1. Introduction
Tea saponins are a mixture of oleanane-type pentacyclic triterpenoid saponins, whose basic structure consists of three parts: saponins, glycosides, and organic acids [
1], and are distributed in the flowers, leaves, and seeds of
C. oleifera [
2]. Tea saponins are excellent natural non-ionic surfactants, which are widely used in the food, chemical, and medicine industries [
3]. The
C. oleifera seed meal is a by-product of oil extraction from
C. oleifera seeds. In China, the annual output of
C. oleifera seed meal is relatively high, which can reach 690,000 metric tons [
4]. And the
C. oleifera seed meal is an excellent source of tea saponins [
5]. The extraction of tea saponins from
C. oleifera seed meals can also improve the utilization value of
C. oleifera seed meals.
At present, the main extraction methods for tea saponins from
C. oleifera seed meals are hot water extraction [
6], ethanol extraction [
7], ultrasonic and microwave-assisted extraction [
8,
9], and new auxiliary extraction methods such as subcritical water. Subcritical water refers to water at temperatures ranging from 100 °C to 374 °C. The polarity of subcritical water is regulated by varying the temperature and pressure to realize the selective extraction of substances with different polarities [
10]. Moreover, subcritical water extraction uses only water as the extraction solvent, so it has the characteristics of being green and protecting the environment [
11]. In addition, subcritical water extraction technology is also characterized by high efficiency. For example, a study has shown that the subcritical water extraction method had a higher yield of polysaccharides extracted from lily, compared with the ultrasonic-assisted extraction [
12].
Currently, the extraction of tea saponins in subcritical water is mainly focused on the optimization of extraction conditions from
C. oleifera seed meals and the simultaneous extraction of oil and tea saponins from
C. oleifera seeds [
13,
14]. Subcritical water extraction temperature, time, pressure, and the solid–liquid ratio are external factors affecting the extraction rate of tea saponins, while the composition of raw materials is an internal factor affecting the extraction rate of the target substance. For example, there are differences in the polysaccharide yield of different varieties of the
Gastrodia elata Bl aerial parts under the same extraction conditions [
15]. The composition of meals is related to various factors such as varieties and growth environment [
16]. Moreover, the extraction conditions of the oil from seeds affect the composition of the meals [
17].
C. oleifera is widely distributed in southern China, and there are many varieties of
C. oleifera [
18,
19]. So, there are differences in the composition of different
C. oleifera seed meals. At present, there are few studies on the effect of the composition of
C. oleifera seed meals on the extraction rate of tea saponins under subcritical water extraction conditions.
In this study, we aimed to investigate the effect of the composition of C. oleifera seed meals on the extraction rate of tea saponins in subcritical water, to improve the extraction rate of tea saponins. Therefore, the extraction rates of tea saponins from three C. oleifera seed meals purchased from Hubei, Hunan, and Guizhou provinces in subcritical water were studied, and the components affecting the extraction of tea saponins were screened. To further explore the mechanism of the influence of the screened components on the extraction rate of tea saponins in subcritical water, the simulated system of commercially available tea saponins, whey protein isolate (WPI), and glucose was reacted under subcritical water conditions, and the mechanism of the effect of WPI and glucose on tea saponin content was analyzed. In order to verify these mechanisms and achieve efficient extraction of tea saponins, alkaline protease was used to change the composition of C. oleifera seed meal, and the changes in the extraction rate of tea saponins were analyzed. This paper provides theoretical and technical guidance for the efficient extraction of tea saponins from C. oleifera seed meals.
2. Materials and Methods
2.1. Materials
Tea saponin standard (HPLC ≥ 98%) was provided by Shanghai Ru ji Biotechnology (Shanghai, China). Whey protein isolate (WPI) and alkaline protease were supplied by Shanghai Yuan ye Biotechnology (Shanghai, China). Commercially available tea saponins were purchased from Shanxi Ze lang Biotechnology (Xi’an China), named CA-tea saponins. 8-Anilino-1-naphthalenesulfonic acid (ANS), 3,5-dinitrosalicylic acid, and vanillin were supplied by Macklin (Shanghai, China). Petroleum ether, sulfuric acid, ethyl acetate, anhydrous ethanol, sodium hydroxide, glucose, hydrogen peroxide, phenol, sodium metabisulfite, hydrochloric acid, potassium sodium tartrate, and potassium bromide were purchased from Sinopharm Chemical Reagent (Shanghai, China). All chemicals were of analytical grade unless otherwise stated.
2.2. Samples
The three kinds of C. oleifera seed meal were purchased from Hubei province, Hunan province, and Guizhou province in China, named HB C. oleifera seed meal, HN C. oleifera seed meal, and GZ C. oleifera seed meal, respectively. The C. oleifera seed meals were crushed and passed through a 60-mesh sieve. The residual oil in the C. oleifera seed meals was removed with petroleum ether, and the degreased and dried C. oleifera seed meals were put into a sealed bag and placed in a desiccator.
2.3. Composition of C. oleifera Seed Meals
2.3.1. Determination of Ash, Crude Protein, Crude Fiber, and Amino Acid Content
The determination methods of ash, crude protein, crude fiber, and amino acid content in the
C. oleifera seed meal were GB 5009.4-2016, 5009.5-2016, 5009.124-2016, GB/T 8310-2013 [
20,
21,
22,
23].
2.3.2. Determination of Total Sugar Content
The total sugar extraction procedure from the
C. oleifera seed meals was determined following the method in [
24], with slight modifications, as follows: 0.3 g of
C. oleifera seed meal was added to a mixture of 15 mL HCl and 50 mL distilled water, then heated at 100 °C for 3 h. After cooling to room temperature, the residue was washed with distilled water, the wash solution was centrifuged, and the supernatant-total sugar solution was collected in a 250 mL volumetric flask. Next, 1 mL of 5% (
w/
v) phenol solution and 5 mL of concentrated sulfuric acid were sequentially added to 1 mL of the total sugar solution. The mixture was vortexed thoroughly, left to stand for 10 min, and then incubated in a 30 °C water bath for 20 min. The absorbance of the solution was measured at 490 nm using a UV-1800 spectrophotometer (UV-1800, Shimadzu, Tokyo, Japan). The standard working curve of total sugars was y = 10.987x + 0.05, R
2 = 0.9956. Based on Equation (1), the total sugar content was quantitatively analyzed, with the results being expressed as grams of total sugars per 100 g of
C. oleifera seed meal.
where
C1 is the total sugar concentration (mg/mL);
V1 is the volume of the total sugar solution (mL);
N is the number of dilutions;
m0 is the weight of the
C. oleifera seed meal (g).
2.3.3. Determination of Reducing Sugar Content
The reducing sugar extraction procedure from the
C. oleifera seed meals was determined following the method in [
25] with slight modifications, as follows: 1 g of
C. oleifera seed meal was added to 20 mL of distilled water and subjected to ultrasound treatment at 50 °C for 30 min, and centrifuged at 4000 r/min for 20 min. This process was repeated three times, and the supernatant-reducing sugar solution was combined in a 100 mL volumetric flask. Next, 2 mL of 3,5-dinitrosalicylic acid (DNS) reagent was added to 1 mL of the reducing sugar solution, which was heated in a boiling water bath for 15 min. After heating, the solution was cooled to room temperature under running water, and distilled water was added to bring the final volume to 10 mL. The absorbance of the solution was measured at 540 nm using a UV-1800 spectrophotometer (UV-1800, Shimadzu, Tokyo, Japan). The standard working curve of reducing sugars was y = 1.9204x + 0.2026, R
2 = 0.9948. Based on Equation (2), the reducing sugar content was quantitatively analyzed, with the results being expressed as grams of reducing sugars per 100 g of
C. oleifera seed meal.
where
C2 is the reducing sugar concentration (mg/mL);
V2 is the volume of reducing sugar solution (mL);
N is the number of dilutions;
