Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus–Kiwifruit Composite Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Detection of Physicochemical Parameters
2.3. Determination of Polyphenolic Substances and Iridoid Glycosides in Cornus-Kiwi Fruit Wine
2.4. GC-MS Analyses of Volatile Compounds in Wines
2.5. Clarity and Color Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results
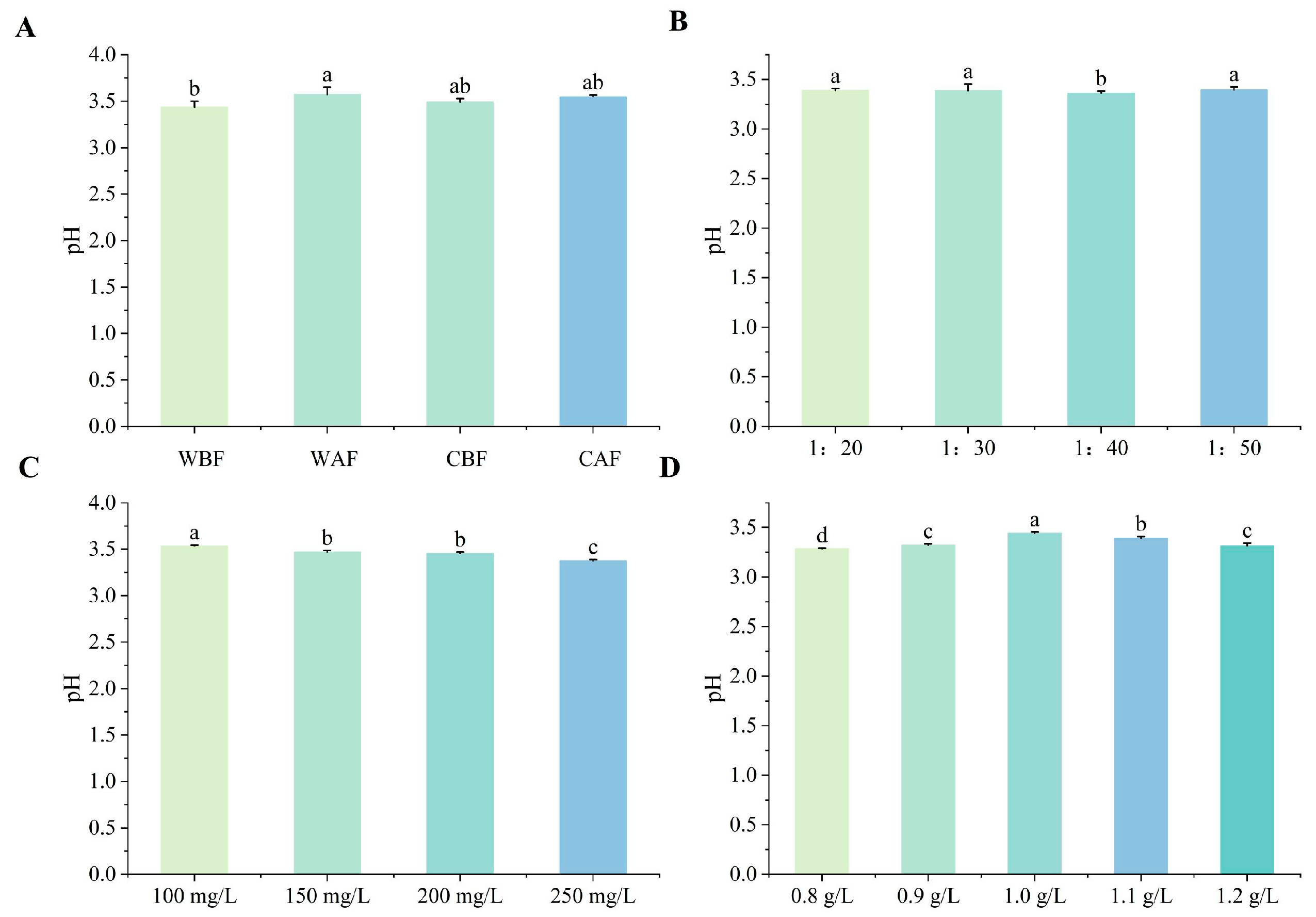
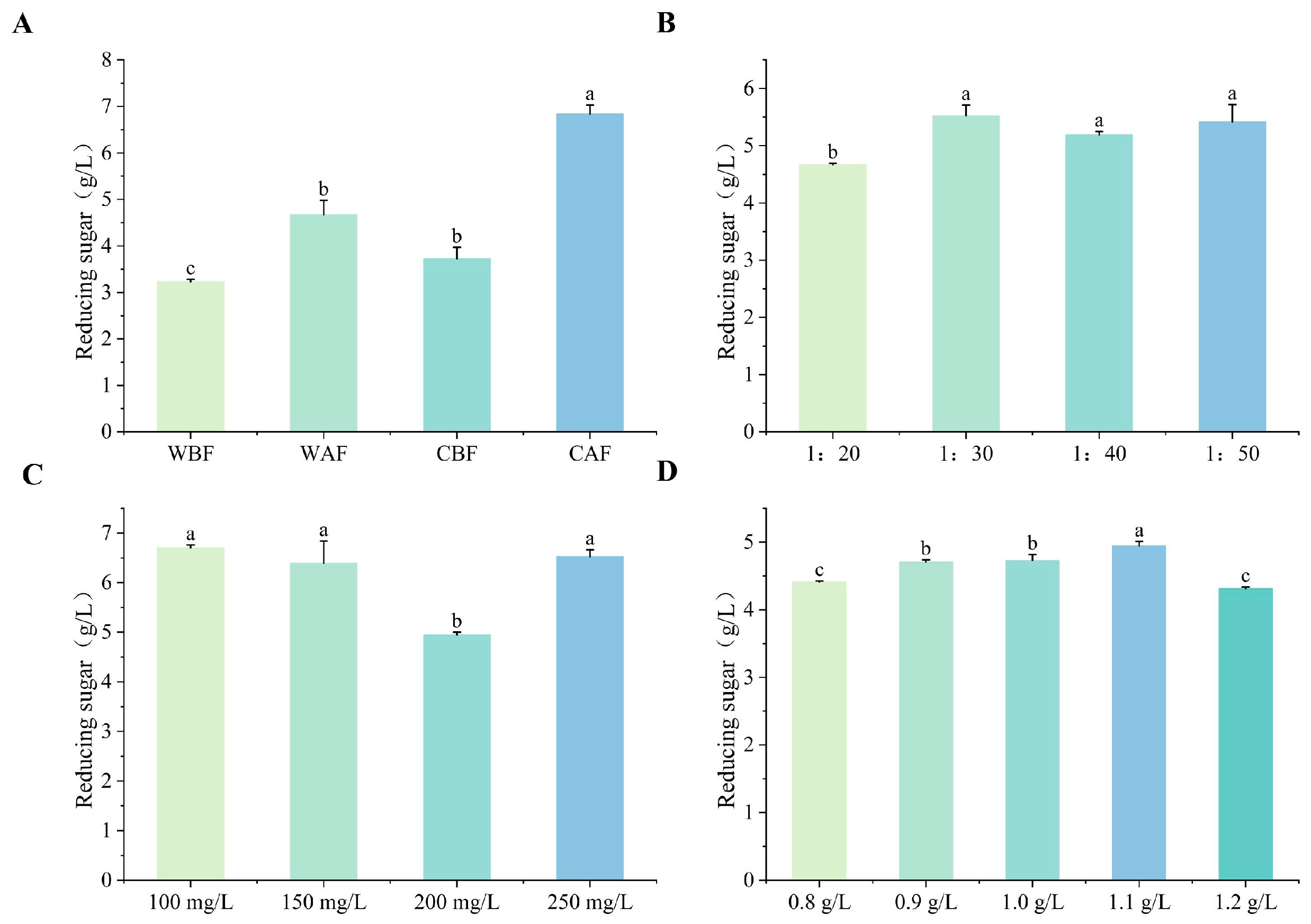
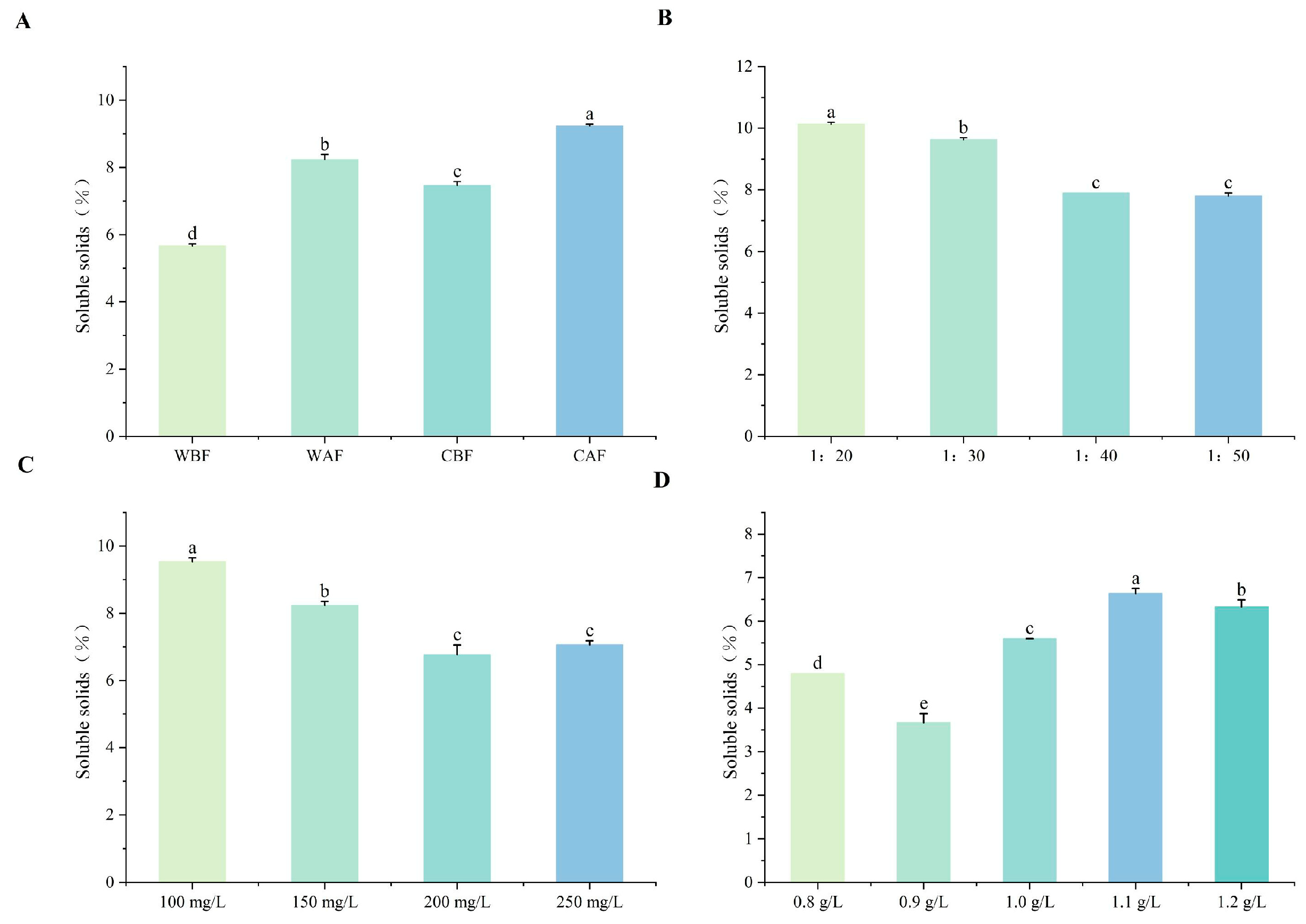
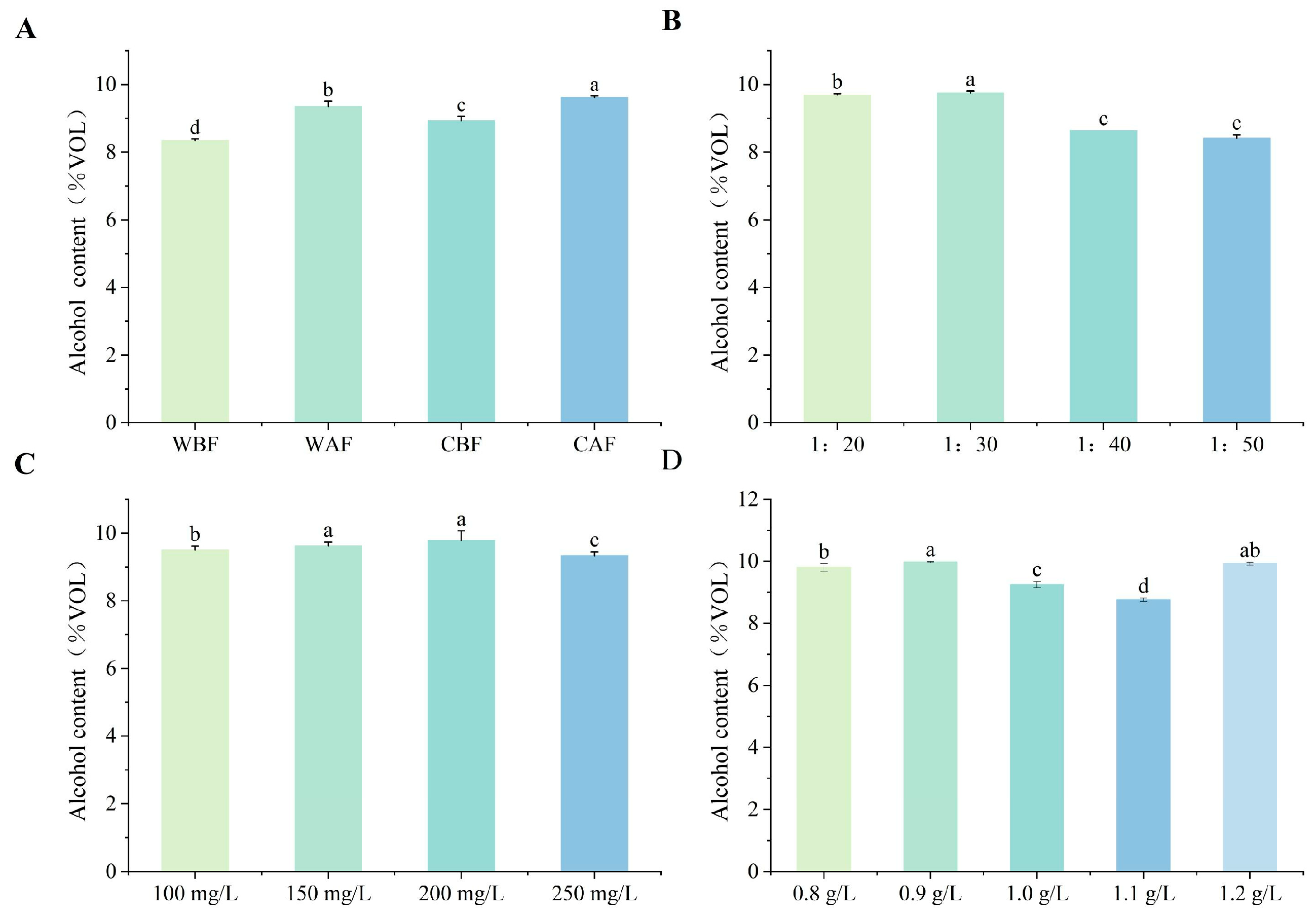
3.1. Effects of Various Treatments on Basic Physicochemical Indexes of Cornus–Kiwifruit Wine
3.2. Effects of Various Treatment Methods on Polyphenolic Substances and Unique Components in Cornus-Kiwifruit Wine
3.3. Different Treatments on Volatile Compounds in Cornus–Kiwifruit Wine
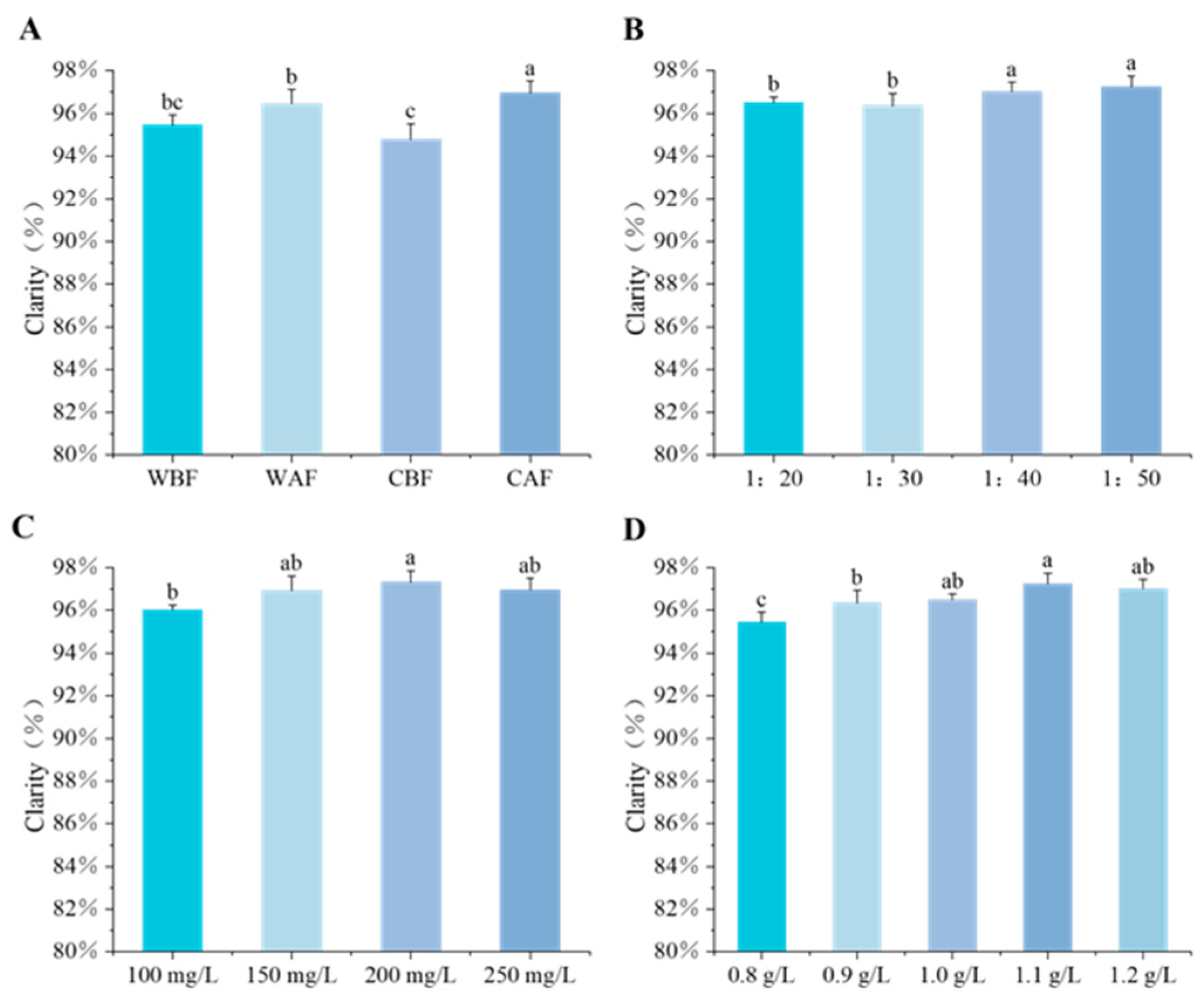
3.4. Effects of Different Treatments on the Color and Clarity of Cornus–Kiwifruit Wine
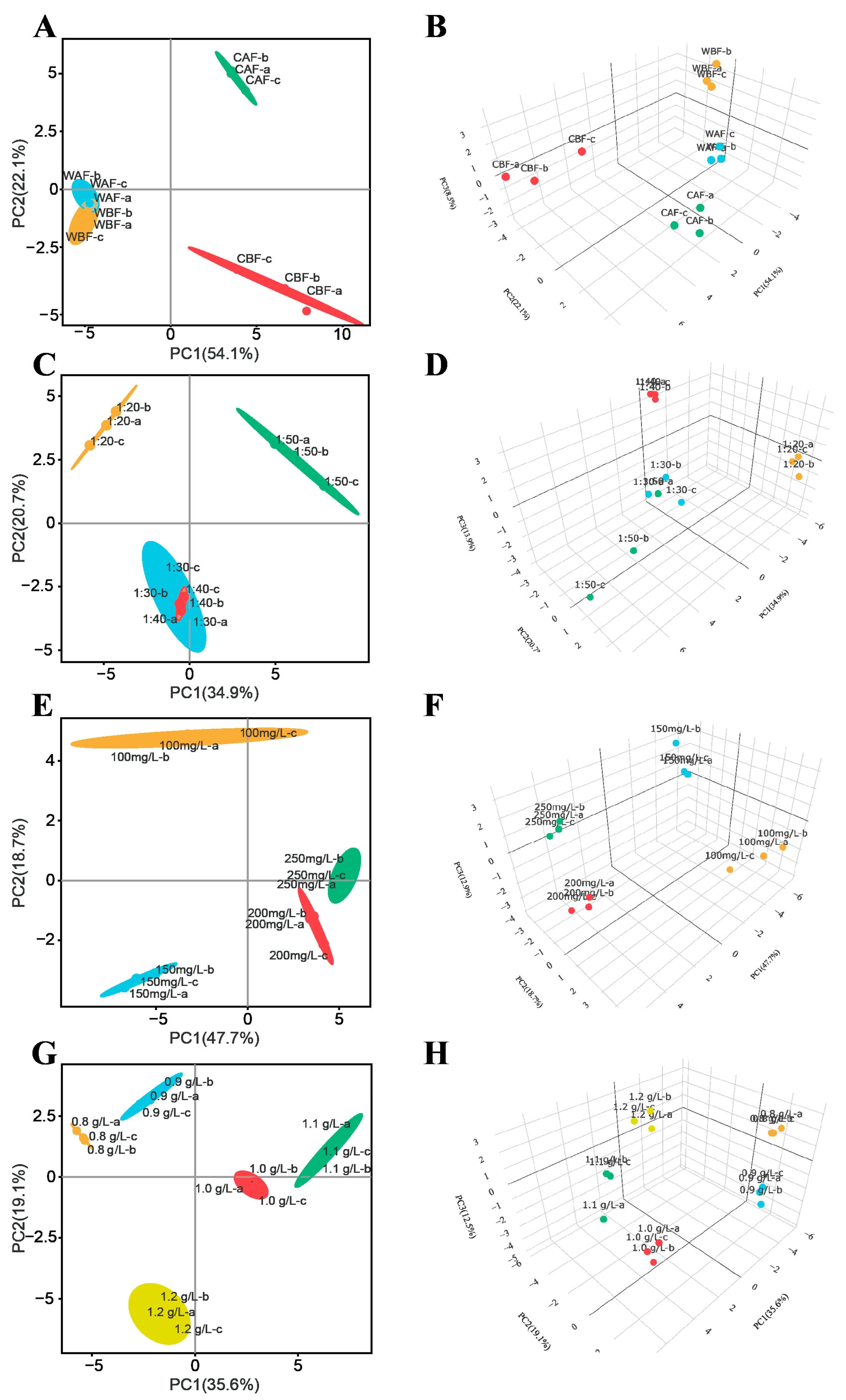
3.5. Comprehensive Quality Analysis of Cornus–Kiwifruit Wine Based on Principal Component Analysis
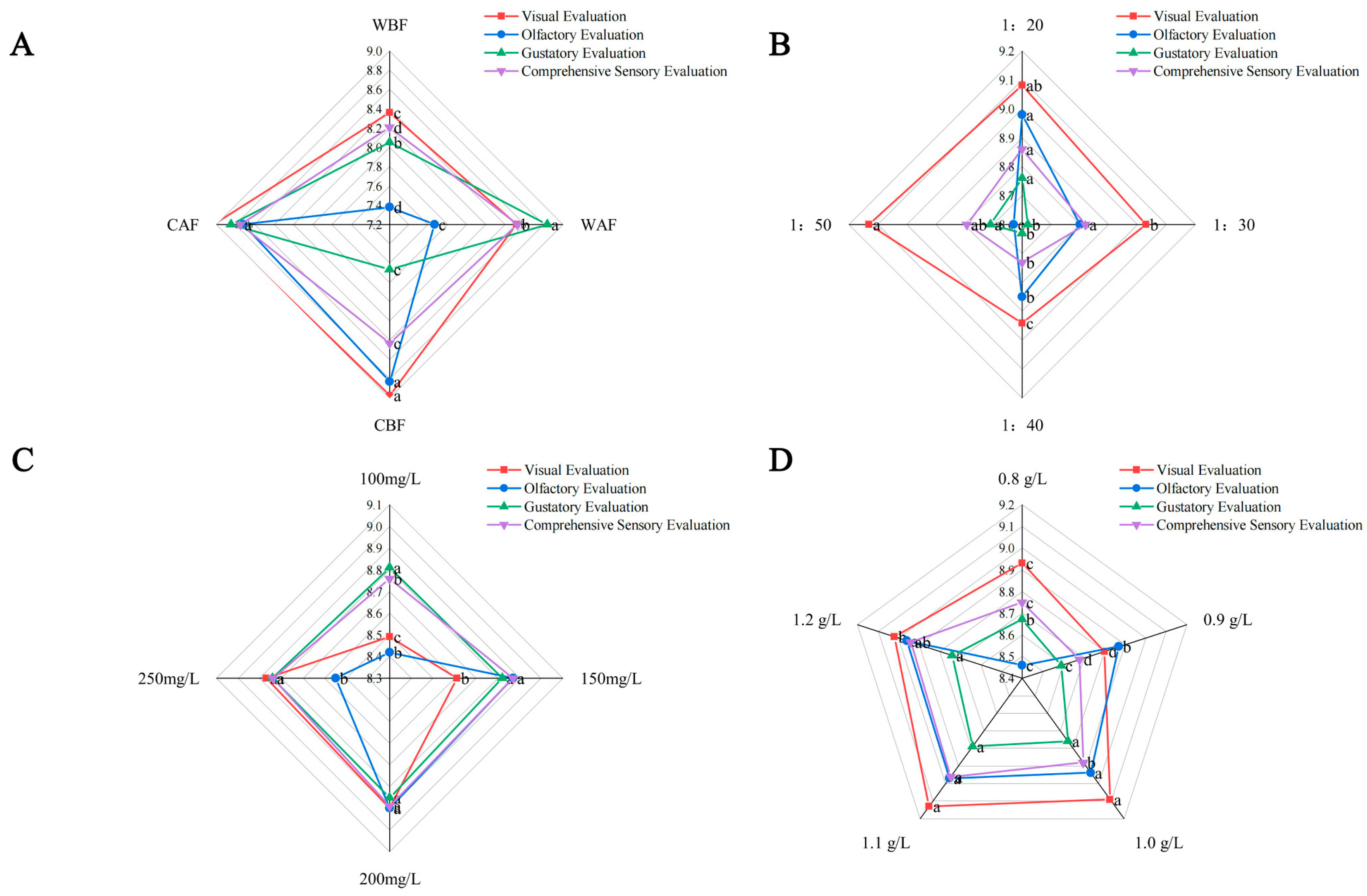
3.6. Effect of Different Treatments on the Sensory Quality of Cornus–Kiwifruit Wine
4. Discussion
4.1. Effects of Different Treatments on Basic Physicochemical Indices of Cornus–Kiwifruit Wine
4.2. Effects of Different Adding Methods of Cornus officinalis on Polyphenolic Substances and Special Components in Cornus–Kiwifruit Wine
4.3. Effects of Different Addition Methods on Aroma Compound Content in Cornus–Kiwifruit Wine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, T.; Lan, T.; Geng, T.; Ju, Y.; Cheng, G.; Que, Z.; Gao, G.; Fang, Y.; Sun, X. Nutritional properties and biological activities of kiwifruit (Actinidia) and kiwifruit products under simulated gastrointestinal in vitro digestion. Food Nutr. Res. 2019, 63, 1674. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Yue, T.; Quek, S.Y. Optimization of polyphenol removal from kiwifruit juice using a macroporous resin. J. Sci. Food Agric. 2017, 97, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Soufleros, E.H.; Pissa, I.; Petridis, D.; Lygerakis, M.; Mermelas, K.; Boukouvalas, G.; Tsimitakis, E. Instrumental analysis of volatile and other compounds of Greek kiwi wine; sensory evaluation and optimisation of its composition. Food Chem. 2001, 75, 487–500. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B.; Petriccione, M.; Martignetti, A.; Malorni, L.; Zampella, L.; Laurino, C.; Pellicano, M.P. Comparative analysis of volatile metabolites, quality and sensory attributes of Actinidia chinensis fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qi, Y.; Zhao, N.; Cao, Y.; Xu, J.; Fan, M. Multivariate analysis reveals effect of glutathione-enriched inactive dry yeast on amino acids and volatile components of kiwi wine. Food Chem. 2020, 329, 127086. [Google Scholar] [CrossRef]
