Stachydrine from Natural Foods Alleviates Hyperuricemia by Modulating Renal Urate Transporters and Suppressing Mitochondrial Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal Experiment
2.3. Biochemical and Inflammatory Marker Analysis
2.4. RT-qPCR
2.5. Histopathological and Immunofluorescence Analysis
2.6. Tissue DHE Analysis
2.7. Cell Culture
2.8. Assessment of MMP and Cellular ROS
2.9. Transmission Electron Microscope (TEM)
2.10. Western Blot
2.11. Statistical Analysis
3. Results
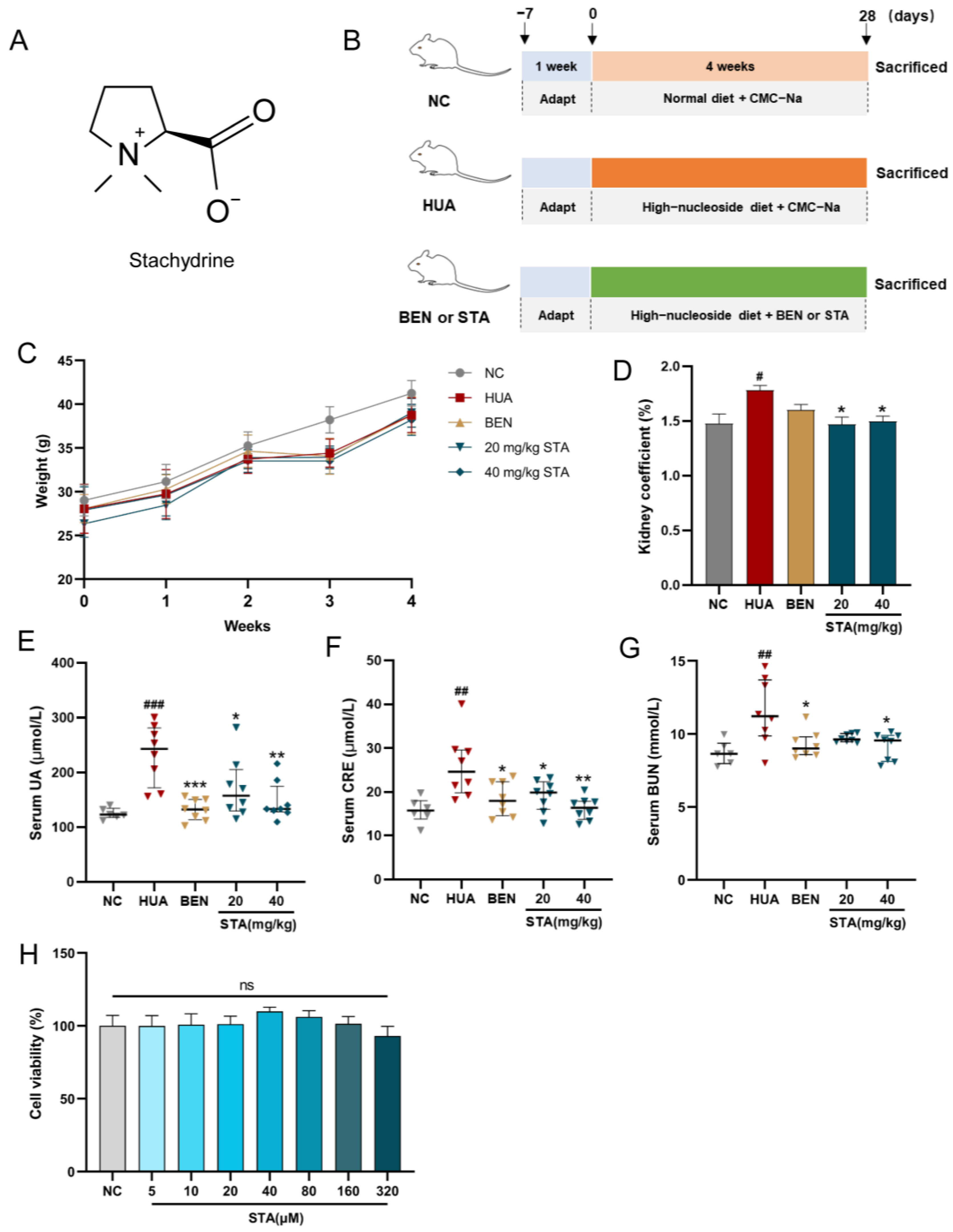
3.1. STA Efficiently Alleviated HUA in Mice
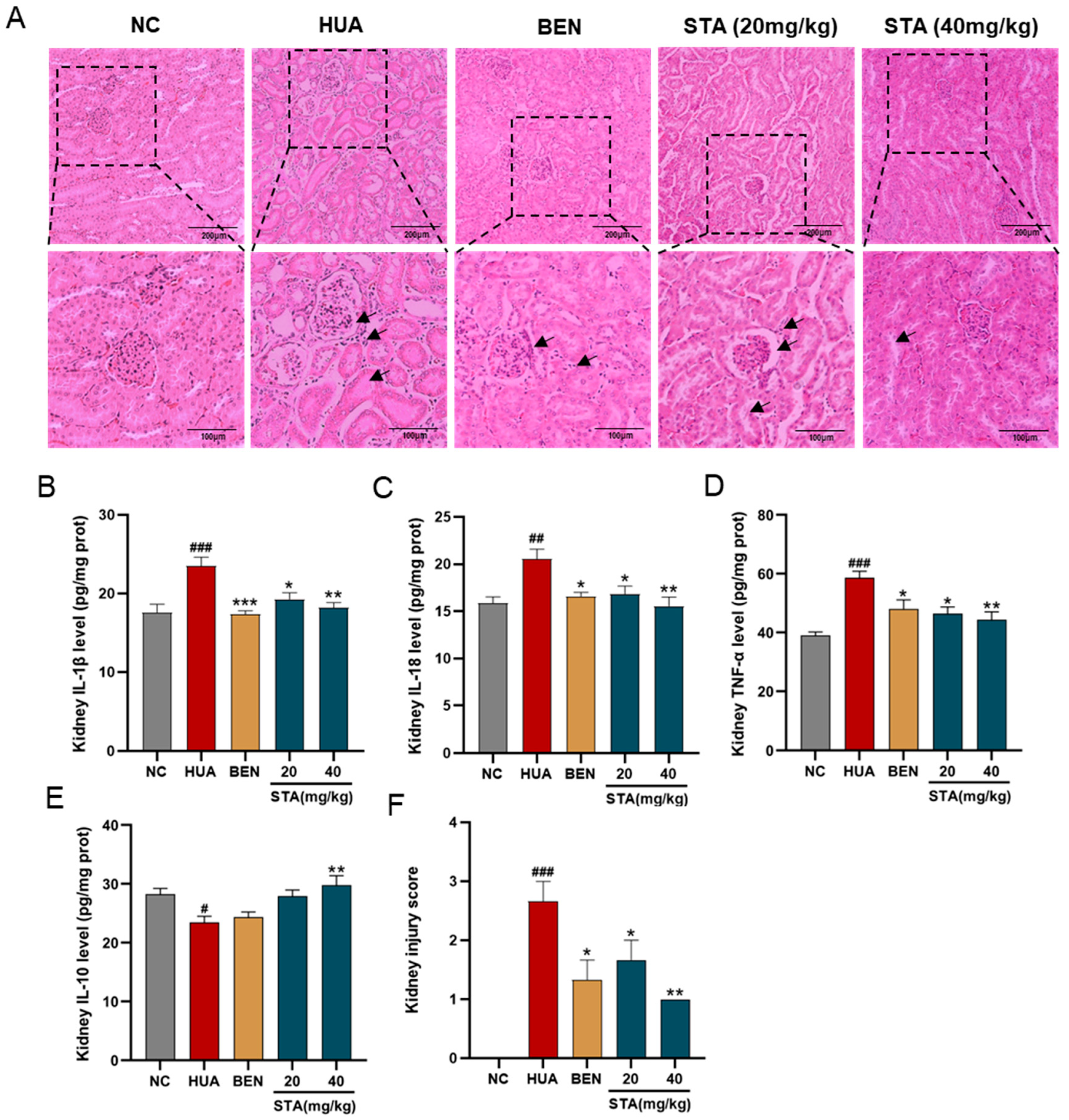
3.2. STA Mitigated HUA-Induced Renal Inflammation and Pathological Damage
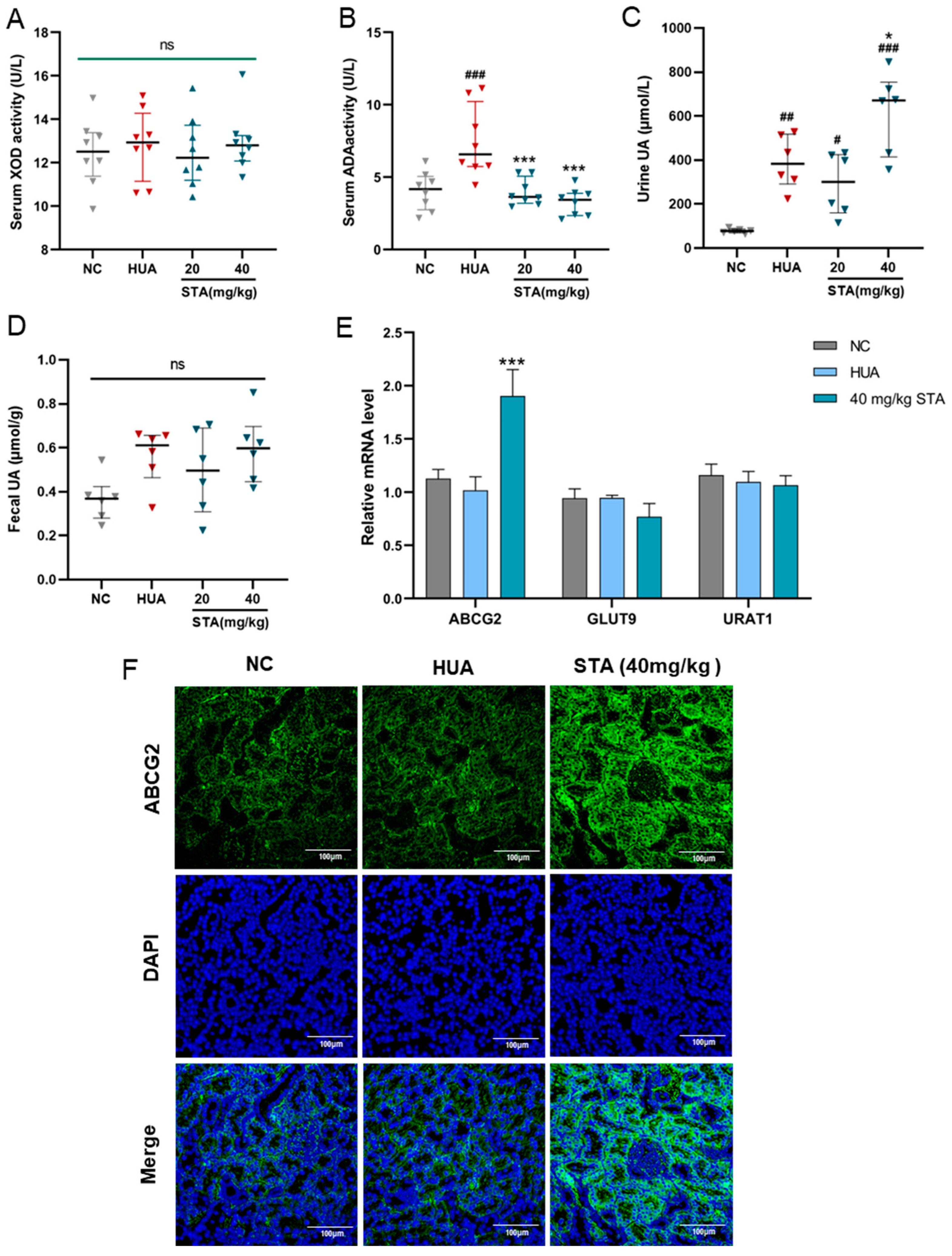
3.3. STA Enhanced UA Excretion by Upregulating ABCG2
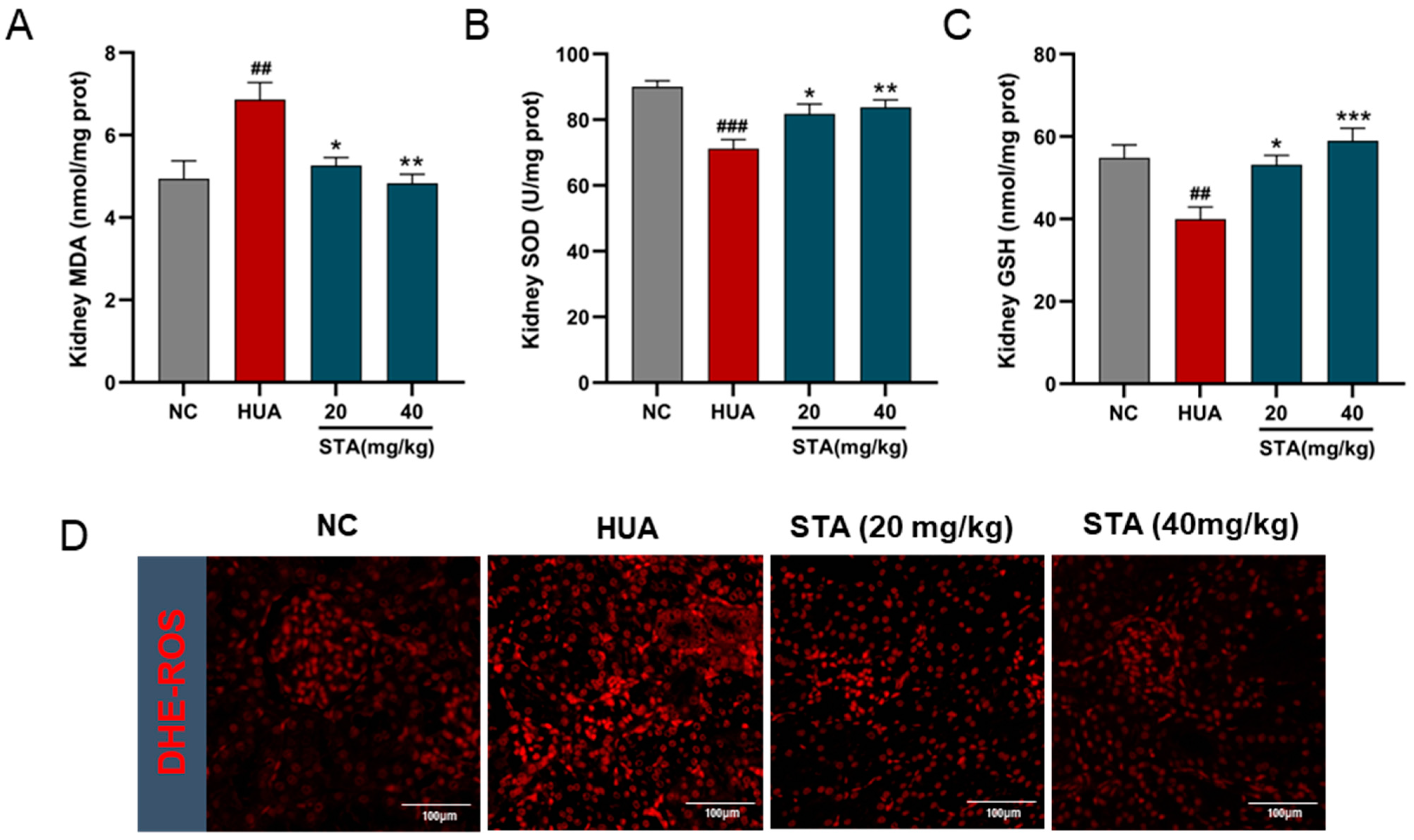
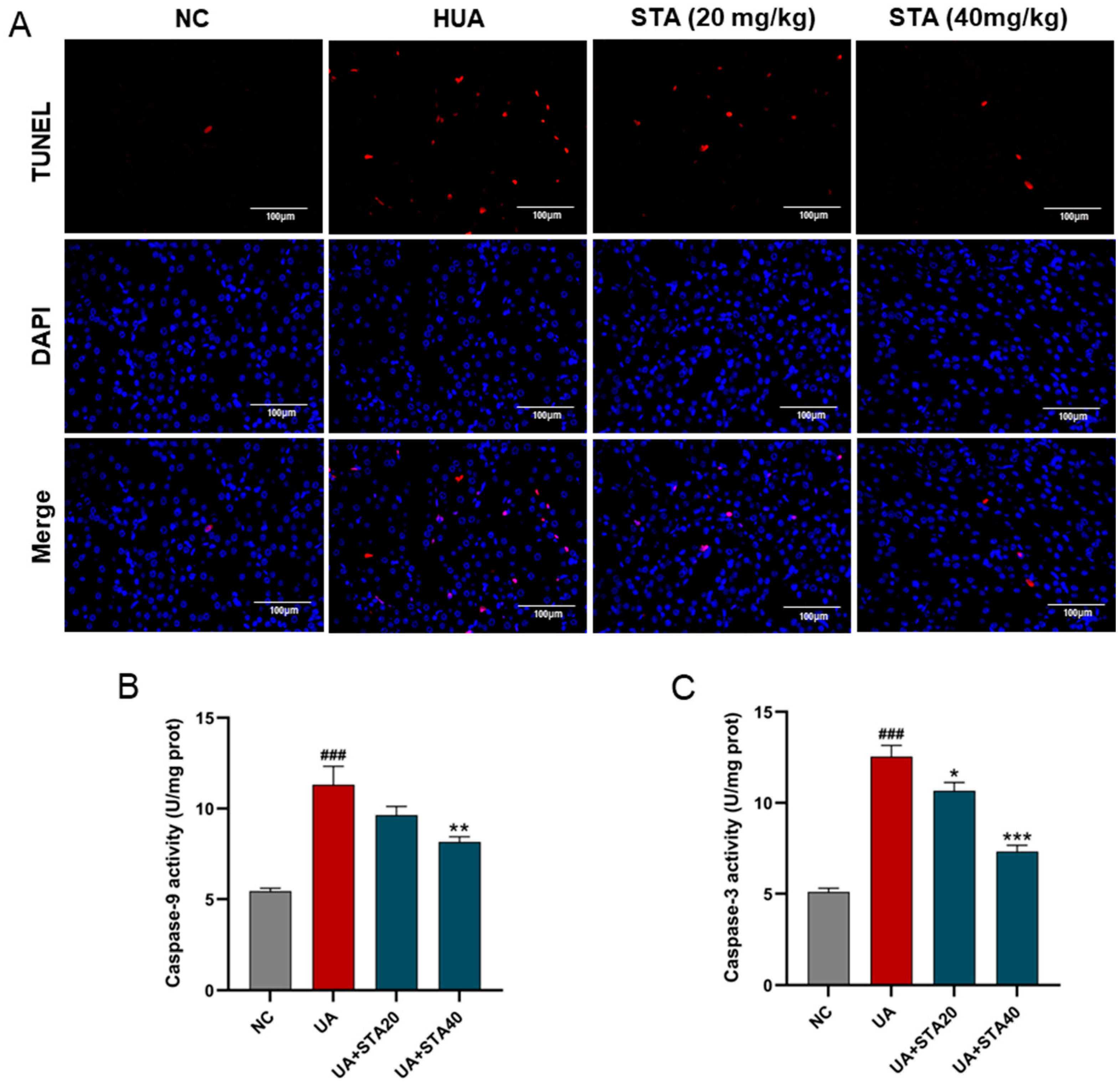
3.4. STA Mitigated HUA-Induced Renal Mitochondrial Oxidative Stress
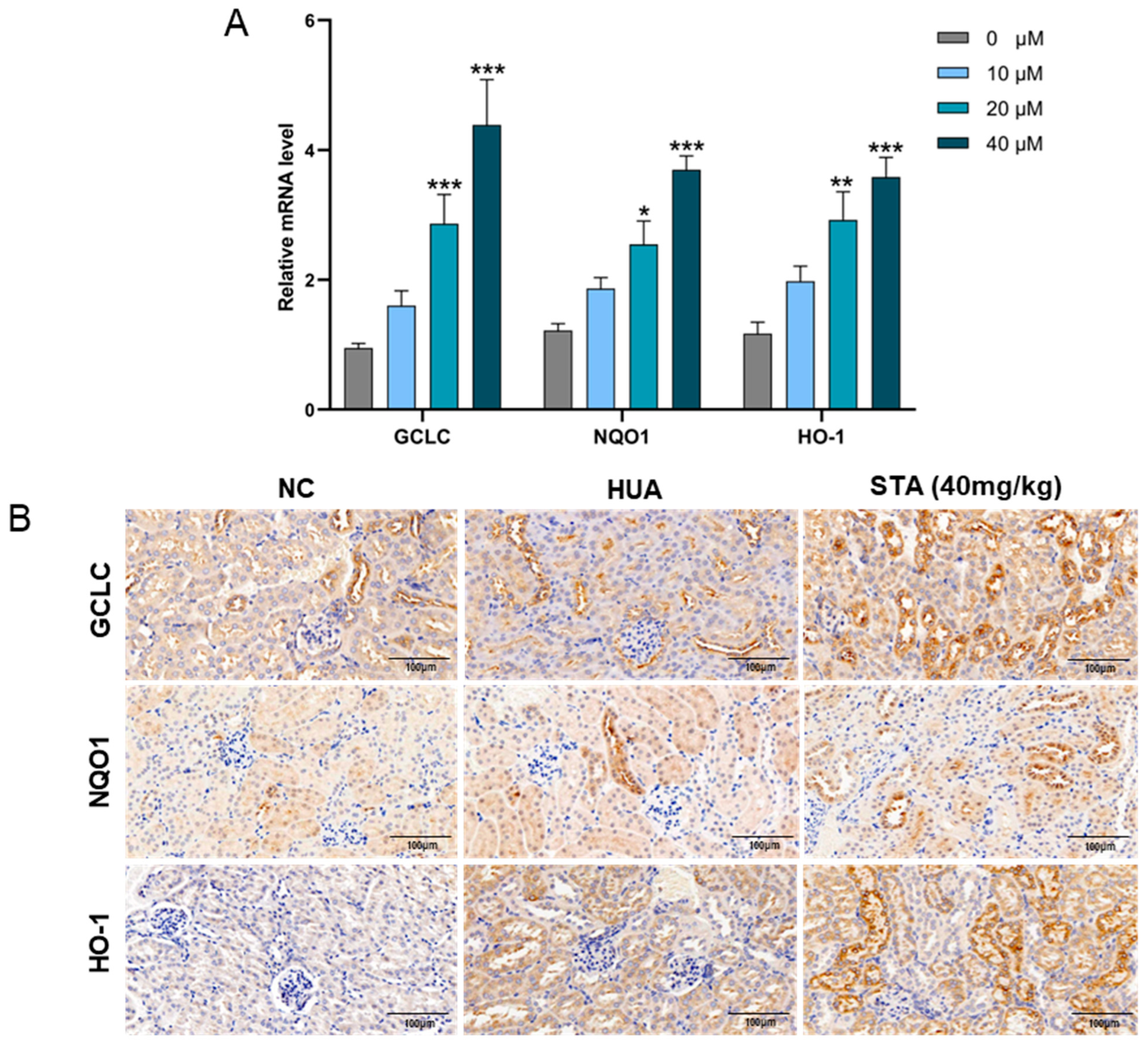
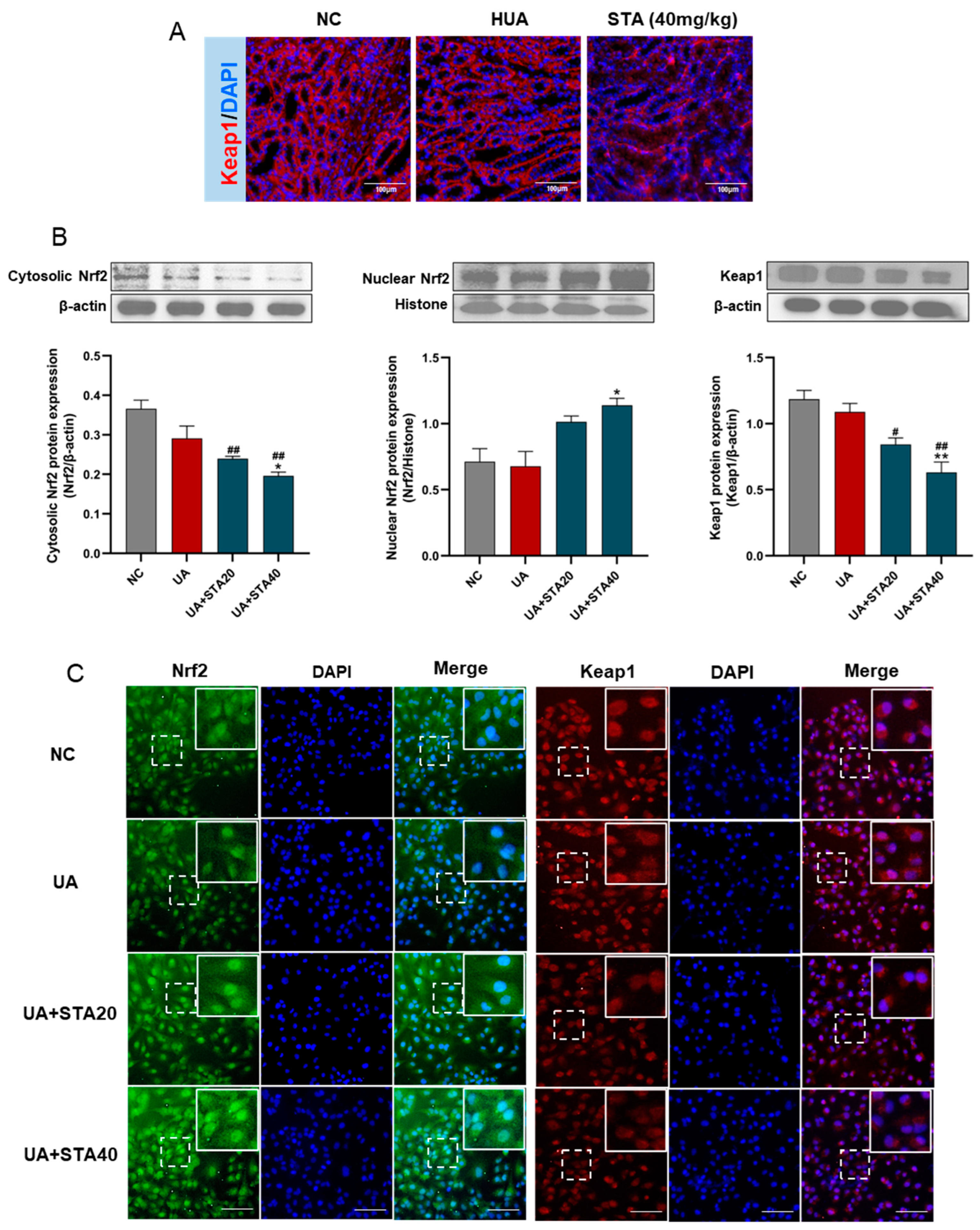
3.5. STA Alleviated HUA-Induced Oxidative Stress by Regulating the Keap1/Nrf2 Signaling Pathway
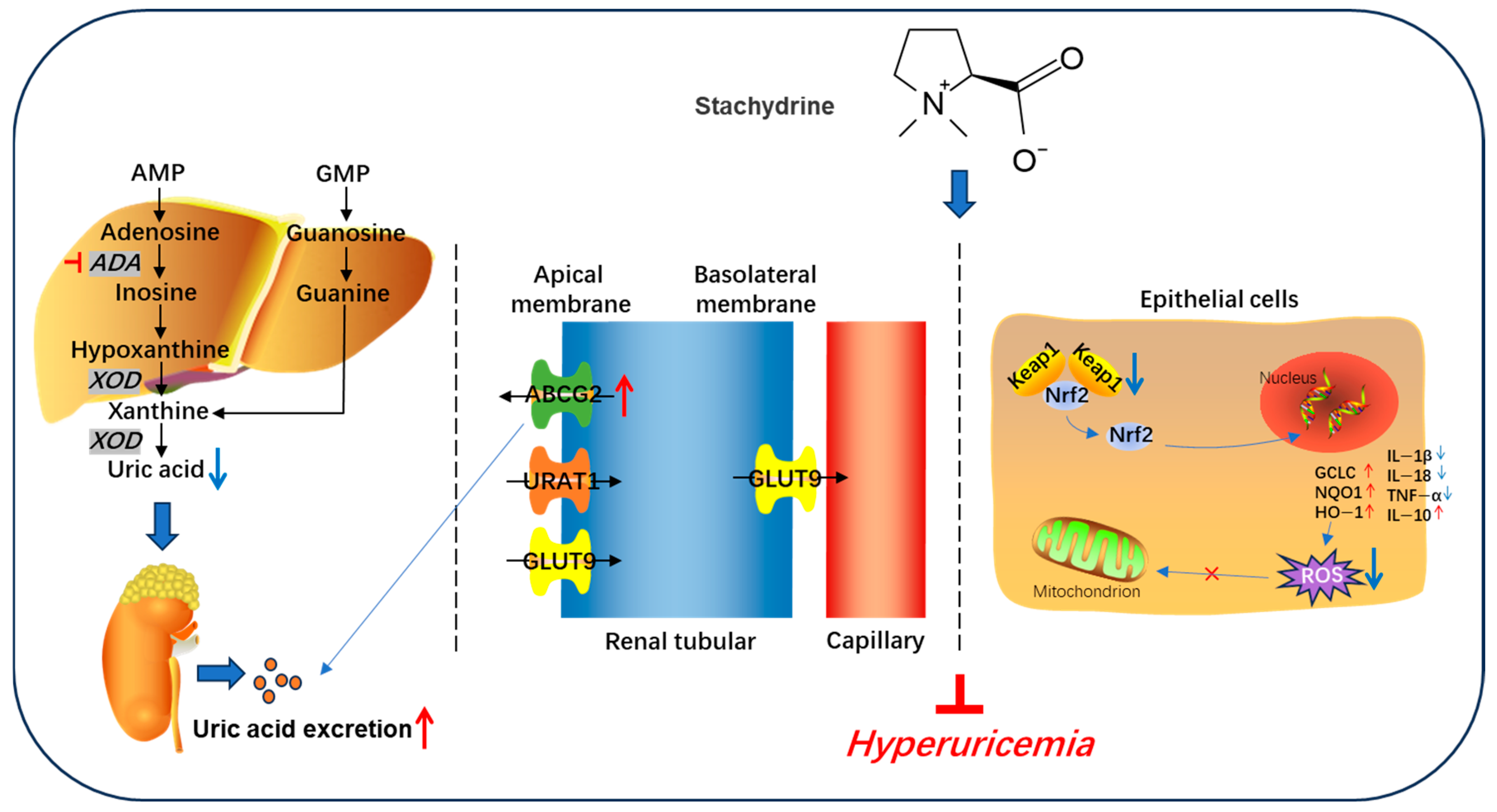
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.L.; Gong, X.; Ji, M.Y.; Wang, C.C.; Wang, J.H.; Li, M.H. Bioactive compounds from plant-based functional foods: A promising choice for the prevention and management of hyperuricemia. Foods 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Ma, L.; Fu, P. Traditional Chinese herbs and natural products in hyperuricemia-induced chronic kidney disease. Front. Pharmacol. 2022, 13, 971032. [Google Scholar] [CrossRef] [PubMed]