m0 is the weight of the
C. oleifera seed meal (g).
2.3.4. Determination of Tea Saponin Content
The extraction procedure was as follows: 0.4 g of
C. oleifera seed meal wrapped in filter paper was subjected to Soxhlet extraction with 80% ethanol in an 80 °C water bath for 5 h, and the ethanol solution–tea saponin solution was combined in a 100 mL volumetric flask. The tea saponin content was determined following the method of Yu et al. [
2] with slight modifications: 0.5 mL of 8% (
w/
v) vanillin-anhydrous ethanol solution and 4 mL of 77% (
v/
v) sulfuric acid solution were sequentially added to 0.5 mL of the tea saponin solution, which was heated in a 60 °C water bath for 15 min. After cooling in an ice water bath for 10 min to reach room temperature, the absorbance of the solution was measured at 550 nm using a UV-1800 spectrophotometer (UV-1800, Shimadzu, Tokyo, Japan). Based on Equation (3), the tea saponins were quantitatively analyzed using a standard calibration curve, y = 1.1694x + 0.1669, R
2 = 0.9961, with the results being expressed as grams of tea saponins per 100 g of
C. oleifera seed meal.
where
C3 is the tea saponin concentration (mg/mL);
V3 is the volume of tea saponin solution (mL);
N is the number of dilutions;
m0 is the weight of the
C. oleifera seed meal (g).
2.4. Subcritical Water Extraction of Tea Saponins
2.4.1. Determination of Tea Saponin Extraction Rate
The extraction procedure was as follows: 5.0 g of
C. oleifera seed meal was dissolved in distilled water, stirred evenly, and tea saponins were extracted at 1 MPa, under the following subcritical water extraction conditions: extraction time (20–60 min), solid–liquid ratio (1:18–1:22,
w/
v), and temperature (120–160 °C). After extraction, the solution was centrifuged at 4000 r/min for 20 min, and the supernatant was concentrated using a rotary evaporator. Next, 50 mL of 95% ethanol was added to the concentrated solution, which was then filtered. The filtrate was further concentrated, and the rotary flask was rinsed with water to bring the final volume of concentration up to 20 mL. The concentrated solution was added to 20 mL of hydrogen peroxide (30%) and heated in a 60 °C water bath for 60 min. Afterward, the solution was dried at 60 °C in a blast drying oven to obtain a light yellow tea saponin solid powder, and the tea saponin sample was stored in a dryer. The method for determining the tea saponin content was the same as described in
Section 2.3.4. Extracted crude tea saponins were dissolved in distilled water, passed through an AB-8 macroporous resin, and eluted sequentially with distilled water followed by 80% ethanol. The eluate was then concentrated and dried to obtain purified tea saponin powder. The purified tea saponin sample was mixed with potassium bromide crystals and scanned using a Fourier transform infrared spectrometer (IS50 Fourier-transform infrared, Suzhou, China) within the spectral range of 400 to 4000 cm
−1 [
2]. Based on Equation (4), the tea saponin extraction rate was quantitatively analyzed using a standard calibration curve, y = 1.1694x + 0.1669, R
2 = 0.9961, with the results being expressed as grams of tea saponins per 100 g of tea saponins in the
C. oleifera seed meal.
where
ω1 is the tea saponins purity of extracted tea saponins (%);
ω2 is the content of tea saponins in the
C. oleifera seed meal (%);
m1 is the weight of extracted tea saponins (g);
m0 is the weight of the
C. oleifera seed meal (g).
2.4.2. Determination of Reducing Sugar Dissolution Rate
The crude tea saponin sample extracted from HB, HN, and GZ three
C. oleifera seed meals was dissolved in distilled water. The content of reducing sugars in the crude tea saponin sample was determined, and the method was the same as described in
Section 2.3.3. Based on Equation (5), the reducing sugar dissolution rate was quantitatively analyzed using a standard calibration curve, y = 1.9204x + 0.2026, R
2 = 0.9948, with the results being expressed as grams of reducing sugars per 100 g of reducing sugars in the
C. oleifera seed meal.
where
ω3 is the reducing sugar content of the extracted tea saponins (%);
ω4 is the content of the reducing sugars in the
C. oleifera seed meal (%);
m1 is the weight of the extracted tea saponins (g);
m0 is the weight of the
C. oleifera seed meal (g).
2.4.3. Determination of Protein Dissolution Rate
The crude tea saponin sample extracted from HB, HN, and GZ three
C. oleifera seed meals was dissolved in distilled water, and the content of proteins in the crude tea saponin sample was determined, using the Bradford assay [
26], with absorbance taken at 595 nm using a Multiskan SkyHigh (Multiskan SkyHigh, Thermo Fisher, Singapore). Based on Equation (6), the protein dissolution rate was quantitatively analyzed using a standard calibration curve, y = 3.6813x + 0.5703, R
2 = 0.9902, with the results being expressed as grams of proteins per 100 g of proteins in the
C. oleifera seed meal.
where
ω5 is the protein content of extracted tea saponins (%);
ω6 is the content of proteins in the
C. oleifera seed meal (%);
m1 is the weight of extracted tea saponins (g);
m0 is the weight of the
C. oleifera seed meal (g).
2.5. Changes in CA-Tea Saponin Content in Simulation System
At 120 °C and 1 MPa, the following three factors were considered on the residual rate of CA-tea saponins: (1) the concentration of CA-tea saponins was 3 mg/mL, the mass ratio of WPI and CA-tea saponins was 0.2:3–1.0:3, and the control group was a CA-tea saponin solution at a concentration of 3 mg/mL. (2) In the CA-TS group, the concentration of CA-tea saponins was 3 mg/mL, in the WPI group, the concentration of WPI was 1 mg/mL, and in the WPI+CA-TS group, the mass ratio of WPI and CA-tea saponins was 1.0:3. The reaction time was 20–70 min. (3) The concentration of CA-tea saponins was 1 mg/mL, the mass ratio of glucose and WPI and CA-tea saponins was 1:1:1–5:1:1. In the control group, the concentration of CA-tea saponins was 1 mg/mL and the mass ratio of WPI to CA-tea saponins was 1:1.
CA-tea saponins, WPI, and glucose were dissolved in 100 mL of distilled water according to the above experimental conditions, the pH was adjusted to 4, and the solution was then heated in a reaction kettle at 120 °C and 1 MPa. After heating, the solution was cooled and stored in the refrigerator at 4° C for further use. The method for determining CA-tea saponin content in the solution was the same as described in
Section 2.3.4. Based on Equation (7), the CA-tea saponin residual rate was quantitatively analyzed, with the results being expressed as grams of residual CA-tea saponins per 100 g of CA-tea saponins before the reaction.
where
C4 is the concentration of the CA-tea saponins after subcritical water heating (mg/mL);
C5 is the concentration of the CA-tea saponins before subcritical water heating (mg/mL);
N is the number of dilutions;
W is the purity of CA-tea saponins (%).