- Santoni, F.; Barboni, T.; Paolini, J.; Costa, J. Influence of Cultivation Parameters on the Mineral Composition of Kiwi Fruit from Corsica. Chem. Biodivers. 2016, 13, 748–754. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Zhang, K.; Du, M.; Zhang, H.; Zhang, X.; Cao, S.; Wang, X.; Wang, W.; Guan, X.; Zhou, P.; Li, J.; et al. The haplotype-resolved T2T genome of teinturier cultivar Yan73 reveals the genetic basis of anthocyanin biosynthesis in grapes. Hortic. Res. 2023, 10, uhad205. [Google Scholar] [CrossRef]
- Eltorki, M.; Leong, R.; Ratcliffe, E.M. Kiwifruit and Kiwifruit Extracts for Treatment of Constipation: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2022, 2022, 7596920. [Google Scholar] [CrossRef]
- Wang, B.; Peng, B. A Feasibility Study on Monitoring Residual Sugar and Alcohol Strength in Kiwi Wine Fermentation Using a Fiber-Optic FT-NIR Spectrometry and PLS Regression. J. Food Sci. 2017, 82, 358–363. [Google Scholar] [CrossRef]
- Ozgen, F.; Celik, N. Evaluation of Design Parameters on Drying of Kiwi Fruit. Appl. Sci. 2018, 9, 10. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef] [PubMed]
- Čakar, U.; Grozdanić, N.; Pejin, B.; Vasić, V.; Čakar, M.; Petrović, A.; Djordjević, B. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, T.; Yang, H.; Li, E. Isolation and Selection of Non-Saccharomyces Yeasts Being Capable of Degrading Citric acid and Evaluation Its Effect on Kiwifruit Wine Fermentation. Fermentation 2020, 6, 25. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Ye, P.; Lin, F.; Huang, J.; Wang, H.; Zhou, R.; Zhang, S.; Zhou, J.; Cai, L. Characterization of major properties and aroma profile of kiwi wine co-cultured by Saccharomyces yeast (S. cerevisiae, S. bayanus, S. uvarum) and T. delbrueckii. Eur. Food Res. Technol. 2020, 246, 807–820. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Li, H.; Li, F.; Song, M.; Li, Z.; Zhang, T.; Han, S.; Pan, C. Optimization of fermentation technology for composite fruit and vegetable wine by response surface methodology and analysis of its aroma components. RSC Adv. 2022, 12, 35616–35626. [Google Scholar] [CrossRef]
- Liu, J.; Guan, W.; Sun, Z.; Ni, Y.; He, L.; Tian, F.; Cai, L. Application of Cyclocarya paliurus-Kiwifruit Composite Fermented to Enhance Antioxidant Capacity, Flavor, and Sensory Characteristics of Kiwi Wine. Molecules 2023, 29, 32. [Google Scholar] [CrossRef]