- Barman, Z.; Hasan, M.; Miah, R.; Mou, A.D.; Hafsa, J.M.; Trisha, A.D.; Mahmud, F.; Ali, N. Association between hyperuricemia and chronic kidney disease: A cross-sectional study in Bangladeshi adults. BMC Endocr. Disord. 2023, 23, 45. [Google Scholar] [CrossRef]
- Riaz, M.; Al Kury, L.T.; Atzaz, N.; Alattar, A.; Alshaman, R.; Shah, F.A.; Li, S. Carvacrol alleviates hyperuricemia-induced oxidative stress and inflammation by modulating the NLRP3/NF-κB pathway. Drug Des. Dev. Ther. 2023, 16, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xin, M.; Liang, S.; Xu, X.; Cai, T.; Dong, L.; Wang, C.; Wang, M.; Cui, Y.; Song, X.; et al. New insight into the management of renal excretion and hyperuricemia: Potential therapeutic strategies with natural bioactive compounds. Front. Pharmacol. 2022, 13, 1026246. [Google Scholar] [CrossRef]
- Su, H.Y.; Yang, C.; Liang, D.; Liu, H.F. Research advances in the mechanisms of hyperuricemia-induced renal injury. Biomed. Re. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef]
- Liu, Y.L.; Gong, S.T.; Li, K.J.; Wu, G.; Zheng, X.H.; Zheng, J.N.; Lu, X.W.; Zhang, L.Y.; Li, J.C.; Sun, Z.R.; et al. Coptisine protects against hyperuricemic nephropathy through alleviating inflammation, oxidative stress and mitochondrial apoptosis via PI3K/Akt signaling pathway. Biomed. Pharmacother. 2022, 156, 113941. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhang, W.; Cao, Q.T.; Wang, Z.Y.; Zhao, M.Y.; Xu, L.Y.; Zhuang, Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Cristóbal-García, M.; García-Arroyo, F.E.; Tapia, E.; Osorio, H.; Arellano-Buendía, A.S.; Madero, M.; Rodríguez-Iturbe, B.; Pedraza-Chaverrí, J.; Correa, F.; Zazueta, C.; et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxid. Med. Cell. Longev. 2015, 2015, 535686. [Google Scholar] [CrossRef]