2.6. Measurement of Browning Degree in Simulation System
Following the method of Tai et al. [
27] was used with slight modifications: the reaction solution from
Section 2.5, was diluted 16 times, and its absorbance was measured at 294 nm and 420 nm using a UV-1800 spectrophotometer (UV-1800, Shimadzu, Tokyo, Japan).
2.7. The Surface Hydrophobicity of WPI in the Simulation System
Surface hydrophobicity is an important indicator that reflects the tertiary structure of proteins [
28]. Following the method of Yang et al. [
29] with slight modifications, 0.2, 0.4, 0.6, 0.8, and 1.0 mL of the reaction solution from
Section 2.5. was added to 5 mL brown test tubes, respectively. Distilled water was added to bring the total volume to 4 mL, followed by the addition of 20 μL (8 mmol/L) of ANS, which was then placed in the dark for 15 min. Fluorescence intensity was measured using an F-4600 spectrometer (F-4600, Hitachi, Tokyo, Japan) at an excitation wavelength of 390 nm and an emission wavelength of 470 nm. The initial slope of fluorescence intensity versus WPI concentration, calculated by linear regression analysis, was used as an indicator of surface hydrophobicity.
2.8. Alkaline Protease Enzymatic Hydrolysis of HB C. oleifera Seed Meal
Alkaline protease can break down proteins into small peptides, increasing the content of proteins in the solution [
30]. Alkaline protease treatment of the HB
C. oleifera seed meal can increase the protein content in the solution, which is conducive to the occurrence of the Maillard reaction. The procedure was as follows: 5.0 g of HB
C. oleifera seed meal was added to 110 mL of distilled water, followed by the alkaline protease dosage of 2%, 3%, 4%, 5% (
w/
w) at pH 8 and a 50 °C water bath for 1 h. After enzymatic hydrolysis, the pH of the solution was adjusted to 5.5, and the control group was the HB
C. oleifera seed meal without alkaline protease treatment. The tea saponins were extracted in subcritical water at 120 °C and 1 MPa for 30 min. The extracted tea saponin sample was obtained according to the experimental method described in
Section 2.4.1. The method for determining the tea saponin extraction rate was the same as described in
Section 2.4.1.
2.9. Statistical Analysis
All assays were performed in triplicate. Data are presented as mean values with standard deviations. Statistical analysis was conducted using Duncan’s test at a significance level of p < 0.05, and ANOVA with SPSS 25.0 software.
3. Results
3.1. Composition of C. oleifera Seed Meals
The contents of the basic components of the HB, HN, and GZ
C. oleifera seed meals are shown in
Table 1. There were differences in the composition of the three
C. oleifera seed meals. The total sugar content in the HB, HN, and GZ
C. oleifera seed meals was 28.31%, 24.81%, and 25.62%, and the crude fiber content was 17.06%, 20.91%, and 24.67%, respectively. The content of tea saponins in the HB
C. oleifera seed meal was the highest, which was 19.30%. The crude protein content in the HB, HN, and GZ
C. oleifera seed meals was 13.75%, 11.66%, and 10.75%, respectively. The proteins in the HN and GZ
C. oleifera seed meals consisted of 17 amino acids (
Table A1), which agrees with previous studies [
31], while the protein in the HB
C. oleifera seed meal lacked histidine. The ratios of basic amino acids to total amino acids were as follows: HB (12.12%) < HN (15.67%) < GZ (19.06%), and the ratios of non-polar hydrophobic amino acids to total amino acids were as follows: HB (41.93%) > GZ (36.05%) > HN (34.18%).
3.2. Extraction Rate of Tea Saponins from HB, HN, and GZ C. oleifera Seed Meals
The extraction rates of tea saponins from the HB, HN, and GZ
C. oleifera seed meals in subcritical water are shown in
Table 2. The extraction rate of tea saponins from the HB
C. oleifera seed meal decreased from 21.62% to 1.78% as temperature increased from 120 °C to 150 °C. Similarly, the extraction rate of tea saponins from the HN
C. oleifera seed meal decreased from 29.89% to 4.86% as temperature increased from 120 °C to 160 °C. In contrast, the extraction rate of tea saponins from the GZ
C. oleifera seed meal initially increased from 17.01% to 22.81% as temperature increased from 120 °C to 130 °C, but then decreased to 1.79% at 160 °C. This is because the plant cell wall was disrupted with increasing temperature [
32] to dissolve more tea saponins, whereas the excessive temperature of subcritical water resulted in the degradation of the tea saponins in the solution, which is consistent with previous results reported in the literature [
14]. On the other hand, the polarity of water decreases with increasing temperature in subcritical water [
33], which may be detrimental to the extraction of tea saponins since tea saponins are polar molecules [
34].
At 120 °C and 1 MPa and a solid–liquid ratio of 1:20, the extraction rate of tea saponins from the HN C. oleifera seed meal was the highest from 30 min to 60 min compared to the HB and GZ C. oleifera seed meals. The extraction rate of tea saponins from the HB and HN C. oleifera seed meals increased from 15.42% to 21.62% and 12.30% to 29.89% as the time increased from 20 min to 30 min, respectively. However, the extraction rate of tea saponins decreased to 6.32% and 23.45% at 60 min, respectively. In contrast, the extraction rate of tea saponins from the GZ C. oleifera seed meal decreased from 21.13% to 10.29% as the time increased from 20 min to 60 min.
At 120 °C and 1 MPa for 30 min, the extraction rate of tea saponins from the HB and GZ C. oleifera seed meals increased from 15.59% to 21.62% and 16.29% to 17.01%, respectively, as the solid–liquid ratio increased from 1:18 to 1:20, at 1:22, the extraction rate of tea saponins decreased to 19.35% and 13.98%, respectively. In contrast, the extraction rate of tea saponins from the HN C. oleifera seed meal increased from 25.47% to 31.69% as the solid–liquid ratio increased from 1:18 to 1:22. Under the above subcritical water extraction conditions, the maximum tea saponin extraction rate (21.62%) from the HB C. oleifera seed meal was achieved at 120 °C and 1 MPa for 30 min, and a solid–liquid ratio of 1:20; the maximum tea saponin extraction rate (31.69%) from the HN C. oleifera seed meal was achieved at 120 °C and 1 MPa for 30 min, and a solid–liquid ratio of 1:22; the maximum tea saponin extraction rate (22.81%) from the GZ C. oleifera seed meal was achieved at 130 °C and 1 MPa for 30 min, and a solid–liquid ratio of 1:20.
