masry, E.M.S.; Chandran, D.; Pandiselvam, R.; et al. Apple (Malus domestica Borkh.) seed: A review on health promoting bioactivities and its application as functional food ingredient. Food Biosci. 2022, 50, 102155. [Google Scholar] [CrossRef]
- Asif, M.; Javaid, T.; Razzaq, Z.U.; Khan, M.K.I.; Maan, A.A.; Yousaf, S.; Usman, A.; Shahid, S. Sustainable utilization of apple pomace and its emerging potential for development of functional foods. Environ. Sci. Pollut. Res. 2024, 31, 17932–17950. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus × domestica) cultivars. Hortic. Res. 2014, 1, 14019. [Google Scholar] [CrossRef]
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, S.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic acid content in apple pulp, peel, and monovarietal cloudy juices of 64 different cultivars. Int. J. Food Prop. 2017, 20, S2626–S2634. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef] [PubMed]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Medica 2017, 83, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple pomace: A versatile substrate for biotechnological applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Munekata, P.E.; Domínguez, R.; Pateiro, M.; Nawaz, A.; Hano, C.; Walayat, N.; Lorenzo, J.M. Strategies to Increase the Value of Pomaces with Fermentation. Fermentation 2021, 7, 299. [Google Scholar] [CrossRef]
- Nawawi, M.H.; Ismail, K.I.; Sa’ad, N.; Mohamad, R.; Tahir, P.M.; Asa’ari, A.Z.; Saad, W.Z. Optimisation of Xylanase–Pectinase Cocktail Production with Bacillus amyloliquefaciens ADI2 Using a Low-Cost Substrate via Statistical Strategy. Fermentation 2022, 8, 119. [Google Scholar] [CrossRef]
- Marín, M.; Sánchez, A.; Artola, A. Production and recovery of cellulases through solid-state fermentation of selected lignocellulosic wastes. J. Clean. Prod. 2019, 209, 937–946. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kolotylo, V.; Kieliszek, M. Use of apple pomace, glycerine, and potato wastewater for the production of propionic acid and vitamin B12. Appl. Microbiol. Biotechnol. 2022, 106, 5433–5448. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.F.; Pascuta, M.S.; Varvara, R.A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Pakulska, A.; Bartosiewicz, E.; Galus, S. The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings 2023, 13, 1409. [Google Scholar] [CrossRef]
- Carpes, S.T.; Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Siddique, M.A.B.; Harrison, S.M.; Brunton, N.P. Bio-based films prepared with apple pomace: Volatiles compound composition and mechanical, antioxidant and antibacterial properties. LWT 2021, 144, 111241. [Google Scholar] [CrossRef]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of Bio-Based Films and 3D Objects from Apple Pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; Friedman, M.; McHugh, T.H. Physical and antibacterial properties of edible films formulated with apple skin polyphenols. J. Food Sci. 2011, 76, 149–155. [Google Scholar] [CrossRef]
- Espitia, P.J.; Avena-Bustillos, R.J.; Du, W.X.; Chiou, B.S.; Williams, T.G.; Wood, D.; McHugh, T.H.; Soares, N.F. Physical and antibacterial properties of açaí edible films formulated with thyme essential oil and apple skin polyphenols. J. Food Sci. 2014, 79, 903–910. [Google Scholar] [CrossRef]
- Rebolledo-Leiva, R.; Estévez, S.; Hernández, D.; Feijoo, G.; Moreira, M.T.; González-García, S. Apple Pomace Integrated Biorefinery for Biofuels Production: A Techno-Economic and Environmental Sustainability Analysis. Resources 2024, 13, 156. [Google Scholar] [CrossRef]