- Zhuang, C.; Ni, S.; Yang, Z.C.; Liu, R.P. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of angelica sinensis polysaccharide. Oxid. Med. Cell Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef]
- Sun, H.J.; Ding, S.; Guan, D.X.; Ma, L.Q. Nrf2/Keap1 pathway in countering arsenic-induced oxidative stress in mice after chronic exposure at environmentally-relevant concentrations. Chemosphere 2022, 303, 135256. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, H.; Sun, Q.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.Z.; Lu, W. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid. Med. Cell. Longev. 2021, 2021, 1470380. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Meng, J.; Li, F.; Yu, H.F.; Lin, D.M.; Lin, S.Q.; Li, M.; Zhou, H.; Yang, B.X. Ganoderma lucidum polysaccharide peptide alleviates hyperuricemia by regulating adenosine deaminase and urate transporters. Food Funct. 2022, 13, 12619–12631. [Google Scholar] [CrossRef] [PubMed]
- Adomako, E.A.; Moe, O.W. Uric acid transport, transporters, and their pharmacological targeting. Acta Physiol. 2023, 238, e13980. [Google Scholar] [CrossRef]
- Rey, A.; Batteux, B.; Laville, S.M.; Marienne, J.; Masmoudi, K.; Gras-Champel, V.; Liabeuf, S. Acute kidney injury associated with febuxostat and allopurinol: A post-marketing study. Arthritis Res. Ther. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Ye, X.; Wu, J.; Tang, K.; Li, W.; Xiong, C.; Zhuo, L. Benzbromarone as a possible cause of acute kidney injury in patients with urolithiasis: Two case reports. Medicine 2019, 98, e15214. [Google Scholar] [CrossRef]