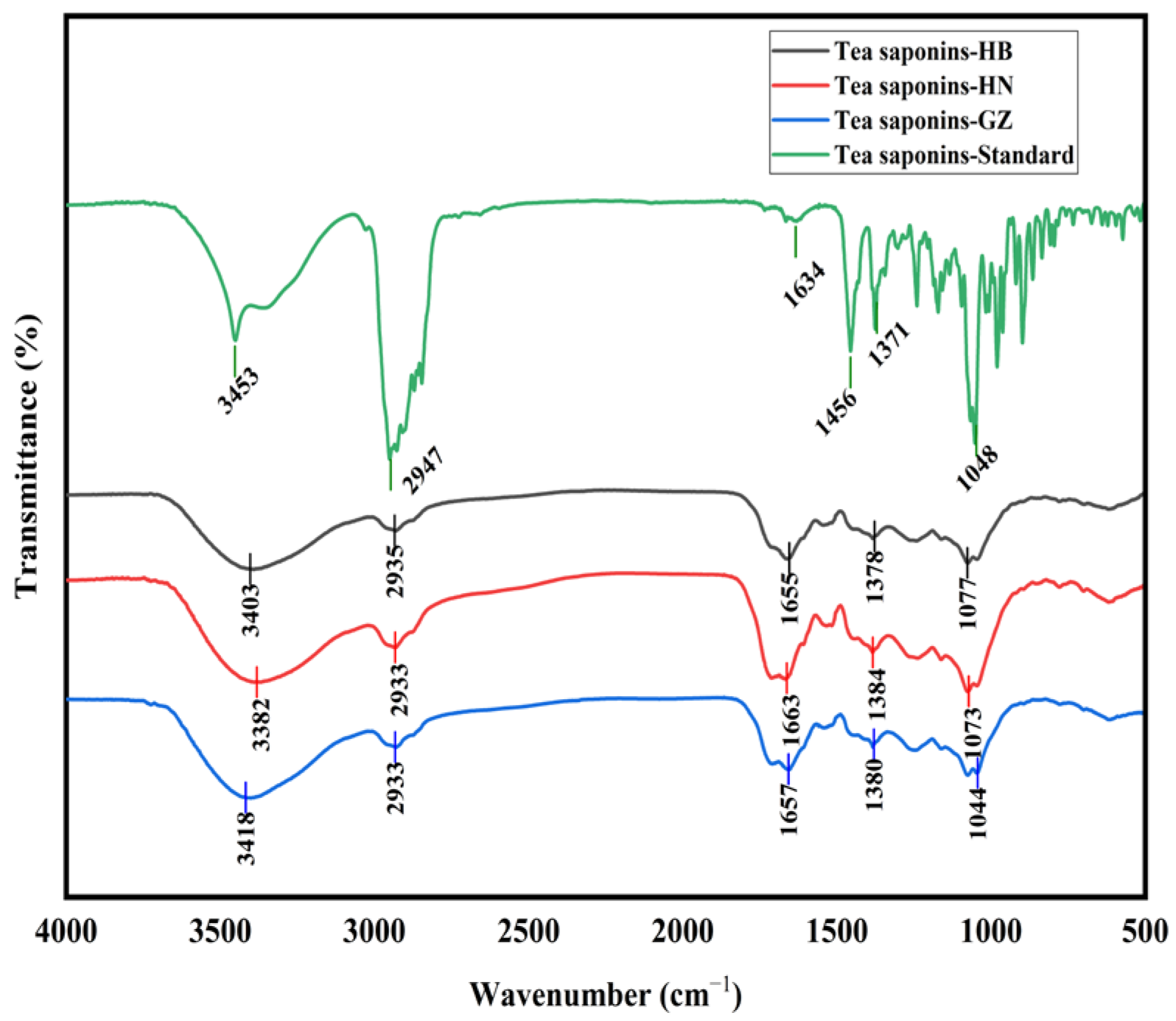
The FT-IR spectra of the tea saponin samples are shown in
Figure 1. The tea saponins extracted from the HB, HN, and GZ
C. oleifera seed meals using subcritical water exhibited the same infrared characteristic peaks as the standard and closely resembled the infrared spectra of tea saponins reported in previous studies [
2]. Consequently, these findings confirm that the subcritical water extraction method used in this study successfully extracted tea saponins, which belong to the class of oleanane pentacyclic triterpene saponins.
3.3. Dissolution Rates of Reducing Sugars and Proteins from HB, HN, and GZ C. oleifera Seed Meals
The dissolution rates of reducing sugars and proteins from the HB, HN, and GZ
C. oleifera seed meals are shown in
Table 2, at 120 °C and 1 MPa, and a solid–liquid ratio of 1:18–1:22, over 20–60 min. The dissolution rate of proteins from the GZ
C. oleifera seed meal was relatively high, reaching up to 18.10% at a solid–liquid ratio of 1:20 for 20 min. The dissolution rate of reducing sugars from the HB
C. oleifera seed meal increased from 27.21% to 39.39% as the time increased from 20 min to 50 min, then decreased to 37.53% at 60 min. The dissolution rate of reducing sugars from the HN
C. oleifera seed meal increased from 18.96% to 20.49% as the time increased from 20 min to 30 min, decreased to 13.11% at 50 min, and then slowly rose to 15.85% at 60 min. The dissolution rate of reducing sugars from the GZ
C. oleifera seed meal was 11.99–14.44% (20–60 min). The dissolution rate of reducing sugars was influenced by the composition of the three
C. oleifera seed meals and the consumption extent of the reducing sugars during subcritical water extraction because reducing sugars can participate in the Maillard reaction with proteins [
35]. The order of the dissolution rate of reducing sugars in the three
C. oleifera seed meals was GZ < HN < HB (20–60 min). The solid–liquid ratio from 1:18 to 1:22 at 120 °C and 1 MPa for 30 min did not significantly affect the dissolution rate of reducing sugars.
Tea saponins contain hydrophilic glycans and hydrophobic saponins [
36], which may be less hydrophilic than reducing sugars. In subcritical water, compared with HB and HN
C. oleifera seed meals, the large dissolution rate of reducing sugars from the HB
C. oleifera seed meal was not conducive to the extraction of tea saponins. This may be due to the reducing sugars in the HB
C. oleifera seed meal competing with tea saponins for dissolution. But compared with the GZ and HN
C. oleifera seed meals, the dissolution rate of reducing sugars from the GZ
C. oleifera seed meal was lower, which was also not conducive to the extraction of tea saponins. Previous studies have shown that basic amino acids have higher reactivity in the Maillard reaction [
37]. Due to the relatively high content of basic amino acids in the GZ
C. oleifera seed meal (2.35 g/100 g), as shown in
Table A1, although the reducing sugar content in the GZ
C. oleifera seed meal is higher, more reducing sugars can be consumed by the Maillard reaction, which may be the reason for the lower dissolution rate of reducing sugars from the GZ
C. oleifera seed meal.
Based on the analysis of the experimental results, it can be concluded that the content of reducing sugars and basic amino acids impacted the tea saponin extraction rate in C. oleifera seed meals. For the same raw material of C. oleifera seed meal, the basic amino acid content can be classified as the protein content, so the content of reducing sugars and proteins in the C. oleifera seed meal was the internal factor of the extraction rate of tea saponins in subcritical water.
3.4. Changes in CA-Tea Saponins and Maillard-Reaction Products Content in the Simulation System
In order to further study the effects of reducing sugars and proteins on the content of tea saponins in subcritical water, glucose and WPI were selected as the reducing sugar and protein, respectively. Glucose, WPI, and CA-tea saponins composed the simulation system in subcritical water. At 120 °C and 1 MPa for 30 min, the concentration of CA-tea saponins was 3 mg/mL, the residual rate of CA-tea saponins was 92.78%, which was attributed to the hydrolysis of tea saponins in subcritical water.