- Pathania, S.; Sharma, N.; Handa, S. Immobilization of co-culture of Saccharomyces cerevisiae and Scheffersomyces stipitis in sodium alginate for bioethanol production using hydrolysate of apple pomace under separate hydrolysis and fermentation. Biocatal. Biotransformation 2017, 35, 450–459. [Google Scholar] [CrossRef]
- Kut, A.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Second generation bioethanol production from hemicellulolytic hydrolyzate of apple pomace by Pichia stipitis. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 5574–5585. [Google Scholar]
- Hernández, D.; Rebolledo-Leiva, R.; Fernández-Puratich, H.; Quinteros-Lama, H.; Cataldo, F.; Muñoz, E.; Tenreiro, C. Recovering Apple Agro-Industrial Waste for Bioethanol and Vinasse Joint Production: Screening the Potential of Chile. Fermentation 2021, 7, 203. [Google Scholar] [CrossRef]
- Demiray, E.; Kut, A.; Karatay, S.E.; Dönmez, G. Usage of soluble soy protein on enzymatically hydrolysis of apple pomace for cost-efficient bioethanol production. Fuel 2021, 289, 119785. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Ampese, L.C.; Sganzerla, W.G.; Di Domenico Ziero, H.; Costa, J.M.; Martins, G.; Forster-Carneiro, T. Valorization of apple pomace for biogas production: A leading anaerobic biorefinery approach for a circular bioeconomy. Biomass Convers. Biorefinery 2024, 14, 14843–14857. [Google Scholar] [CrossRef]
- Abbasi-Riyakhuni, M.; Hashemi, S.S.; Alavijeh, R.S.; Mojoodi, S.; Shavandi, A.; Okoro, O.V.; Tabatabaei, M.; Aghbashlo, M.; Denayer, J.F.; Karimi, K. Comparative analysis of bioenergy and mycoprotein production from apple pomace: Strategies for enhancement and environmental benefits. Process Saf. Environ. Prot. 2024, 190, 123–134. [Google Scholar] [CrossRef]
- Bravo-Venegas, J.; Prado-Acebo, I.; Gullón, B.; Lú-Chau, T.A.; Eibes, G. Avoiding acid crash: From apple pomace hydrolysate to butanol through acetone-butanol-ethanol fermentation in a zero-waste approach. Waste Manag. 2023, 164, 47–56. [Google Scholar] [CrossRef]
- Raina, N.; Chuetor, S.; Elalami, D.; Tayibi, S.; Barakat, A. Biomass Valorization for Bioenergy Production: Current Techniques, Challenges, and Pathways to Solutions for Sustainable Bioeconomy. BioEnergy Res. 2024, 17, 1999–2028. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological activity of phenolics enriched extracts from industrial apple pomace. Ind. Crops Prod. 2021, 160, 113158. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Sudha, M.L. Chapter 36—Apple Pomace (By-Product of Fruit Juice Industry) as a Flour Fortification Strategy. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 395–405. [Google Scholar]
- Bartkiene, E.; Vizbickiene, D.; Bartkevics, V.; Pugajeva, I.; Krungleviciute, V.; Zadeike, D.; Zavistanaviciute, P.; Juodeikiene, G. Application of Pediococcus acidilactici LUHS29 immobilized in apple pomace matrix for high value wheat-barley sourdough bread. LWT—Food Sci. Technol. 2017, 83, 157–164. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabríny, L.; Kačániová, M. Selected Physico-Chemical, Nutritional, Antioxidant and Sensory Properties of Wheat Bread Supplemented with Apple Pomace Powder as a By-Product from Juice Production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Toledo, N.D.; Mondoni, J.; Harada-Padermo, S.D.S.; Vela-Paredes, R.S.; Berni, P.R.D.A.; Selani, M.M.; Canniatti-Brazaca, S.G. Characterization of apple, pineapple, and melon by-products and their application in cookie formulations as an alternative to enhance the antioxidant capacity. J. Food Process. Preserv. 2019, 43, 14100. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Pollini, L.; Blasi, F.; Ianni, F.; Grispoldi, L.; Moretti, S.; Di Veroli, A.; Cossignani, L.; Cenci-Goga, B.T. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules 2022, 27, 1933. [Google Scholar] [CrossRef] [PubMed]