- He, Z.; Li, P.; Liu, P.; Xu, P. Exploring stachydrine: From natural occurrence to biological activities and metabolic pathways. Front. Plant Sci. 2024, 15, 1442879. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Borah, J.C.; Manna, P. Stachydrine, a pyrrole alkaloid with promising therapeutic potential against metabolic syndrome and associated organ dysfunction. RSC Med. Chem. 2024, 15, 3652–3673. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhao, P.; Zhao, W.X.; Tian, J.; Guo, W.; Xu, M.; Zhang, C.; Lu, R. Stachydrine ameliorates pressure overload-induced diastolic heart failure by suppressing myocardial fibrosis. Am. J. Transl. Res. 2017, 9, 4250–4260. [Google Scholar]
- Liao, L.; Tang, Y.; Li, B.; Tang, J.; Xu, H.; Zhao, K.; Zhang, X. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed. Pharmacother. 2023, 161, 114489. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Tong, Q.Q.; Zhang, L.; Guan, Y.F.; Wang, S.J.; Xing, Z.H. Effect of stachydrine on endoplasmic reticulum stress-induced apoptosis in rat kidney after unilateral ureteral obstruction. J. Asian Nat. Prod. Res. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Q.; Zhang, Y.; Xue, M.; Yan, H.; Qiu, X.; Tian, Y.J.; Zhang, H.Q.; Liang, H. Fucoidan from Saccharina japonica alleviates hyperuricemia-induced renal fibrosis through inhibiting the JAK2/STAT3 signaling pathway. J. Agric. Food Chem. 2023, 71, 11454–11465. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Wang, C.; Nadeem, M.; Raza, A.; Ali, N.; Shah, A.A. Management of hyperuricemia through dietary polyphenols as a natural medicament: A comprehensive review. Crit. Rev. Food Sci. 2019, 59, 1433–1455. [Google Scholar] [CrossRef]