- Martin-Gomez, J.; Garcia-Martinez, T.; Varo, M.A.; Merida, J.; Serratosa, M.P. Enhance Wine Production Potential by Using Fresh and Dried Red Grape and Blueberry Mixtures with Different Yeast Strains for Fermentation. Foods 2023, 12, 3925. [Google Scholar] [CrossRef]
- Czerwinska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis-Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhang, T.Y.; Yang, F.Q.; Ito, Y. Separation of gallic acid from Cornus officinalis Sieb. et Zucc by high-speed counter-current chromatography. J. Chromatogr. A. 2000, 886, 309–312. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Feng, Q.-M.; Li, Q.X.; Wang, F.; Wang, P.; Li, H.B.; Zhang, L.X.; Chi, J.; Dai, L.P. 5-Hydroxymethylfurfural derivatives from wine-processed Corni fructus. J. Mol. Struct. 2023, 1294, 136322. [Google Scholar] [CrossRef]
- Klongová, L.; Kovár, M.; Navrátilová, A.; Fialkova, V.; Požgajová, M. Cornus mas L. Extract-Mediated Modulations of the Redox State Induce Cytotoxicity in Schizosaccharomyces pombe. Appl. Sci. 2024, 14, 4049. [Google Scholar] [CrossRef]
- Moldovan, R.; Mitrea, D.R.; Florea, A.; Chis, I.C.; Suciu, S.; David, L.; Moldovan, B.E.; Muresan, L.E.; Lenghel, M.; Ungur, R.A.; et al. Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet-A Preliminary Study. Antioxidants 2022, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Popovic, B.M.; Stajner, D.; Slavko, K.; Sandra, B. Antioxidant capacity of cornelian cherry (Cornus mas L.)—Comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, X.G.; Hu, Z.D.; Ju, Y. Analysis of three effective components in Fructus corni and its preparations by micellar electrokinetic capillary chromatography. Biomed. Chromatogr. 2003, 17, 306–311. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.L.; Chen, H.B.; Wang, F.S.; Lu, J.H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Kim, S.Y.; Hwangbo, H.; Park, C.; Hong, S.H.; Kim, G.Y.; Choi, Y.H. The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5. Nutrients 2021, 13, 2986. [Google Scholar] [CrossRef]
- Wang, F.; Chi, J.; Guo, H.; Wang, J.; Wang, P.; Li, Y.X.; Wang, Z.M.; Dai, L.P. Revealing the effects and mechanism of wine processing on Corni Fructus using chemical characterization integrated with multi-dimensional analyses. J. Chromatogr. A 2024, 1730, 465100. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Peng, K.; Yang, B.; Yang, M.; Jia, X.; Wang, N.; Zhang, Q.; Kong, D.; Du, Y. The therapeutic effect of wine-processed Corni Fructus on chronic renal failure in rats through the interference with the LPS/IL-1-mediated inhibition of RXR function. J. Ethnopharmacol. 2024, 321, 117511. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Jiang, Z.; Wang, M.; Jiang, H.; Zhao, F.; Ding, X.; Cai, B.; Zhan, Z. 5-Hydroxymethylfurfural from wine-processed Fructus corni inhibits hippocarnpal neuron apoptosis. Neural Regen. Res. 2013, 8, 2605–2614. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z.; Glowacki, A.; Piorecki, N. Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads. Biomolecules 2021, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Yuan, Y.; Dai, L.; Yue, T. Characteristic fruit wine production via reciprocal selection of juice and non-Saccharomyces species. Food Microbiol. 2019, 79, 66–74. [Google Scholar] [CrossRef]
- Zhou, W.; Sun-Waterhouse, D.; Xiong, J.; Cui, C.; Wang, W.; Dong, K. Desired soy sauce characteristics and autolysis of Aspergillus oryzae induced by low temperature conditions during initial moromi fermentation. J. Food Sci. Technol. 2019, 56, 2888–2898. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbiol. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, T.; Li, J.; Zhang, B.; Yu, Y.; Wang, Y.; Niu, H. Variations in Main Flavor Compounds of Freshly Distilled Brandy during the Second Distillation. Int. J. Food Eng. 2014, 10, 809–820. [Google Scholar] [CrossRef]
- Li, M.Y.; Wang, W.; Meng, X.C.; Wang, X.J. Determination of content of iridoid glycosides in the enrichment of Cornus officinalis macroporous adsorption resin. Chin. Med. Pharmacol. 2010, 38, 43–45. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Evaluation of pH differential and HPLC methods expressed as cyanidin-3-glucoside equivalent for measuring the total anthocyanin contents of berries. J. Food Meas. Charact. 2016, 10, 562–568. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.Z.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M.A. Characterization, phenolic profile, nitrogen compounds and antioxidant activity of Carignano wines. J. Food Compos. Anal. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Chen, K.; Ju, Y.; Min, Z.; Sun, X.; Cheng, Z.; Liao, Z.; Zhang, K.; Fang, Y. Effect of foliar application of fulvic acid antitranspirant on sugar accumulation, phenolic profiles and aroma qualities of Cabernet Sauvignon and Riesling grapes and wines. Food Chem. 2021, 351, 129308. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odor Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; pp. 207–358. [Google Scholar]
- Baca-Bocanegra, B.; Goncalves, S.; Nogales-Bueno, J.; Mansinhos, I.; Heredia, F.J.; Hernandez-Hierro, J.M.; Romano, A. Influence of Wine pH and Ethanol Content on the Fining Efficacy of Proteins from Winemaking By-Products. Foods 2022, 11, 1688. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of glutathione during bottle storage of sparkling wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef]
- ISO 3591:1997; Sensory Analysis—Apparatus—Wine-Tasting Glass. International Organization for Standardization: Geneva, Switzerland, 1997.