- Kęska, P.; Wójciak, K.; Stadnik, J.; Kluz, M.I.; Kačániová, M.; Čmiková, N.; Solska, E.; Mazurek, K. Influence of apple pomace on the oxidation status, fatty acid content, colour stability and microbiological profile of baked meat products. Int. J. Food Sci. Technol. 2024, 59, 1591–1604. [Google Scholar] [CrossRef]
- Koishybayeva, A.; Korzeniowska, M. Utilization and Effect of Apple Pomace Powder on Quality Characteristics of Turkey Sausages. Foods 2024, 13, 2807. [Google Scholar] [CrossRef]
- Grispoldi, L.; Ianni, F.; Blasi, F.; Pollini, L.; Crotti, S.; Cruciani, D.; Cenci-Goga, B.T.; Cossignani, L. Apple Pomace as Valuable Food Ingredient for Enhancing Nutritional and Antioxidant Properties of Italian Salami. Antioxidants 2022, 11, 1221. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- de Souza Neves Ellendersen, L.; Granato, D.; Bigetti Guergoletto, K.; Wosiacki, G. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Li, Y.; Wu, S.; Li, D.; Liu, X.; Han, Y.; Manickam, S.; Show, P.L. Application of ultrasonication at different microbial growth stages during apple juice fermentation by Lactobacillus plantarum: Investigation on the metabolic response. Ultrason. Sonochemistry 2021, 73, 105486. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial Growth, Metabolism of Bioactives and in vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Keshani. Fermentation of Apple Juice with a Selected Yeast Strain Isolated from the Fermented Foods of Himalayan Regions and Its Organoleptic Properties. Front. Microbiol. 2016, 7, 1012. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Li, Y.; Tian, L.; Ye, B.; Yan, W.; Liu, G.; Yang, Y. Evaluating the dynamic effects of complex probiotics as cellulase replacements during fermentation of apple pomace. Int. J. Food Microbiol. 2024, 425, 110896. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Shiels, K.; Saha, S.K.; Abu-Reidah, I.M.; Thomas, R.H.; Redfern, S. Enrichment of Whole-Grain Breads with Food-Grade Extracted Apple Pomace Bioactives Enhanced Their Anti-Inflammatory, Antithrombotic and Anti-Oxidant Functional Properties. Antioxidants 2024, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From Byproduct to Resource: Fermented Apple Pomace as Beer Flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Choi, I. Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup. Foods 2023, 12, 1856. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Gaur, P.; Khan, F.N.; Yadava, P.; Rohatgi, B.; Shrivastava, M.; Khandelwal, A.; Darjee, S.; et al. Optimization and production of alpha-amylase using Bacillus subtilis from apple peel: Comparison with alternate feedstock. Food Biosci. 2022, 49, 101978. [Google Scholar] [CrossRef]
- Amorim, L.F.; Li, L.; Gomes, A.P.; Fangueiro, R.; Gouveia, I.C. Sustainable bacterial cellulose production by low cost feedstock: Evaluation of apple and tea by-products as alternative sources of nutrients. Cellulose 2023, 30, 5589–5606. [Google Scholar] [CrossRef]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical optimization of cultural parameters for the optimized production of alginic acid using apple (Malus domestica) peels through solid-state fermentation. Biomass Convers. Biorefinery 2023, 13, 1269–1277. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Zhou, Y.; Lei, L.; Chen, J.; Li, S.; Zhao, G. Tailoring the composition, antioxidant activity, and prebiotic potential of apple peel by Aspergillus oryzae fermentation. Food Chem. X 2024, 21, 101134. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, S.; Waterhouse, G.I.; Zhou, T.; Du, Y.; Sun-Waterhouse, D.; Wu, P. Yeast fermentation of apple and grape pomaces affects subsequent aqueous pectin extraction: Composition, structure, functional and antioxidant properties of pectins. Food Hydrocoll. 2022, 133, 107945. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef]