- Wang, M.X.; Liu, Y.L.; Yang, Y.; Zhang, D.M.; Kong, L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 2015, 747, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Fachriyah, E.; Ghifari, M.A.; Anam, K. Isolation, Identification, and Xanthine oxidase inhibition activity of alkaloid compound from Peperomia pellucida. IOP Mater. Sci. Eng. 2018, 349, 012017. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Zhao, Y.L.; He, Y.J.; Yang, X.W.; Luo, X.D. Tuberindine a, a truffle alkaloid with an unprecedented skeleton exhibiting anti-hyperuricemic bioactivity. Org. Lett. 2022, 24, 4333–4337. [Google Scholar] [CrossRef]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Tech. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Mileti, L.N.; Baleja, J.D. The role of purine metabolism and uric acid in postnatal neurologic development. Molecules 2025, 30, 839. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.L.; Li, K.X.; Zhang, M.Z.; Liu, X.Q.; Li, L.; Guo, Z.; Li, H. Investigating the role of food-derived peptides in hyperuricemia: From mechanisms of action to structural effects. Foods 2024, 14, 58. [Google Scholar] [CrossRef]
- Takada, T.; Miyata, H.; Toyoda, Y.; Nakayama, A.; Ichida, K.; Matsuo, H. Regulation of urate homeostasis by membrane transporters. Gout Urate Cryst. Depos. Dis. 2024, 2, 206–219. [Google Scholar] [CrossRef]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Takada, T.; Nakayama, A.; Shimizu, T.; Sakiyama, M.; Shimizu, S.; Seiko, S.; Chiba, T.; Nakashima, H.; Nakamura, T.; et