- Li, H. Wine Tasting; Science Press: Beijing, China, 2006. [Google Scholar]
- Chen, X.; Peng, M.; Wu, D.; Cai, G.; Yang, H.; Lu, J. Physicochemical indicators and sensory quality analysis of kiwi wines fermented with different Saccharomyces cerevisiae. J. Food Process. Preserv. 2022, 46, e17132. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, Q.; An, X.; Chitrakar, B.; Li, J.; Zhao, Z.; Ao, C.; Sun, J. Optimization of Mopan Persimmon Wine Fermentation with Pectinase and Analysis of Its Mechanism of Action. Foods 2023, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Fan, X.H.; Zhao, W.Q.; Wang, X.Y.; Liu, H.Z. Evolution of some physicochemical properties in Cornus officinalis wine during fermentation and storage. Eur. Food Res. Technol. 2013, 237, 711–719. [Google Scholar] [CrossRef]
- Chen, A.J.; Fu, Y.Y.; Jiang, C.; Zhao, J.L.; Liu, X.P.; Liu, L.; Ma, J.; Liu, X.Y.; Shen, G.H.; Li, M.L.; et al. Effect of mixed fermentation (Jiuqu and Saccharomyces cerevisiae EC1118) on the quality improvement of kiwi wine. CyTA—J. Food 2019, 17, 967–975. [Google Scholar] [CrossRef]
- Cheng, Y.; Watrelot, A.A. Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines. Molecules 2022, 27, 3482. [Google Scholar] [CrossRef]
- Ren, M.; Liu, S.; Li, R.; You, Y.; Huang, W.; Zhan, J. Clarifying effect of different fining agents on mulberry wine. Int. J. Food Sci. Technol. 2019, 55, 1578–1585. [Google Scholar] [CrossRef]
- Pascoal, A.; Oliveira, J.M.; Pereira, A.P.; Féas, X.; Anjos, O.; Estevinho, L.M. Influence of fining agents on the sensorial characteristics and volatile composition of mead. J. Inst. Brew. 2017, 123, 562–571. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Chen, Y.; Liu, J.; Zhang, B.; Zhu, B.; Qian, X. Lactiplantibacillus plantarum inoculation enhanced the color stabilization and aroma quality of blueberry wine. LWT 2024, 208, 116750. [Google Scholar] [CrossRef]
- Varo, M.A.; Martín-Gómez, J.; Merida, J.; Serratosa, M.P. Exploring the Impact of Temperature and Fermentation Time on the Evolution of Bioactive Compounds, Antioxidant Activity, and Color Evolution in Blueberry Wines. ACS Food Sci. Technol. 2024, 4, 1301–1309. [Google Scholar] [CrossRef]
- Yao, R.Q.; Wang, F. Study on the Clarification Effect of Composite Clarifying Agents on Cornus officinalis Wine. Brew. Sci. Technol. 2018, 6, 30–33+46. [Google Scholar] [CrossRef]
- Song, Y.; Xu, B. Diffusion Profiles of Health Beneficial Components from Goji Berry (Lyceum barbarum) Marinated in Alcohol and Their Antioxidant Capacities as Affected by Alcohol Concentration and Steeping Time. Foods 2013, 2, 32–42. [Google Scholar] [CrossRef]
- Roufa, P.; Evangelou, A.; Beris, E.; Karagianni, S.; Chatzilazarou, A.; Dourtoglou, E.; Shehadeh, A. Increase in Total Phenolic Content and Antioxidant Capacity in Wines with Pre- and Post-Fermentation Addition of Melissa officinalis, Salvia officinalis and Cannabis sativa. Horticulturae 2023, 9, 956. [Google Scholar] [CrossRef]
- Ferrero, L.; Beria D’argentina, S.; Paissoni, M.A.; Río Segade, S.; Rolle, L.; Giacosa, S. Phenolic budget in red winemaking: Influence of maceration temperature and time. Food Chem. 2025, 482, 144159. [Google Scholar] [CrossRef]
- Giordano, M.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Stojković, D.; Soković, M.; Tavares, D.; Cánepa, A.L.; Ferreira, I.C.F.R.; Caleja, C.; et al. Ultrasound-Assisted Extraction of Flavonoids from Kiwi Peel: Process Optimization and Bioactivity Assessment. Appl. Sci. 2021, 11, 6416. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J.; Tan, L.; Wang, Y.; Li, J.; Wang, Y.; Ding, C.; Long, H. Changes in metabolites in raw and wine processed Corni Fructus combination metabolomics with network analysis focusing on potential hypoglycemic effects. Front. Pharmacol. 2023, 14, 1173747. [Google Scholar] [CrossRef]
- Ju, C.G.; Zhu, L.; Wang, W.; Gao, H.; Xu, Y.B.; Jia, T.Z. Cornus officinalis prior and post-processing: Regulatory effects on intestinal flora of diabetic nephropathy rats. Front. Pharmacol. 2022, 13, 1039711. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of chemical constitution and aroma properties of kiwi wines obtained from pure and mixed fermentation with Wickerhamomyces anomalus and Saccharomyces cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Ren, Y.; Wang, X.; Li, H.; Liu, Z.; Yue, T.; Gao, Z. Effect of inoculation method on the quality and nutritional characteristics of low-alcohol kiwi wine. LWT 2022, 156, 113049. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Liu, Y.; Liu, S.; Yang, X.; Wang, X. Process Optimization for Production of Persimmon Wine with Lower Methanol. Foods 2024, 13, 748. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Zhang, J.; Liu, M.; Wei, X.; Fan, M. Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef]
- Lola, D.; Kalloniati, C.; Dimopoulou, M.; Kanapitsas, A.; Papadopoulos, G.; Dorignac, E.; Flemetakis, E.; Kotseridis, Y. Impact of Assimilable Nitrogen Supplementation on Saccharomyces cerevisiae Metabolic Response and Aromatic Profile of Moschofilero Wine. J. Agric. Food Chem. 2023, 71, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontés Riojano wines. LWT 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of ultrasonic-assisted extraction of flavonoids, polysaccharides, and eleutherosides from Acanthopanax senticosus using response surface methodology in development of health wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation.pdf. Foods 2022, 11, 3789. [Google Scholar] [CrossRef]
- Mumford, A.; Pliakoni, E.D.; Hale, I. Effects of Harvest Maturity on Storability, Ripening Dynamics, and Fruit Quality of ‘Geneva 3’ Kiwiberries.pdf. HortScience 2023, 58, 761–767. [Google Scholar] [CrossRef]
- Tilahun, S.; Choi, H.R.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Hyok, K.; Park, S.M.; Jeong, C.S. Ripening quality of kiwifruit cultivars is affected by harvest time. Sci. Hortic. 2020, 261, 108936. [Google Scholar] [CrossRef]