- Shahzadi, I.; Mubarak, S.; Farooq, A.; Hussain, N. Apple peels as a potential adsorbent for removal of Cu and Cr from wastewater. AQUA—Water Infrastruct. Ecosyst. Soc. 2023, 72, 914–929. [Google Scholar] [CrossRef]
- Gomravi, Y.; Karimi, A.; Azimi, H. Adsorption of heavy metal ions via apple waste low-cost adsorbent: Characterization and performance. Korean J. Chem. Eng. 2021, 38, 1843–1858. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T.; Niculescu, V.C.; Marinas, I.C.; Pircalabioru, G.G.; Dutu, D.; Trusca, R.; Oprea, O.C.; Ficai, A.; Andronescu, E. Valorization of apple pomace by obtaining some bioactive ingredients with antioxidant, antimicrobial and prebiotic activities. Food Bioprod. Process. 2025, 150, 182–197. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Plamada, D.; Simon, E.; Nemes, S.A.; Teleky, B.E.; Odocheanu, R.; Szabo, K.; Ranga, F.; Dulf, F.V.; Vodnar, D.C. Exploring the in vitro prebiotic potential of two different freeze-dried apple pomace cultivars. Food Biosci. 2025, 64, 105892. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; De la Peña Armada, R.; Pérez-Cózar, M.L.; Rupérez, P.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100219. [Google Scholar] [CrossRef]
- Wilkowska, A.; Nowak, A.; Antczak-Chrobot, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally Different Pectic Oligosaccharides Produced from Apple Pomace and Their Biological Activity In Vitro. Foods 2019, 8, 365. [Google Scholar] [CrossRef]
- Wilkowska, A.; Nowak, A.; Motyl, I.; Oracz, J. The Molecular Weight of Enzymatically Modified Pectic Oligosaccharides from Apple Pomace as a Determinant for Biological and Prebiotic Activity. Molecules 2025, 30, 46. [Google Scholar] [CrossRef] [PubMed]
- Jagelaviciute, J.; Staniulyte, G.; Cizeikiene, D.; Basinskiene, L. Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Apple Pomace and Its Application for Wheat Bread Making. Plant Foods Hum. Nutr. 2023, 78, 307–313. [Google Scholar] [CrossRef]
- Rößle, C.; Brunton, N.; Gormley, R.T.; Ross, P.R.; Butler, F. Development of potentially synbiotic fresh-cut apple slices. J. Funct. Foods 2010, 2, 245–254. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Margolles, A.; Ruiz, L. Fecal microbiota cooperative metabolism of pectins derived from apple pomace: A functional metagenomic study. LWT 2023, 187, 115362. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef]
- Eder, M.; Sanchez, I.; Brice, C.; Camarasa, C.; Legras, J.L.; Dequin, S. QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation. BMC Genom. 2018, 19, 166. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquie-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Gomes, R.J.; de Fatima Borges, M.; de Freitas Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Suarez, L.; Querejeta, N.; Rodriguez Madrera, R.; Suarez, B.; Centeno, T.A. Apple Waste: A Sustainable Source of Carbon Materials and Valuable Compounds. ACS Sustain. Chem. Eng. 2019, 7, 17335–17343. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Sakadani, E.; Kandylis, P. Enhancing Probiotic Viability in Yogurt: The Role of Apple Fibers in Supporting Lacticaseibacillus casei ATCC 393 During Storage and Gastrointestinal Transit. Foods 2025, 14, 376. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Wang, Q.; Laaksonen, O.; Pujol, E.X.; Heinonen, M.; Yang, B.; Kelanne, N. Impact of yeast selection on composition of vinegar fermented from pomace of a Finnish apple cultivar. Food Biosci. 