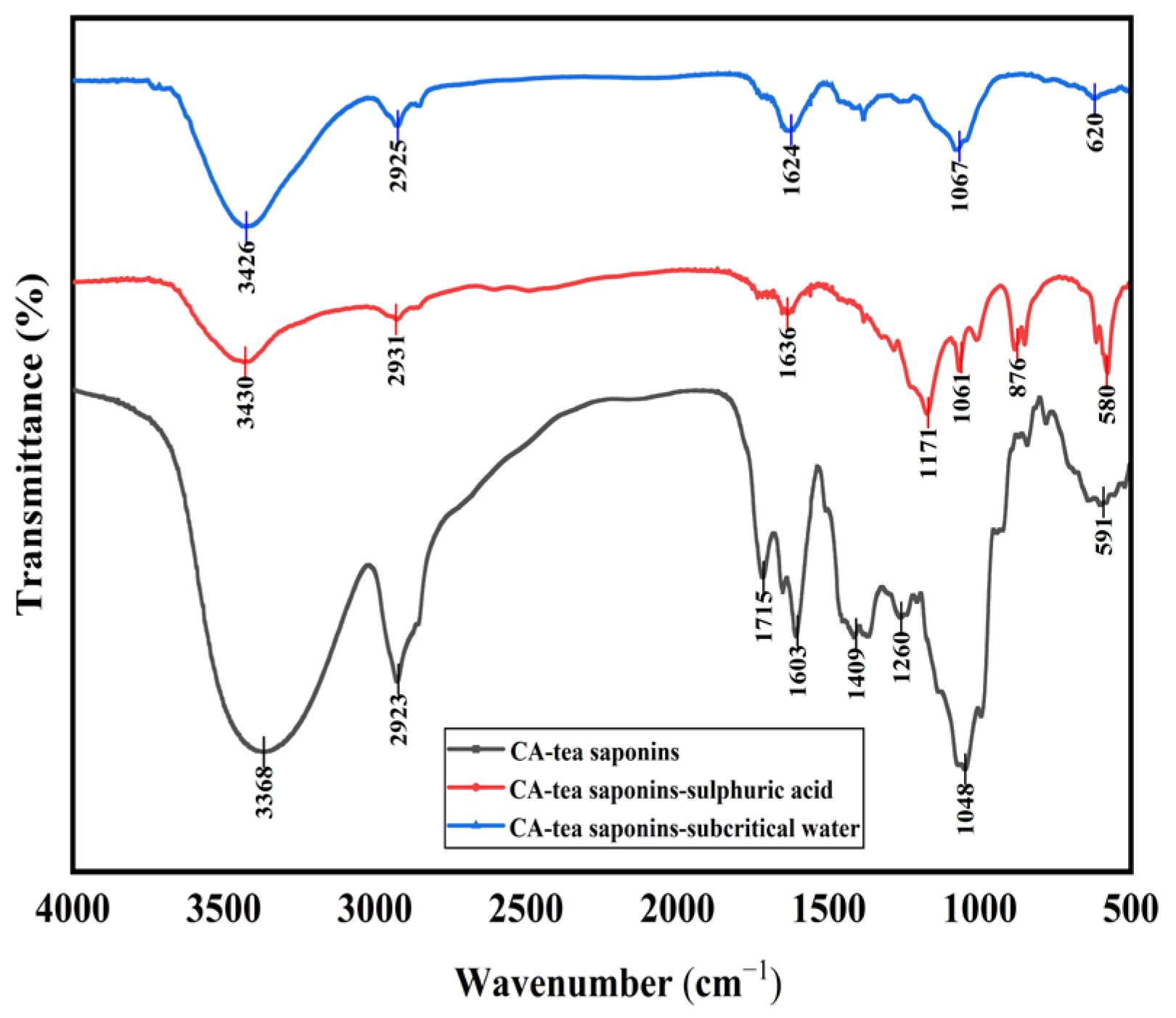
The hydrolysate of CA-tea saponins in subcritical water was consistent with the product catalyzed by sulfuric acid at atmospheric pressure, as confirmed by infrared spectroscopy (
Figure 2). Specifically, the intensity of the absorption peaks around 3400 cm
−1 and 1045 cm
−1 (ether bond) weakened after the hydrolysis of CA-tea saponins, suggesting the detachment of sugar moieties from the CA-tea saponins in subcritical water. Previous studies have also shown that tea saponins are hydrolyzed into glycogen and saponins in the presence of sulfuric acid under atmospheric pressure [
38], and the results of this paper are consistent with their experimental results.
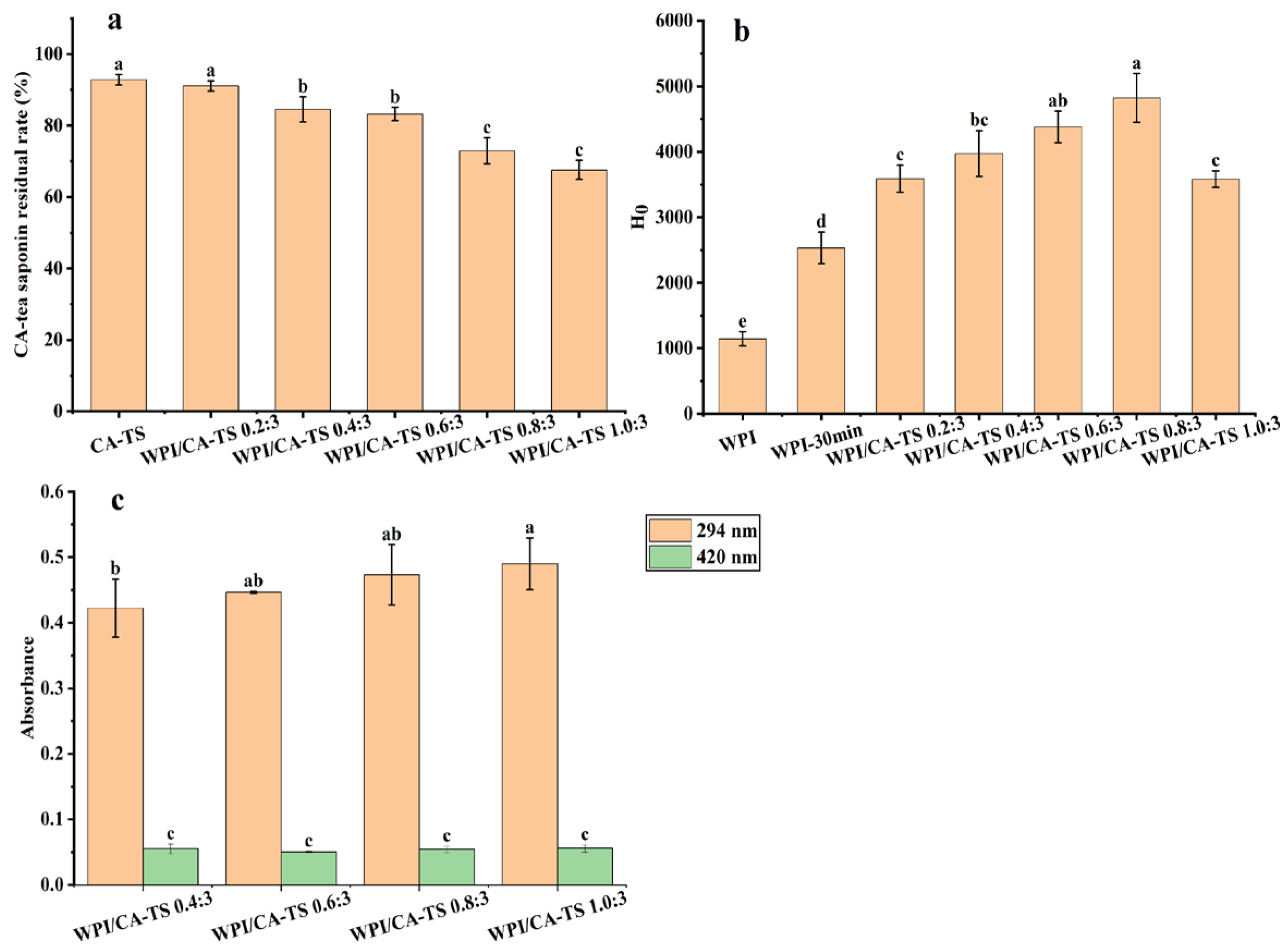
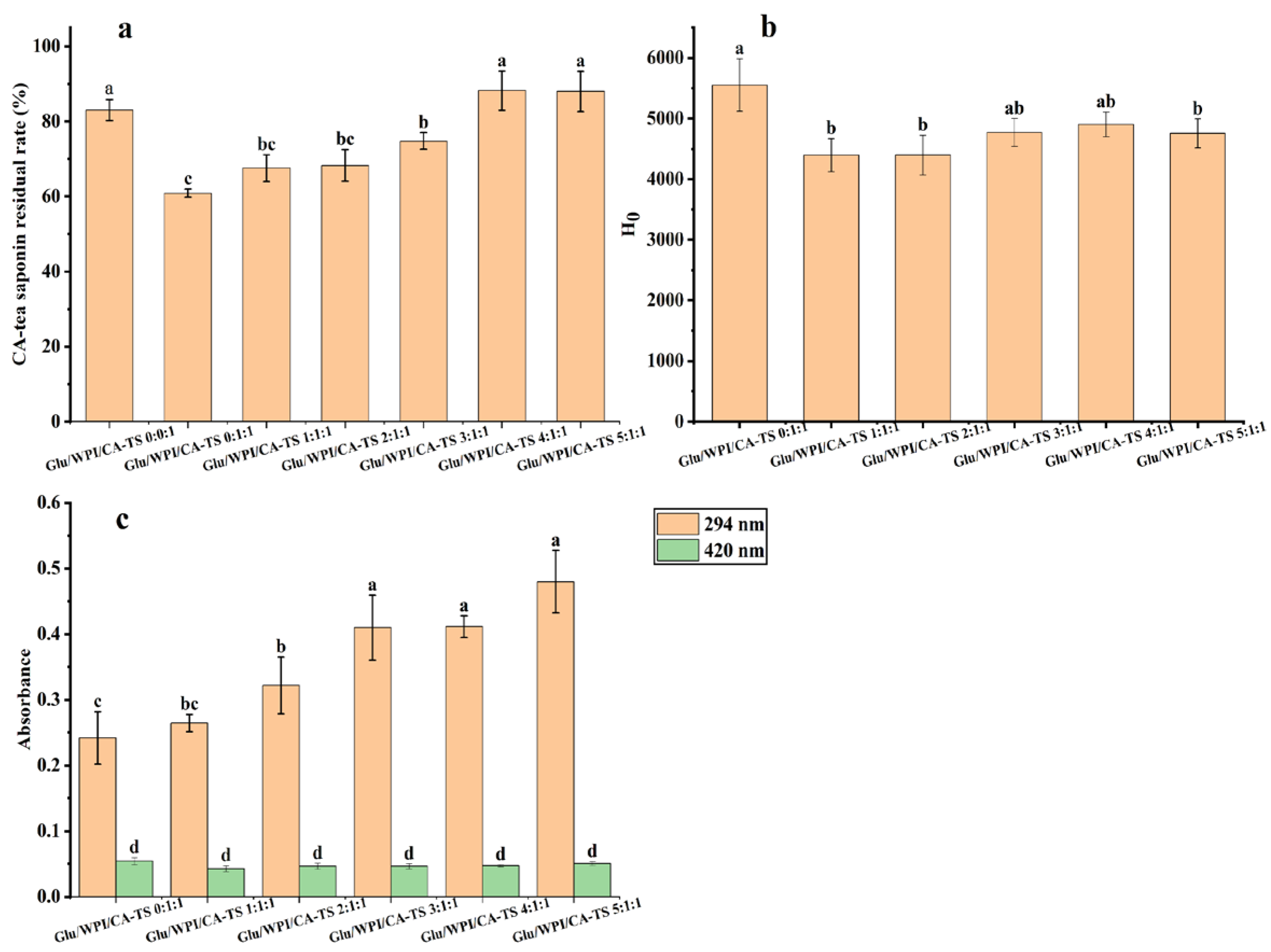
Figure 3a. shows that the residual rate of CA-tea saponins gradually decreased from 91.06% to 67.55% when the mass ratio of WPI and CA-tea saponins increased from 0.2:3 to 1.0:3, indicating that WPI can reduce the residual rate of CA-tea saponins in subcritical water.
A study has shown that ginsenosides Re containing glycans can react with alanine under heating conditions, and the content of ginsenosides Re decreased after the Maillard reaction with alanine [
39]. Tea saponins contain glycans, and the mixed solution of CA-tea saponins and WPI in subcritical water also produced browning. The absorbance at 294 nm and 420 nm, which indicates the contents of intermediate and final products of the Maillard reaction, respectively [
40]. The contents of the Maillard-reaction products in the mixed solution of WPI and CA-tea saponins are shown in
Figure 3c. The absorbance increased from 0.422 to 0.490 at 294 nm and remained in the range of 0.05 to 0.056 at 420 nm as the mass ratio of WPI and CA-tea saponins increased from 0.4:3 to 1.0:3. This indicated the involvement of CA-tea saponins in the Maillard reaction with WPI in subcritical water. The degree of the Maillard reaction between WPI and CA-tea saponins increased with the increasing mass ratio of WPI and CA-tea saponins. At this time, the absorbance of the WPI and tea saponin mixed solution at 294 nm and 420 nm with a WPI and CA-tea saponins mass ratio of 0.2:3 could not be used because the solution under this condition appeared cloudy with a light yellow color.