- Choi, H.R.; Tilahun, S.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Jeong, C.S. Harvest time affects quality and storability of kiwifruit (Actinidia spp.). Sci. Hortic. 2019, 256, 108523. [Google Scholar] [CrossRef]


| Treatments | Total Anthocyanins | Total Flavonoids | Total Phenolics | Total Iridoid Glycosides | ||
|---|---|---|---|---|---|---|
| Cornus officinalis added in different ways and times | 1 | WBF | 1.53 ± 0.10 d | 274.24 ± 19.17 d | 676.47 ± 20.21 d | 2591.75 ± 88.84 d |
| 2 | WAF | 6.90 ± 0.28 b | 433.01 ± 8.36 b | 1317.62 ± 49.31 b | 4353.99 ± 23.62 b | |
| 3 | CBF | 2.99 ± 0.44 c | 322.82 ± 3.14 c | 777.30 ± 33.59 c | 3760.86 ± 120.59 c | |
| 4 | CAF | 10.14 ± 0.52 a | 670.90 ± 7.43 a | 1462.55 ± 37.80 a | 5761.85 ± 20.41 a | |
| Different ratio of Cornus officinalis and kiwi | 5 | 1:20 | 18.49 ± 1.08 a | 575.38 ± 6.47 a | 1406.65 ± 69.03 a | 4486.49 ± 210.64 a |
| 6 | 1:30 | 17.41 ± 0.46 a | 553.13 ± 27.05 a | 1357.76 ± 32.17 a | 3897.45 ± 121.90 b | |
| 7 | 1:40 | 7.89 ± 1.69 b | 507.92 ± 11.08 b | 1321.77 ± 31.10 a | 3246.98 ± 245.47 c | |
| 8 | 1:50 | 5.30 ± 0.52 c | 417.68 ± 22.46 c | 1018.51 ± 59.44 b | 2655.78 ± 329.68 d | |
| Different yeast additions | 9 | 100 mg/L | 6.27 ± 0.66 b | 438.90 ± 15.56 d | 1339.87 ± 48.70 b | 3716.39 ± 390.30 c |
| 10 | 150 mg/L | 7.31 ± 1.08 b | 494.91 ± 16.60 c | 1432.42 ± 10.48 ab | 4829.95 ± 117.85 b | |
| 11 | 200 mg/L | 10.13 ± 0.74 a | 561.66 ± 14.88 b | 1500.12 ± 77.85 a | 5648.48 ± 197.32 a | |
| 12 | 250 mg/L | 9.02 ± 0.23 a | 641.76 ± 11.79 a | 1459.44 ± 83.62 ab | 5237.21 ± 138.66 ab | |
| Different amounts of bentonite additive | 13 | 0.8 g/L | 2.95 ± 0.16 c | 408.29 ± 14.38 c | 897.13 ± 24.15 d | 2604.05 ± 108.22 b |
| 14 | 0.9 g/L | 2.85 ± 0.12 c | 487.44 ± 9.86 b | 996.34 ± 24.60 c | 2615.81 ± 59.55 ab | |
| 15 | 1.0 g/L | 4.60 ± 0.39 a | 499.44 ± 10.87 b | 1174.31 ± 21.66 b | 2894.50 ± 38.24 b | |
| 16 | 1.1 g/L | 3.89 ± 0.12 b | 520.02 ± 3.05 a | 1425.69 ± 41.98 a | 3114.52 ± 73.03 a | |
| 17 | 1.2 g/L | 3.86 ± 0.06 b | 496.26 ± 6.47 b | 918.18 ± 17.23 d | 2723.22 ± 56.12 c | |
| Aroma Substance | Cornus officinalis Added in Different Ways and Times | Different Ratio of Cornus officinalis and kiwi | Different Yeast Additions | Different Amounts of Bentonite Additive | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| WBF | WAF | CBF | CAF | 1:20 | 1:30 | 1:40 | 1:50 | 100 mg/L | 150 mg/L | 200 mg/L | 250 mg/L | 0.8 g/L | 0.9 g/L | 1.0 g/L | 1.1 g/L | 1.2 g/L | |
| Esters | |||||||||||||||||
| Methyl Octanoate | 24.26 ± 0.06 b | 24.20 ± 0.09 b | 24.06 ± 0.15 b | 26.28 ± 0.41 a | 26.22 ± 0.03 a | 25.40 ± 0.23 b | 25.40 ± 0.04 c | 23.66 ± 0.016 d | 26.20 ± 0.056 a | 26.28 ± 0.40 a | 24.83 ± 0.07 b | 24.75 ± 0.08 b | 24.28 ± 0.14 ab | 24.55 ± 0.09 b | 24.57 ± 0.40 b | 25.08 ± 0.12 a | 24.16 ± 0.03 c |
| Ethyl Caprylate | 18.68 ± 0.00 b | 38.61 ± 3.57 b | 125.22 ± 27.78 a | 126.45 ± 14.80 a | 113.08 ± 3.02 a | 78.20 ± 2.24 b | 111.51 ± 3.01 a | 67.46 ± 2.93 c | 132.34 ± 4.78 a | 126.45 ± 14.80 a | 69.80 ± 0.29 b | 60.28 ± 1.32 b | 118.92 ± 1.76 c | 124.31 ± 3.00 b | 125.15 ± 1.87 b | 134.04 ± 2.99 a | 128.29 ± 1.90 b |
| Ethyl Butyrate | 46.80 ± 0.54 b | 62.95 ± 15.20 b | 120.30 ± 27.76 a | 149.16 ± 25.22 a | 123.67 ± 3.09 a | 57.41 ± 1.65 b | 45.46 ± 4.48 c | 47.20 ± 1.91 c | 164.27 ± 3.61 a | 159.16 ± 11.08 a | 89.56 ± 2.59 b | 84.91 ± 0.67 b | 115.46 ± 1.99 b | 119.12 ± 2.53 b | 127.60 ± 3.32 a | 129.90 ± 3.19 a | 128.62 ± 1.98 a |
| Isoamyl Acetate | 650.27 ± 48.51 b | 570.39 ± 58.25 b | 1382.80 ± 99.87 a | 1485.68 ± 99.25 a | 1577.76 ± 82.24 a | 694.38 ± 25.48 c | 709.35 ± 3.09 c | 954.41 ± 3.42 b | 1139.58 ± 54.35 a | 1185.68 ± 42.16 a | 863.82 ± 26.58 a | 759.52 ± 18.96 a | 1076.59 ± 28.26 d | 1012.41 ± 15.52 e | 1238.56 ± 24.45 c | 1374.69 ± 24.15 a | 1310.71 ± 8.17 b |
| Ethyl Hexanoate | 215.13 ± 3.57 b | 258.71 ± 2.49 b | 684.02 ± 13.79 a | 707.13 ± 36.88 a | 309.25 ± 14.35 a | 223.33 ± 6.75 c | 276.14 ± 0.64 b | 311.82 ± 7.83 a | 439.67 ± 59.73 a | 507.13 ± 36.88 a | 319.32 ± 7.95 b | 261.20 ± 19.78 b | 437.74 ± 13.75 d | 583.95 ± 10.02 c | 690.75 ± 5.90 b | 731.25 ± 8.04 a | 701.13 ± 3.28 b |
| Hexyl Acetate | 48.13 ± 1.25 c | 42.49 ± 10.15 c | 153.48 ± 34.61 b | 215.05 ± 13.27 a | 324.41 ± 14.14 a | 143.03 ± 13.03 b | 130.76 ± 3.82 b | 126.89 ± 7.07 b | 179.28 ± 13.10 b | 208.27 ± 3.67 a | 103.39 ± 0.85 c | 64.66 ± 4.34 d | 260.69 ± 9.56 b | 283.41 ± 6.56 a | 260.24 ± 3.40 b | 262.40 ± 9.00 b | 265.46 ± 2.73 b |
| Butyl Acrylate | 7.64 ± 0.08 a | 7.70 ± 0.02 a | 7.65 ± 0.08 a | 7.63 ± 0.03 a | 7.71 ± 0.00 a | 7.76 ± 0.06 a | 7.66 ± 0.09 a | 7.66 ± 0.10 a | 7.60 ± 0.00 b | 7.63 ± 0.039 a | 7.58 ± 0.00 b | 7.67 ± 0.00 a | 7.67 ± 0.08 a | 7.62 ± 0.04 a | 7.70 ± 0.01 a | 7.72 ± 0.05 a | 7.65 ± 0.05 a |
| Methyl Aalicylate | 11.46 ± 0.96 b | 11.11 ± 0.48 b | 14.67 ± 0.54 a | 11.47 ± 0.96 b | 11.43 ± 0.95 a | 11.37 ± 11.37 a | 11.73 ± 1.36 a | 10.23 ± 0.75 a | 11.84 ± 0.41 a | 11.46 ± 0.97 a | 11.52 ± 0.06 a | 10.76 ± 0.00 a | 10.77 ± 0.00 c | 11.28 ± 0.43 ab | 11.77 ± 0.01 a | 11.27 ± 0.45 ab | 10.78 ± 0.01 c |
| Ethyl Acetate | 499.04 ± 15.28 b | 275.43 ± 18.68 c | 514.88 ± 76.82 a | 665.13 ± 1.98 a | 338.33 ± 11.81 b | 333.46 ± 27.24 b | 298.61 ± 9.39 b | 446.79 ± 22.66 a | 470.91 ± 18.12 b | 654.68 ± 16.75 a | 460.93 ± 1.91 b | 671.55 ± 12.18 a | 328.26 ± 2.01 c | 388.91 ± 4.95 a | 365.12 ± 4.47 b | 393.23 ± 3.30 a | 294.29 ± 2.36 d |
| Methyl Benzoate | 11.04 ± 0.00 b | 11.29 ± 0.01 b | 11.96 ± 0.19 a | 11.26 ± 0.30 b | 11.14 ± 0.14 a | 11.18 ± 0.18 a | 11.33 ± 0.17 a | 11.05 ± 0.01 a | 11.35 ± 0.11 a | 11.16 ± 0.16 a | 11.13 ± 0.12 a | 11.04 ± 0.00 a | 11.29 ± 0.20 a | 11.05 ± 0.00 a | 11.04 ± 0.00 a | 11.20 ± 0.26 a | 11.22 ± 0.15 a |
| Aldehyde ketone | |||||||||||||||||