2024, 62, 105447. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Fischer, T.E.; Zardo, D.M.; Nogueira, A.; Zielinski, A.A.F.; Alberti, A. Bioactive compounds recovered from apple pomace as ingredient in cider processing: Monitoring of compounds during fermentation. J. Food Sci. Technol. 2022, 59, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H.; Yuan, L.; Li, W. Changes in polyphenol fractions and bacterial composition after in vitro fermentation of apple peel polyphenol by gut microbiota. Int. J. Food Sci. Technol. 2022, 57, 4268–4276. [Google Scholar] [CrossRef]
- Gaharwar, S.S.; Kumar, A.; Rathod, K.S.; Shinde, S.V. Valorization of Malus domestica L. (Apple) peels: A case study of circular bioeconomy. Sustain. Chem. Pharm. 2023, 36, 101301. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Raczkowska, E.; Serek, P. Health-Promoting Properties and the Use of Fruit Pomace in the Food Industry-A Review. Nutrients 2024, 16, 2757. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef] [PubMed]
- Sair, A.T.; Li, Y.; Zhao, W.; Li, T.; Liu, R.H. Anticancer activity of apple peel extracts against human breast cancer cells through insulin-like growth factor-1 signal transduction pathway. J. Agric. Food Res. 2023, 11, 100507. [Google Scholar] [CrossRef]
- Watanabe, A.; Shimada, M.; Maeda, H.; Narumi, T.; Ichita, J.; Itoku, K.; Nakajima, A. Apple Pomace Extract Improves MK-801-Induced Memory Impairment in Mice. Nutrients 2024, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Anh, N.H.; Jung, C.W.; Long, N.P.; Park, S.; Cho, Y.H.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Son, E.Y.; et al. Metabolic and Cardiovascular Benefits of Apple and Apple-Derived Products: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 766155. [Google Scholar] [CrossRef]
- Tian, J.; Wu, X.; Zhang, M.; Zhou, Z.; Liu, Y. Comparative study on the effects of apple peel polyphenols and apple flesh polyphenols on cardiovascular risk factors in mice. Clin. Exp. Hypertens. 2018, 40, 65–72. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Urso, E.; D’Avino, M.; Campiglia, P.; Marinelli, L.; Novellino, E. Annurca (Malus pumila Miller cv. Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: Results of a randomized clinical trial. J. Sci. Food Agric. 2017, 97, 2107–2115. [Google Scholar] [CrossRef]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef]
- Younis, K.; Ahmad, S. Waste utilization of apple pomace as a source of functional ingredient in buffalo meat sausage. Cogent Food Agric. 2015, 1, 1119397. [Google Scholar] [CrossRef]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Utilization of apple pomace powder as a fat replacer in goshtaba: A traditional meat product of Jammu and Kashmir, India. J. Food Meas. Charact. 2015, 9, 389–399. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kaushik, A.; Sonne, C.; Kim, K.H.; Kumar, S. Emerging nanobiotechnology in agriculture for the management of pesticide residues. J. Hazard. Mater. 2021, 401, 123369. [Google Scholar] [CrossRef] [PubMed]
- Tankiewicz, M. Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS. Foods 2023, 12, 3220. [Google Scholar] [CrossRef] [PubMed]
- Hrynko, I.; Kaczyński, P.; Pietruszyńska, M.; Łozowicka, B. The effect of food thermal processes on the residue concentration of systemic and non-systemic pesticides in apples. Food Control 2023, 143, 109267. [Google Scholar] [CrossRef]
- Naman, M.; Masoodi, F.A.; Wani, S.M.; Ahad, T. Changes in concentration of pesticide residues in fruits and vegetables during household processing. Toxicol. Rep. 2022, 9, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef]