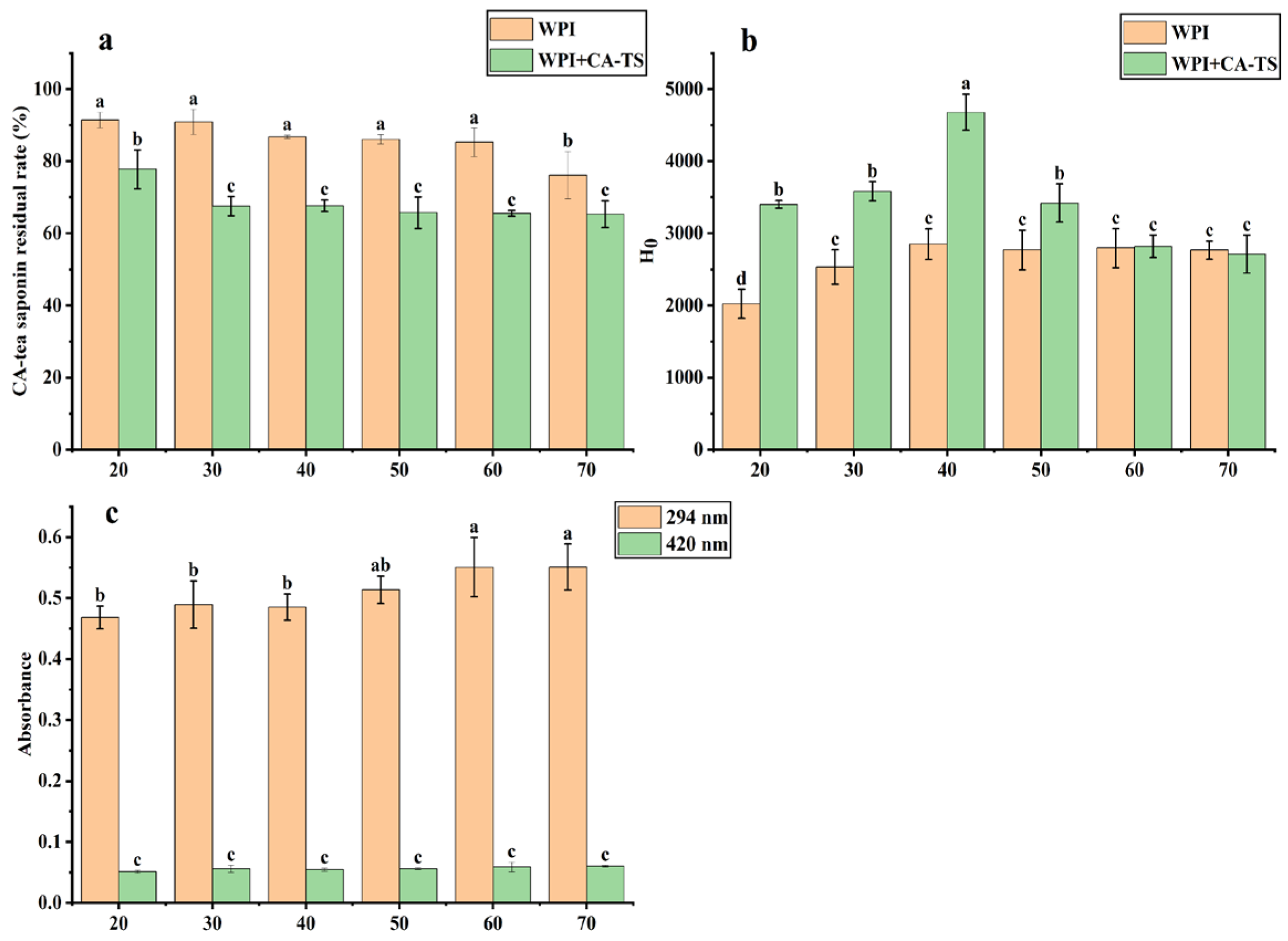
The effect of the reaction time on the residual rate of CA-tea saponins in subcritical water is shown in
Figure 4a. In the CA-TS group, the residual rate of CA-tea saponins was 92.08% at 20 min, decreased from 92.78% to 76.14% as the reaction time increased from 30 min to 70 min, due to the hydrolysis of CA-tea saponins in subcritical water. In contrast, the residual rate of CA-tea saponins decreased from 77.79% to 65.32% over the same time period in the WPI+CA-TS group. The effect of the reaction time on the content of Maillard-reaction products in the WPI and CA-tea saponins mixed solution is shown in
Figure 4c. The absorbance increased from 0.468 to 0.551 at 294 nm and from 0.051 to 0.06 at 420 nm as the time increased from 20 min to 70 min, indicating that the content of Maillard-reaction intermediate products by WPI and CA-tea saponins increased with the extension of reaction time.
The effect of reducing sugars on the residual rate of CA-tea saponins is shown in
Figure 5a. The residual rate of CA-tea saponins increased from 60.87% to 88.19% as the mass ratio of glucose and WPI and CA-tea saponins increased from 0:1:1 to 4:1:1, and was 88.00% at the glucose and WPI and CA-tea saponins mass ratio of 5:1:1. The content of Maillard-reaction products in the glucose, WPI, and CA-tea saponins mixed solution is shown in
Figure 5c. The absorbance increased from 0.242 to 0.480 at 294 nm and varied between 0.043 and 0.054 at 420 nm as the mass ratio of glucose and WPI and CA-tea saponins increased from 0:1:1 to 5:1:1, indicating an increase in the content of Maillard-reaction intermediate products. This may be due to the low molecular weight of glucose, which can easily expose the carbonyl group for more efficient and flexible reaction with WPI [
41]. However, it had an insignificant effect on the content of the final products (
p > 0.05). Glucose increased the residual rate of CA-tea saponins and the degree of Maillard reaction, suggesting that glucose may compete with CA-tea saponins to bind to WPI and participate in the Maillard reaction.
3.5. Effect of CA-Tea Saponins on the Surface Hydrophobicity of WPI in Subcritical Water
It has been reported in the literature that the surface hydrophobicity of proteins changes when they undergo the Maillard reaction with sugars [
42]. To further investigate the mechanism of the Maillard reaction between CA-tea saponins and WPI in subcritical water, the surface hydrophobicity of WPI was investigated.
As shown in
Figure 3b, the surface hydrophobicity of WPI increased from 1143.08 to 2533.23 after subcritical water treatment, indicating an exposure of the hydrophobic groups in WPI, which is consistent with previous literature [
43]. The surface hydrophobicity of WPI/CA-TS increased from 3587.90 to 4821.17 as the mass ratio of WPI and CA-tea saponins increased from 0.2:3 to 0.8:3, then decreased to 3583.30 at 1.0:3, indicating a further increase in the hydrophobic groups in WPI by the CA-tea saponins as the mass ratio of WPI and CA-tea saponins increased from 0.2:3 to 0.8:3 in subcritical water, in contrast, the decrease in the surface hydrophobicity of WPI/CA-TS at ratio of 1.0:3 was attributed to the Maillard reaction between WPI and CA-tea saponins, formed more WPI-sugar combinations, which is also consistent with the literature [
44].
The effect of reaction time on the surface hydrophobicity of WPI is shown in
Figure 4b. In the WPI group, the surface hydrophobicity of WPI increased from 2022.70 to 2852.27 as the time increased from 20 min to 40 min and remained stable from 50 min to 70 min. This is because the structural disruption of the protein increased, exposing more hydrophobic groups as the heating time increased [
45]. In the WPI+CA-TS group, the surface hydrophobicity of WPI/CA-TS increased from 3401.47 to 4679.33 as the time increased from 20 min to 40 min, then decreased to 2711.30 at 70 min. This trend can be explained by the increased structural disruption of the proteins and exposure of more hydrophobic amino acids as the reaction time increased from 20 min to 40 min, whereas when the reaction time increased from 50 min to 70 min, more WPI underwent a Maillard reaction with the CA-tea saponins and the reducing sugars in them, resulting in a decrease in the surface hydrophobicity of the WPI/CA-TS, which is in agreement with the literature [
46].
The effect of glucose on the surface hydrophobicity of WPI is shown in
Figure 5b. The surface hydrophobicity of WPI/CA-TS/Glucose was reduced after adding glucose, due to the introduction of -OH groups to WPI by glucose, which is also consistent with previous studies [
47].