| (E)-2-Hexenal | 532.71 ± 2.87 c | 533.16 ± 12.81 c | 2304.27 ± 69.93 a | 1638.57 ± 76.44 b | 773.16 ± 29.29 a | 558.28 ± 1.04 c | 612.78 ± 10.09 b | 579.78 ± 21.87 c | 1060.75 ± 33.61 a | 792.95 ± 0.45 b | 766.691 ± 47.34 b | 500.27 ± 24.94 c | 538.26 ± 4.30 c | 600.91 ± 7.03 b | 699.78 ± 5.90 a | 707.92 ± 3.95 a | 440.07 ± 6.36 d |
| (E,E)-2,4-Hexadienal | 19.45 ± 0.05 b | 19.41 ± 0.05 b | 23.40 ± 1.24 a | 20.97 ± 1.05 b | 19.23 ± 0.02 a | 19.28 ± 0.08 a | 19.26 ± 0.01 a | 19.39 ± 0.13 a | 19.36 ± 0.11 a | 19.92 ± 0.43 a | 19.59 ± 0.15 a | 19.76 ± 0.68 a | 19.30 ± 0.08 a | 19.35 ± 0.09 a | 19.39 ± 0.14 a | 19.35 ± 0.22 a | 19.26 ± 0.04 a |
| Hexanal | 947.08 ± 3.18 c | 595.21 ± 36.57 a | 1929.25 ± 46.75 a | 1530.70 ± 96.65 b | 940.51 ± 13.41 a | 950.44 ± 39.33 a | 425.64 ± 15.82 c | 835.05 ± 10.67 b | 977.16 ± 7.50 b | 1530.708 ± 96.65 a | 858.38 ± 42.33 c | 825.02 ± 15.57 c | 1554.53 ± 3.20 c | 1673.37 ± 11.10 b | 1641.14 ± 25.80 b | 1860.08 ± 43.20 a | 951.24 ± 43.04 d |
| Octanal | 17.71 ± 0.65 b | 16.69 ± 2.32 b | 33.64 ± 3.89 a | 20.06 ± 1.20 b | 13.141 ± 1.92 a | 12.98 ± 1.03 a | 13.52 ± 1.59 a | 17.08 ± 2.14 a | 18.56 ± 2.62 a | 20.66 ± 0.34 a | 18.26 ± 0.71 a | 14.67 ± 0.32 b | 17.75 ± 0.61 a | 14.24 ± 0.60 b | 12.42 ± 0.78 c | 18.55 ± 0.85 a | 18.27 ± 1.49 a |
| Nonanal | 244.59 ± 34.86 d | 318.66 ± 13.35 c | 633.02 ± 24.29 a | 471.11 ± 5.66 b | 215.00 ± 6.58 a | 190.20 ± 9.08 b | 185.02 ± 6.36 b | 216.02 ± 8.63 a | 345.67 ± 7.34 c | 463.76 ± 4.73 a | 396.19 ± 11.75 b | 244.51 ± 5.48 d | 330.68 ± 3.75 c | 318.63 ± 2.45 d | 309.11 ± 2.09 e | 362.34 ± 4.10 a | 341.76 ± 1.37 b |
| (E)-2-Octenal | 34.21 ± 3.32 c | 29.96 ± 6.48 c | 79.15 ± 0.22 b | 121.25 ± 20.52 a | 30.16 ± 1.56 b | 31.57 ± 5.39 b | 29.38 ± 0.65 b | 45.76 ± 5.51 a | 47.63 ± 0.72 a | 47.53 ± 2.50 a | 42.99 ± 0.31 b | 38.58 ± 0.04 c | 75.15 ± 1.99 ab | 68.87 ± 2.49 c | 77.10 ± 2.27 ab | 78.84 ± 3.06 a | 72.14 ± 3.09 bc |
| (E,E)-2,4-Heptadienal | 5.88 ± 0.02 a | 6.03 ± 0.04 a | 7.40 ± 2.0 a | 6.42 ± 0.14 a | 5.97 ± 0.17 a | 5.75 ± 0.09 a | 6.04 ± 0.13 a | 5.77 ± 0.12 a | 5.87 ± 0.02 b | 6.42 ± 0.14 a | 5.77 ± 0.23 b | 5.89 ± 0.10 b | 5.78 ± 0.16 a | 5.76 ± 0.09 a | 5.65 ± 0.02 a | 5.77 ± 0.07 a | 5.69 ± 0.02 a |
| Benzaldehyde | 45.96 ± 0.97 b | 43.90 ± 1.55 b | 78.21 ± 14.29 a | 69.39 ± 0.87 a | 32.43 ± 1.98 a | 17.07 ± 2.53 b | 28.92 ± 0.19 a | 15.60 ± 0.46 b | 38.34 ± 4.81 b | 69.39 ± 0.87 a | 36.38 ± 0.02 b | 38.82 ± 0.29 b | 32.73 ± 1.86 c | 44.88 ± 1.76 b | 45.39 ± 0.91 b | 57.30 ± 1.79 a | 55.36 ± 0.13 a |
| (E,E)-2,4-Nonadienal | 15.84 ± 0.01 a | 15.98 ± 0.18 a | 17.83 ± 2.68 a | 15.87 ± 0.00 a | 15.84 ± 0.00 a | 15.97 ± 0.18 a | 15.83 ± 0.01 a | 16.09 ± 0.35 a | 15.86 ± 0.03 b | 15.87 ± 0.00 b | 16.27 ± 0.03 a | 15.85 ± 0.02 b | 15.85 ± 0.02 a | 15.88 ± 0.05 a | 15.93 ± 0.12 a | 15.86 ± 0.03 a | 15.84 ± 0.01 a |
| 1-Octen-3-one | 9.89 ± 0.19 b | 9.49 ± 0.21 b | 17.52 ± 1.87 a | 10.27 ± 0.07 b | 10.13 ± 0.13 a | 9.54 ± 0.31 a | 9.98 ± 0.19 a | 10.87 ± 1.44 a | 9.32 ± 0.01 b | 10.27 ± 0.07 a | 9.99 ± 0.07 a | 9.49 ± 0.24 b | 9.53 ± 0.19 a | 9.49 ± 0.15 a | 9.50 ± 0.06 a | 9.58 ± 0.27 a | 9.80 ± 0.42 a |
| Carvone | 1.95 ± 0.02 a | 1.95 ± 0.00 a | 2.00 ± 0.00 a | 1.98 ± 0.03 a | 1.96 ± 0.01 a | 1.95 ± 0.01 a | 1.96 ± 0.01 a | 1.95 ± 0.00 a | 1.96 ± 0.00 a | 1.98 ± 0.03 a | 1.94 ± 0.01 a | 1.96 ± 0.01 a | 1.95 ± 0.01 a | 1.95 ± 0.01 a | 1.94 ± 0.01 a | 1.95 ± 0.00 a | 1.95 ± 0.00 a |
| β-Damascone | 33.68 ± 2.14 c | 35.22 ± 5.75 c | 183.20 ± 21.15 a | 76.32 ± 3.01 b | 64.16 ± 0.91 a | 42.59 ± 0.83 b | 42.53 ± 13.18 b | 65.74 ± 15.69 a | 36.11 ± 4.51 b | 76.32 ± 3.01 a | 43.52 ± 1.42 b | 37.167 ± 0.29 b | 57.06 ± 1.74 a | 54.24 ± 1.62 b | 53.20 ± 0.05 b | 53.41 ± 0.16 b | 53.04 ± 0.43 b |
| Acetophenone | 14.09 ± 0.48 a | 13.98 ± 3.39 a | 19.45 ± 2.59 a | 18.70 ± 1.55 a | 15.10 ± 0.41 a | 12.91 ± 1.88 b | 13.97 ± 1.23 a | 11.65 ± 0.09 b | 17.82 ± 1.96 a | 15.70 ± 5.79 a | 11.58 ± 0.00 a | 13.68 ± 2.95 a | 11.54 ± 0.10 b | 11.59 ± 0.01 b | 12.75 ± 0.28 a | 13.25 ± 1.14 a | 11.59 ± 0.00 b |
| Alcohols | |||||||||||||||||
| (Z)-2-Hexenol | 48.66 ± 5.38 b | 46.39 ± 5.97 b | 79.54 ± 4.48 a | 76.05 ± 5.26 a | 29.78 ± 4.73 b | 32.16 ± 9.38 b | 55.14 ± 2.86 a | 26.80 ± 3.48 b | 42.62 ± 7.55 b | 76.04 ± 5.26 a | 30.85 ± 0.63 c | 23.16 ± 0.85 c | 57.61 ± 0.40 d | 73.56 ± 2.29 b | 75.26 ± 0.86 ab | 77.91 ± 1.53 a | 63.82 ± 2.27 c |
| 4-methyl-1-Pentanol | 16.27 ± 1.78 b | 5.68 ± 1.98 c | 46.91 ± 1.27 a | 52.98 ± 5.29 a | 7.55 ± 0.17 b | 12.06 ± 1.79 a | 9.14 ± 0.13 b | 13.08 ± 0.60 a | 46.82 ± 4.27 a | 53.28 ± 4.86 a | 17.68 ± 0.93 b | 15.85 ± 0.20 b | 33.92 ± 0.68 c | 37.23 ± 0.94 b | 43.86 ± 1.41 a | 42.94 ± 2.45 a | 41.18 ± 2.56 a |
| 1-Octen-3-ol | 45.80 ± 4.35 b | 28.59 ± 0.97 c | 147.36 ± 28.90 a | 76.95 ± 5.19 b | 23.63 ± 0.72 a | 18.18 ± 0.13 c | 20.40 ± 0.24 b | 20.25 ± 0.96 b | 26.05 ± 0.76 b | 39.93 ± 4.72 a | 20.47 ± 3.09 b | 24.98 ± 0.39 b | 25.23 ± 1.27 b | 31.35 ± 2.31 a | 32.02 ± 1.02 a | 33.65 ± 1.38 a | 23.17 ± 1.41 b |
| 2-Ethyl-1-hexanol | 9.65 ± 0.27 c | 18.10 ± 0.83 b | 30.92 ± 5.94 a | 33.30 ± 1.17 a | 7.07 ± 0.90 b | 7.85 ± 1.44 b | 10.56 ± 0.71 a | 13.275 ± 1.68 a | 19.59 ± 1.38 a | 14.04 ± 2.22 b | 12.62 ± 0.79 c | 17.24 ± 0.04 a | 26.57 ± 0.88 d | 30.46 ± 2.09 c | 39.07 ± 1.62 b | 44.96 ± 2.62 a | 44.69 ± 2.03 a |
| 1-Octanol | 42.56 ± 4.16 b | 32.39 ± 0.52 c | 47.57 ± 5.47 b | 81.12 ± 0.74 a | 28.94 ± 2.98 a | 23.30 ± 1.62 b | 24.82 ± 0.76 b | 5.85 ± 0.35 c | 47.36 ± 2.51 a | 50.39 ± 1.77 a | 47.40 ± 0.42 a | 43.50 ± 2.47 a | 53.07 ± 1.48 a | 49.54 ± 2.08 ab | 47.00 ± 0.55 b | 46.18 ± 3.22 b | 25.31 ± 1.88 c |
| 1-Nonanol | 27.01 ± 4.21 b | 31.07 ± 5.28 b | 116.02 ± 18.41 a | 89.68 ± 10.47 a | 20.04 ± 0.95 c | 20.49 ± 1.34 c | 24.23 ± 1.54 b | 32.14 ± 3.06 a | 54.33 ± 3.47 a | 54.68 ± 3.40 a | 33.78 ± 1.92 b | 26.30 ± 2.30 b | 77.66 ± 1.44 a | 68.81 ± 0.93 b | 56.68 ± 0.97 c | 54.14 ± 3.45 c | 50.34 ± 2.23 d |