- Gryko, K.; Kalinowska, M.; Świderski, G. The Use of apple pomace in removing heavy metals from water and sewage. Environ. Sci. Proc. 2021, 9, 24. [Google Scholar]
- Jangde, V.; Umathe, P.; Antony, P.S.; Shinde, V.; Pakade, Y. Fixed-bed column dynamics of xanthate-modified apple pomace for removal of Pb(II). Int. J. Environ. Sci. Technol. 2019, 16, 6347–6356. [Google Scholar] [CrossRef]
- He, X.Y.; Wu, L.J.; Wang, W.X.; Xie, P.J.; Chen, Y.H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef]
- Adewusi, S.R.A.; Oke, O.L. On the metabolism of amygdalin. 1. The LD50 and biochemical changes in rats. Can. J. Physiol. Pharmacol. 1985, 63, 1080–1083. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B. Suárez Valles, Analysis of Cyanogenic Compounds Derived from Mandelonitrile by Ultrasound-Assisted Extraction and High-Performance Liquid Chromatography in Rosaceae and Sambucus Families. Molecules 2021, 26, 7563. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]


| Fat (g/100 g) | Protein (g/100 g) | Total Carbohydrate (g/100 g) | Total Fiber (g/100 g) | Insoluble Fiber (g/100 g) | Soluble Fiber (g/100 g) | Total Polyphenols (mg GAE/100 g) | Vitamin C (mg/100 g) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Apple peel (dm) | 2.7–14.8 | 2.8–4.5 | 60.0–81.9 | 40.3–43.9 | 11.9–25.5 | 14.7–32.0 | 1.1–9.6 | 60.3–63.4 | [62,63,64,65] | |
| Apple seed | 19.7 | 35.3–40.1 | n.d. | 19.5–21.1 | n.d. | n.d. | 270.0–1744.0 defatted matter | nd | [66,67] | |
| Apple pulp | 10.0 | 4.7 | 79.0 | 3.0 | n.d. | n.d. | 22.0–62.0 fm | 3.1–4.4 fm | [68,69,70] | |
| Apple pomace | 1.2–3.6 | 1.2–5.9 | 44.5–57.4 * | 27.9–49.5 | 017.4–25.6 | 13.5–25.5 | 266.0–394.0 dm | 12.0–52.0 dm | [63,71,72,73,74,75,76] | |
| Apple pulp + peel | 5.4 | 2.5 | 71.8 | n.d. | n.d. | n.d. | 10.8 dm | n.d. | [63] | |
| Whole apple (fm) | 0.2–0.3 | 0.1–0.2 | 10.0 | 2.0–2.1 | n.d. | n.d. | n.d. | 6.7 | [77,78] | |
| Sodium (mg/100 g) | Potassium (mg/100 g) | Calcium (mg/kg) | Phosphorus (mg/100 g) | Magnesium (mg/100 g) | Iron (mg/100 g) | Zinc (mg/100 g) | Copper (mg/100 g) | Manganese (mg/100 g) | Ref. | |
| Apple peel | <0.01–0.4 | 800.0–1100.0 | 1000.0 | 100.0 | 100.0 | 1.3–1.4 | 1.0 | 0.04–0.2 | 0.02–0.5 | [65,79,80,81] |
| Apple seed | n.d. | 650.0 | 270.0 | 72.0 | 51.0 | 11.0 | 4.4 | 0.2 | 0.5 | [82] |
| Apple pulp | 0.6–0.8 | 68.7 | 0.9–3.9 | n.d. | 0.6–1.8 | n.d. | n.d. | n.d. | n.d. | [68] |
| Apple pomace | 39.3 | 872.8–925.0 | 55.6–92.7 | 50.0–112.0 | 20.0–61.6 | 2.4–23.0 | 0.9–1.8 | 0.6–0.9 | 0.4–1.8 | [73,75] |
| Whole apple | <1.0–1.0 | 59.0–95.0 | 2.0–5.0 | 6.0–9.0 | 3.0–4.7 | <0.1–0.1 | 0.02 | 0.01–0.02 | 0.02–0.03 | [77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmi, H.; Presutto, E.; Totaro, M.; Spano, G.; Capozzi, V.; Fragasso, M. Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review. Foods 2025, 14, 1850. https://doi.org/10.3390/foods14111850
Selmi H, Presutto E, Totaro M, Spano G, Capozzi V, Fragasso M. Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review. Foods. 2025; 14(11):1850. https://doi.org/10.3390/foods14111850
Chicago/Turabian StyleSelmi, Hiba, Ester Presutto, Martina Totaro, Giuseppe Spano, Vittorio Capozzi, and Mariagiovanna Fragasso. 2025. "Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review" Foods 14, no. 11: 1850. https://doi.org/10.3390/foods14111850
APA StyleSelmi, H., Presutto, E., Totaro, M., Spano, G., Capozzi, V., & Fragasso, M. (2025). Apple Waste/By-Products and Microbial Resources to Promote the Design of Added-Value Foods: A Review. Foods, 14(11), 1850. https://doi.org/10.3390/foods14111850