3.6. Validation of the Extraction Mechanism of Tea Saponins
To validate the effect of the reducing sugars and proteins on tea saponin extraction rate and improve the extraction rate of tea saponins, alkaline protease was used to regulate the content of reducing sugars and proteins in the HB
C. oleifera seed meal. Alkaline proteases can break down proteins into small-molecule peptides, increasing the degree of protein hydrolysis and exposing more free amino groups [
48]. The results are shown in
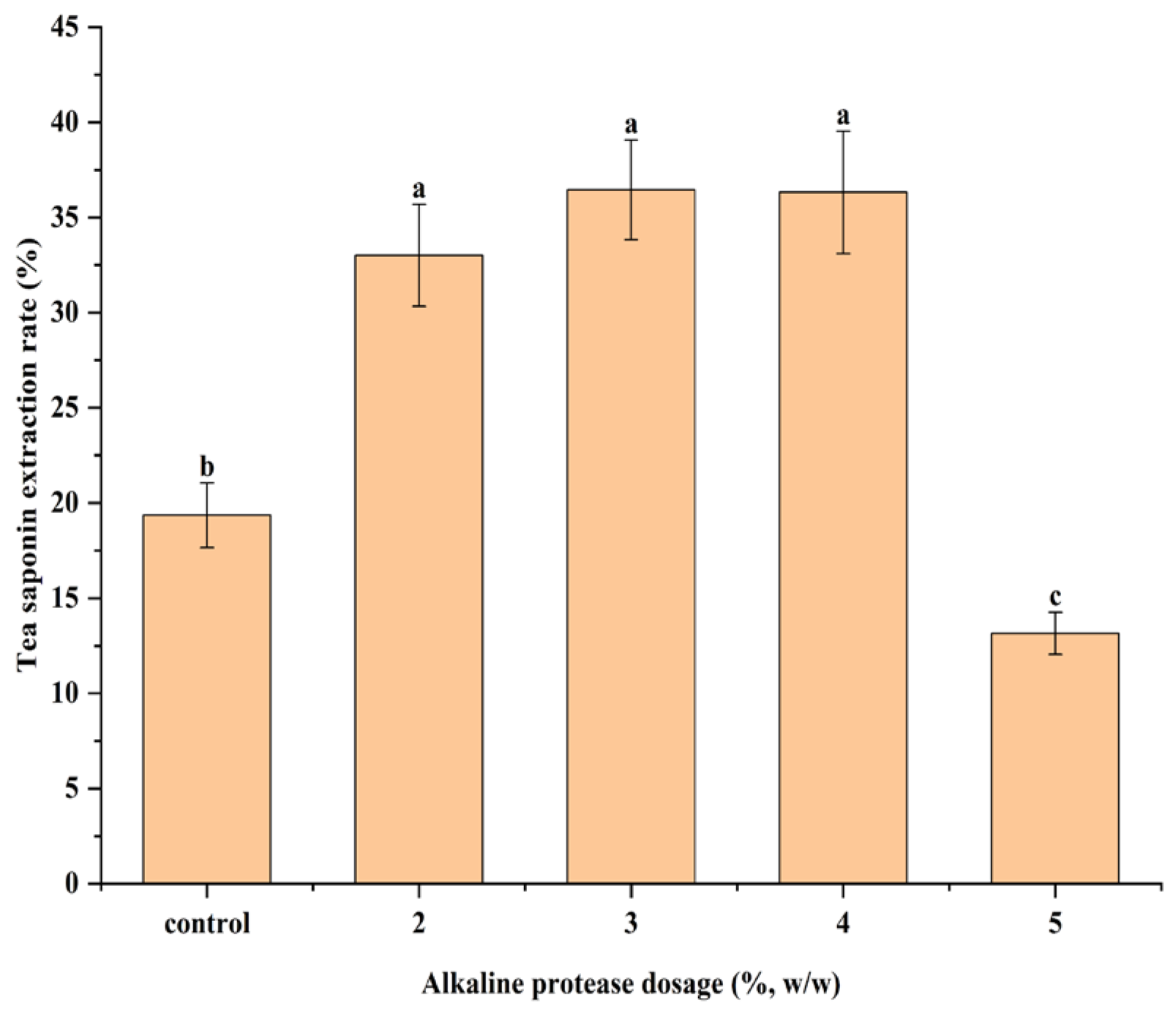
Figure 6. The tea saponin extraction rate increased from 33.01% to 36.45% at the alkaline protease dosage of 2% to 3%, and decreased to 13.15% at the alkaline protease dosage of 5%. The tea saponin extraction rate was 19.35% in the control group.
Compared to the control group, the increase in the tea saponin extraction rate at an alkaline protease dosage of 2% to 3% may be attributed to the reduced amount of reducing sugars, which inhibited the competitive dissolution of reducing sugars. This occurred as the alkaline protease increases protein dissolution, consuming more reducing sugars by participating in a Maillard reaction with proteins in subcritical water. The extraction rate of tea saponins decreased at the alkaline protease dosage of 5%, which may be because the reducing sugars were consumed in large quantities and cannot effectively inhibit the Maillard reaction of tea saponins with proteins.
4. Conclusions
At 120 °C, 1 MPa, and a solid–liquid ratio of 1:20, the tea saponin extraction rate from the HN C. oleifera seed meal was the highest as the time increased from 30 min to 60 min, among the three HB, HN, and GZ C. oleifera seed meals, the maximum tea saponin extraction rate (31.69%) was achieved at 120 °C and 1 MPa for 30 min, and a solid–liquid ratio of 1:22. By comparing the extraction rates of saponins, and the dissolution rates of reducing sugars and proteins in three C. oleifera seed meals, it was found that the content of reducing sugars and proteins can affect the extraction rate of tea saponins. The dissolution rate of reducing sugars in the HB C. oleifera seed meal was high, so the reduced sugar content in the HB C. oleifera seed meal is relatively high in subcritical water, which was not conducive to the extraction of tea saponins. The simulation system showed that CA-tea saponins can be hydrolyzed, and the residual rate of CA-tea saponins decreased. WPI can further reduce the content of CA-tea saponins through the Maillard reaction, and the mechanism of action of WPI is to synergize the degradation of CA-tea saponins. Glucose can inhibit the Maillard reaction between CA-tea saponins and WPI, and the mechanism of action of glucose reverses the protection of CA-tea saponins. The quantitative relationship of these mechanisms is influenced by the reaction conditions. At 120 °C and 1 MPa for 30 min, the CA-tea saponin concentration of 1 mg/mL, the WPI and CA-tea saponins mass ratio of 1:1, the content of CA-tea saponins decreased by 39.13%, of which the hydrolysis of CA-tea saponins accounted for 16.97%, and the Maillard reaction between WPI and CA-tea saponins accounted for 22.16%. The residual rate of CA-tea saponins increased by 44.88% at a glucose to WPI to CA-tea saponins mass ratio of 4:1:1.
After alkaline protease treatment, the content of reducing sugars and proteins in the HB C. oleifera seed meal was changed, and the extraction rate of tea saponins was affected. The extraction rate of tea saponins increased by 88.37% at an alkaline protease dosage of 3%, compared with the control group. These provide theoretical guidance for the efficient extraction of tea saponins from C. oleifera seed meals. In this study, only three kinds of C. oleifera seed meal were selected as experimental materials, which had certain limitations, and the raw materials suitable for tea saponin extraction could be screened out by selecting a variety of experimental materials in a later study.