al. ABCG2 dysfunction increases the risk of renal overload hyperuricemia. Nucleos. Nucleot. Nucl. 2014, 33, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dong, W.; Luo, X.; Xu, L.; Wang, Y. Target screen of anti-hyperuricemia compounds from Cortex Fraxini in vivo based on ABCG2 and bioaffinity ultrafiltration mass spectrometry. Molecules 2023, 28, 7896. [Google Scholar] [CrossRef]
- Bao, R.X.; Chen, Q.; Li, Z.; Wang, D.; Wu, Y.Z.; Liu, M.Y.; Zhang, Y.; Wang, T. Eurycomanol alleviates hyperuricemia by promoting uric acid excretion and reducing purine synthesis. Phytomedicine 2022, 96, 153850. [Google Scholar] [CrossRef]
- Copur, S.; Demiray, A.; Kanbay, M. Uric acid in metabolic syndrome: Does uric acid have a definitive role? Eur. J. Intern. Med. 2022, 103, 4–12. [Google Scholar] [CrossRef]
- Chen, A.; Huang, H.; Fang, S.; Hang, Q. ROS: A “booster” for chronic inflammation and tumor metastasis. BBA-Rev. Cancer 2024, 1879, 189175. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Dhamodharan, U.; Ali, D.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine 2021, 92, 153755. [Google Scholar] [CrossRef]
- Hamdy, S.; Elshopakey, G.E.; Risha, E.F.; Rezk, S.; Ateya, A.I.; Abdelhamid, F.M. Curcumin mitigates gentamicin induced-renal and cardiac toxicity via modulation of Keap1/Nrf2, NF-κB/iNOS and Bcl-2/BAX pathways. Food Chem. Toxicol. 2024, 183, 114323. [Google Scholar] [CrossRef]