| 1-Butanol | 9.71 ± 0.94 a | 7.25 ± 0.07 c | 8.78 ± 0.52 b | 10.47 ± 0.33 a | 7.98 ± 0.48 a | 7.99 ± 0.72 a | 7.63 ± 0.22 a | 8.64 ± 0.02 a | 7.92 ± 0.39 b | 10.47 ± 0.33 a | 8.51 ± 0.37 b | 8.24 ± 0.60 b | 6.93 ± 0.09 bc | 7.45 ± 0.33 b | 6.68 ± 0.35 c | 8.17 ± 0.30 a | 6.96 ± 0.21 bc |
| 3-methyl-1-Butanol | 859.86 ± 42.49 c | 711.01 ± 8.99 c | 2585.19 ± 326.18 a | 1938.93 ± 127.23 b | 759.40 ± 11.32 b | 686.56 ± 14.91 c | 672.19 ± 6.88 c | 939.57 ± 10.90 a | 1490.82 ± 41.95 b | 1988.93 ± 56.52 a | 993.98 ± 8.69 c | 788.99 ± 11.23 d | 1653.48 ± 36.81 a | 787.85 ± 6.02 d | 1429.07 ± 17.20 c | 1683.45 ± 7.85 a | 1544.21 ± 22.97 b |
| (Z)-3-Hexen-1-ol | 10.34 ± 0.34 b | 10.13 ± 0.77 b | 26.76 ± 4.78 a | 23.57 ± 1.43 a | 8.97 ± 1.05 b | 9.07 ± 1.44 b | 7.96 ± 1.20 b | 15.69 ± 2.69 a | 16.94 ± 5.14 a | 23.57 ± 1.43 a | 12.46 ± 1.47 b | 9.68 ± 0.60 b | 16.45 ± 0.19 a | 13.71 ± 0.17 b | 10.34 ± 0.13 c | 16.23 ± 0.96 a | 10.68 ± 0.11 c |
| Phenylethyl Alcohol | 464.60 ± 32.80 c | 522.30 ± 7.63 c | 985.02 ± 45.46 b | 1097.05 ± 8.41 a | 594.29 ± 1.03 a | 446.67 ± 9.16 b | 595.99 ± 20.01 a | 536.77 ± 24.94 a | 805.01 ± 5.84 b | 1047.05 ± 62.29 a | 473.29 ± 16.75 c | 431.59 ± 20.38 c | 969.61 ± 7.48 b | 590.39 ± 7.58 e | 952.94 ± 7.49 c | 1051.62 ± 10.94 a | 690.18 ± 6.82 d |
| Terpene | |||||||||||||||||
| β-Myrcene | 22.69 ± 0.01 a | 22.58 ± 0.01 a | 22.66 ± 0.03 a | 22.73 ± 0.20 a | 22.63 ± 0.05 a | 22.66 ± 0.10 a | 22.60 ± 0.01 a | 23.67 ± 1.54 a | 22.81 ± 0.20 a | 22.73 ± 0.20 a | 22.65 ± 0.09 a | 22.63 ± 0.07 a | 22.59 ± 0.01 a | 22.58 ± 0.01 a | 22.60 ± 0.05 a | 22.66 ± 0.07 a | 22.64 ± 0.09 a |
| α-Phellandrene | 22.64 ± 0.09 a | 22.67 ± 0.02 a | 22.76 ± 0.01 a | 22.73 ± 0.00 a | 22.68 ± 0.02 a | 22.73 ± 0.06 a | 22.68 ± 0.01 a | 22.68 ± 0.02 a | 22.65 ± 0.02 a | 22.73 ± 0.00 a | 22.64 ± 0.09 a | 22.69 ± 0.02 a | 22.69 ± 0.02 ab | 22.70 ± 0.03 ab | 22.70 ± 0.02 ab | 22.72 ± 0.02 a | 22.67 ± 0.02 b |
| D-Limonene | 22.73 ± 0.17 b | 23.05 ± 0.04 b | 23.17 ± 0.08 a | 23.40 ± 0.17 a | 22.74 ± 0.24 a | 23.31 ± 0.50 a | 22.97 ± 0.18 a | 22.62 ± 0.79 a | 23.26 ± 0.94 a | 23.40 ± 0.17 a | 23.05 ± 0.30 a | 22.75 ± 0.32 a | 22.93 ± 0.14 b | 22.99 ± 0.11 ab | 22.85 ± 0.05 b | 23.24 ± 0.16 a | 22.76 ± 0.19 b |
| β-Caryophyllene | 25.97 ± 0.01 b | 25.94 ± 0.01 b | 28.05 ± 0.88 a | 26.02 ± 0.08 b | 25.94 ± 0.03 a | 25.98 ± 0.08 a | 25.97 ± 0.07 a | 27.03 ± 1.49 a | 26.00 ± 0.10 a | 26.02 ± 0.08 a | 26.03 ± 0.06 a | 25.96 ± 0.04 a | 25.96 ± 0.07 a | 25.93 ± 0.01 a | 25.96 ± 0.05 a | 25.96 ± 0.04 a | 25.95 ± 0.03 a |
| (Z)-Rose Oxide | 5.57 ± 0.04 b | 5.54 ± 0.01 b | 5.89 ± 0.10 a | 5.54 ± 0.01 b | 5.56 ± 0.04 a | 5.62 ± 0.02 a | 5.63 ± 0.04 a | 5.58 ± 0.05 a | 5.66 ± 0.04 a | 5.54 ± 0.01 b | 5.57 ± 0.04 b | 5.61 ± 0.00 a | 5.54 ± 0.00 a | 5.58 ± 0.07 a | 5.54 ± 0.01 a | 5.58 ± 0.05 a | 5.57 ± 0.05 a |
| 4-Terpinenol | 33.03 ± 1.35 b | 21.89 ± 0.18 c | 78.46 ± 0.69 a | 46.66 ± 1.55 b | 37.39 ± 4.06 a | 23.59 ± 0.97 b | 23.89 ± 5.63 b | 38.14 ± 4.44 a | 45.15 ± 2.69 c | 46.66 ± 1.55 c | 60.21 ± 3.28 a | 52.85 ± 0.09 b | 41.09 ± 1.10 b | 39.82 ± 1.22 b | 41.18 ± 0.41 b | 49.80 ± 1.21 a | 28.77 ± 2.05 c |
| α-Terpineol | 10.63 ± 0.00 a | 11.46 ± 0.44 a | 13.37 ± 3.73 a | 10.70 ± 0.01 a | 14.26 ± 5.13 a | 10.09 ± 0.74 a | 11.03 ± 0.55 a | 10.74 ± 0.13 a | 10.68 ± 0.03 a | 10.70 ± 0.02 a | 10.64 ± 0.02 a | 10.67 ± 0.035 a | 10.65 ± 0.03 c | 11.63 ± 0.01 b | 12.61 ± 0.12 a | 12.73 ± 0.23 a | 10.50 ± 0.23 c |
| Geraniol | 90.85 ± 8.47 a | 118.80 ± 8.36 a | 159.74 ± 18.62 b | 245.39 ± 38.02 a | 99.41 ± 7.40 a | 60.05 ± 3.28 b | 55.62 ± 5.86 b | 51.98 ± 7.81 b | 179.83 ± 2.30 b | 213.39 ± 7.22 a | 93.83 ± 4.93 c | 82.77 ± 2.63 c | 172.97 ± 3.14 d | 209.41 ± 1.95 b | 206.75 ± 2.10 bc | 255.17 ± 4.69 a | 201.91 ± 2.17 c |
| p-Cymene | 36.12 ± 0.32 b | 36.09 ± 0.24 b | 37.14 ± 0.27 a | 37.15 ± 0.07 a | 36.07 ± 0.26 a | 36.88 ± 0.85 a | 36.25 ± 0.21 a | 34.26 ± 2.26 a | 36.35 ± 0.87 a | 37.15 ± 0.07 a | 36.21 ± 0.51 a | 36.11 ± 0.35 a | 36.16 ± 0.39 a | 36.50 ± 0.61 a | 36.11 ± 0.31 a | 36.78 ± 0.08 a | 36.07 ± 0.31 aa |
| p-Mentha-1,8-dien-9-olen-9-ol | 8.03 ± 0.93 b | 12.88 ± 3.01 a | 10.22 ± 2.12 a | 7.32 ± 0.06 b | 8.82 ± 0.75 a | 7.25 ± 0.37 a | 7.60 ± 0.26 a | 8.43 ± 0.73 a | 9.14 ± 0.16 a | 7.32 ± 0.06 b | 7.72 ± 0.15 b | 7.62 ± 0.39 b | 7.37 ± 0.20 c | 7.31 ± 0.06 c | 9.83 ± 0.16 b | 13.42 ± 0.51 a | 9.44 ± 0.41 b |
| Others | |||||||||||||||||
| Butanedioic acid | 27.81 ± 1.84 c | 46.12 ± 3.60 c | 249.36 ± 52.06 a | 133.73 ± 5.10 b | 149.58 ± 4.27 a | 110.04 ± 5.41 b | 52.01 ± 0.03 c | 20.94 ± 1.94 d | 80.40 ± 12.25 b | 139.23 ± 2.67 a | 77.07 ± 6.62 b | 69.43 ± 8.76 b | 102.07 ± 1.90 a | 104.52 ± 0.95 a | 102.11 ± 1.83 a | 104.91 ± 0.60 a | 104.63 ± 2.57 a |
| 2-Methylpyrazine | 16.85 ± 0.45 b | 16.83 ± 0.07 b | 21.95 ± 3.06 a | 17.92 ± 1.51 b | 16.73 ± 0.77 a | 16.52 ± 0.54 a | 15.67 ± 0.29 a | 16.88 ± 0.34 a | 17.77 ± 1.10 a | 17.92 ± 1.51 a | 17.36 ± 1.08 a | 17.03 ± 0.28 a | 16.37 ± 0.20 b | 17.50 ± 0.42 a | 15.81 ± 0.24 b | 17.48 ± 0.36 a | 16.20 ± 0.42 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Zhang, X.; Zhang, J.; Pan, S.; Chen, K.; Fang, Y. Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus–Kiwifruit Composite Wine. Foods 2025, 14, 1705. https://doi.org/10.3390/foods14101705
Zeng C, Zhang X, Zhang J, Pan S, Chen K, Fang Y. Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus–Kiwifruit Composite Wine. Foods. 2025; 14(10):1705. https://doi.org/10.3390/foods14101705
Chicago/Turabian StyleZeng, Cuiyan, Xueru Zhang, Junxia Zhang, Shuiyan Pan, Keqin Chen, and Yulin Fang. 2025. "Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus–Kiwifruit Composite Wine" Foods 14, no. 10: 1705. https://doi.org/10.3390/foods14101705
APA StyleZeng, C., Zhang, X., Zhang, J., Pan, S., Chen, K., & Fang, Y. (2025). Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus–Kiwifruit Composite Wine. Foods, 14(10), 1705. https://doi.org/10.3390/foods14101705