- Feng, J.Z.; Ji, K.B.; Pan, Y.J.; Huang, P.P.; He, T.; Xing, Y. Resveratrol ameliorates retinal ischemia-reperfusion injury by modulating the Nlrp3 inflammasome and Keap1/Nrf2/Ho-1 signaling pathway. Mol. Neurobiol. 2024, 61, 8454–8466. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Fernando, P.M.D.J.; Kang, K.A.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Kim, Y.R.; Hyun, J.W. Rosmarinic acid inhibits ultraviolet b-mediated oxidative damage via the AKT/ERK-NRF2-GSH pathway in vitro and in vivo. Biomol. Ther. 2024, 32, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Xia, X.X.; Yuan, G.J.; Zhang, T.K.; Deng, B.B.; Feng, X.Y.; Wang, Q.X. Stachydrine, a bioactive equilibrist for synephrine, identified from four citrus Chinese herbs. Molecules 2023, 28, 3813. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Gong, L.Y.; Wang, L.; Zhao, N.; Sun, Q.; Kamara, M.O.; Ma, H.Y.; Meng, F.H. Comparative pharmacokinetics study of leonurine and stachydrine in normal rats and rats with cold-stagnation and blood-stasis primary dysmenorrhoea after the administration of Leonurus japonicus houtt electuary. J. Sep. Sci. 2019, 42, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; Giovane, A.; Casale, R.; Balestrieri, M.L.; Cautela, D.; Paolucci, M.; Siano, F.; Volpe, M.G.; Castaldo, D. Betaines and related ammonium compounds in chestnut (Castanea sativa Mill.). Food Chem. 2016, 196, 1301–1309. [Google Scholar] [CrossRef]
- Heinzmann, S.S.; Brown, I.J.; Chan, Q.; Bictash, M.; Dumas, M.E.; Kochhar, S.; Stamler, J.; Holmes, E.; Elliott, P.; Nicholson, J.K. Metabolic profiling strategy for discovery of nutritional biomarkers: Proline betaine as a marker of citrus consumption. Am. J. Clin. Nutr. 2010, 92, 436–443. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. 2020, 60, 1738–1746. [Google Scholar] [CrossRef]


| Gene | Species | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| ABCG2 | Mouse | GAACTCCAGAGCCGTTAGGAC | CAGAATAGCATTAAGGCCAGGTT |
| GLUT9 | Mouse | TTGCTTTAGCTTCCCTGATGTG | GAGAGGTTGTACCCGTAGAGG |
| URAT1 | Mouse | TGGGTTTACGACCACAGCAC | CTTCTGCGCCCAAACCTATCT |
| GCLC | Human | TACGGAGGAACAATGTCCGA | CAGTGTGAACCCAGGACAGC |
| NQO1 | Human | AAGAAGAAAGGATGGGAGGTGG | GAACAGACTCGGCAGGATACTG |
| HO-1 | Human | GCCAGCAACAAAGTGCAAGA | TAAGGACCCATCGGAGAAGC |
| β-actin | Mouse | GAGACCTTCAACACCCCAGC | ATGTCACGCACGATTTCCC |
| β-actin | Human | TTGGCAATGAGCGGTTCC | AGACAGCACTGTGTTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Jia, J.; Wang, A.; Gu, Y.; Xia, X. Stachydrine from Natural Foods Alleviates Hyperuricemia by Modulating Renal Urate Transporters and Suppressing Mitochondrial Oxidative Stress. Foods 2025, 14, 1718. https://doi.org/10.3390/foods14101718
Guo J, Jia J, Wang A, Gu Y, Xia X. Stachydrine from Natural Foods Alleviates Hyperuricemia by Modulating Renal Urate Transporters and Suppressing Mitochondrial Oxidative Stress. Foods. 2025; 14(10):1718. https://doi.org/10.3390/foods14101718
Chicago/Turabian StyleGuo, Jian, Jinhui Jia, Ailin Wang, Yunqi Gu, and Xiaodong Xia. 2025. "Stachydrine from Natural Foods Alleviates Hyperuricemia by Modulating Renal Urate Transporters and Suppressing Mitochondrial Oxidative Stress" Foods 14, no. 10: 1718. https://doi.org/10.3390/foods14101718
APA StyleGuo, J., Jia, J., Wang, A., Gu, Y., & Xia, X. (2025). Stachydrine from Natural Foods Alleviates Hyperuricemia by Modulating Renal Urate Transporters and Suppressing Mitochondrial Oxidative Stress. Foods, 14(10), 1718. https://doi.org/10.3390/foods14101718






