Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
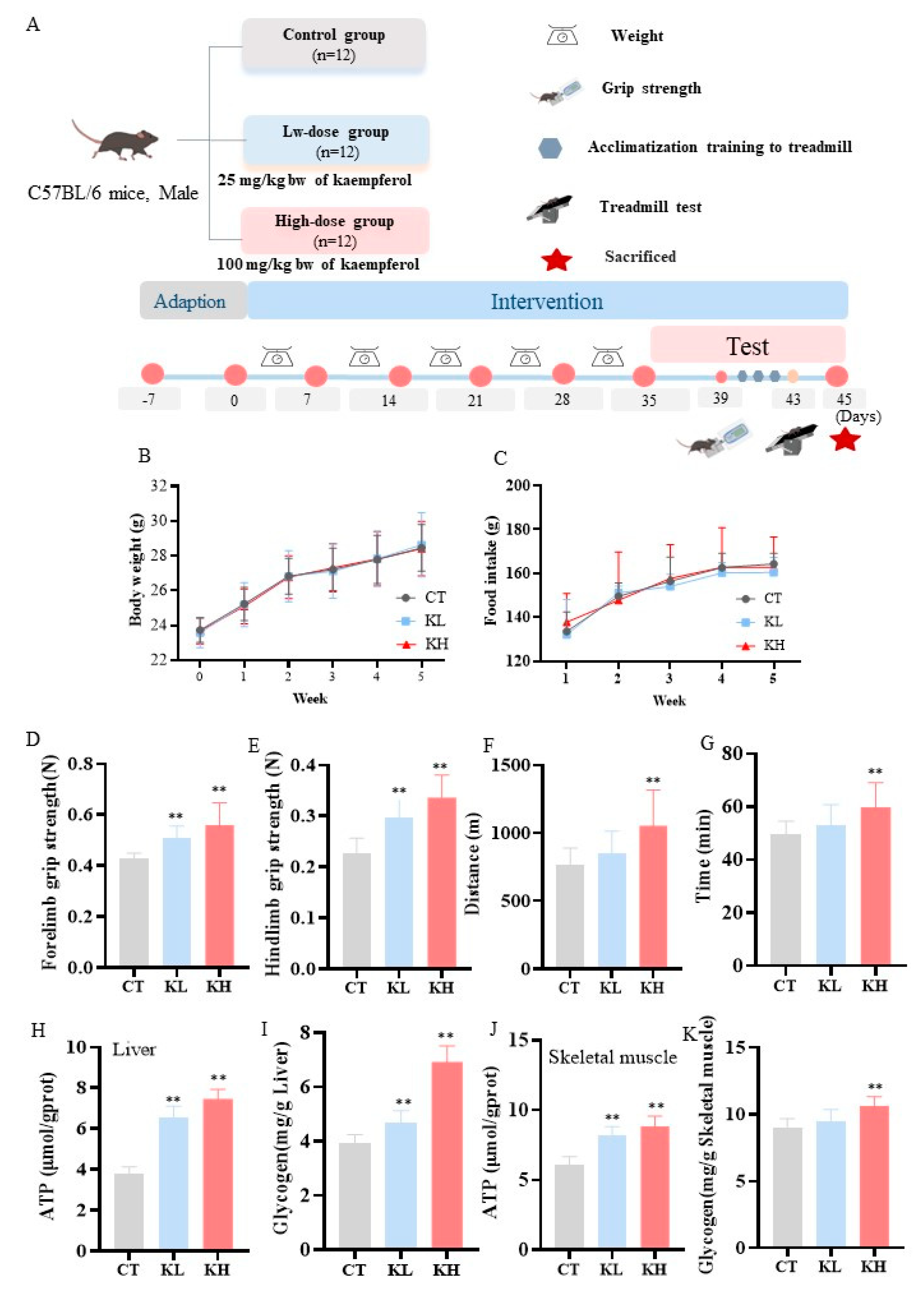
2.2. Animals and Treatment
2.3. Grip Strength
2.4. Exercise Capacity
2.5. Measurement of Tissue Glycogen and ATP
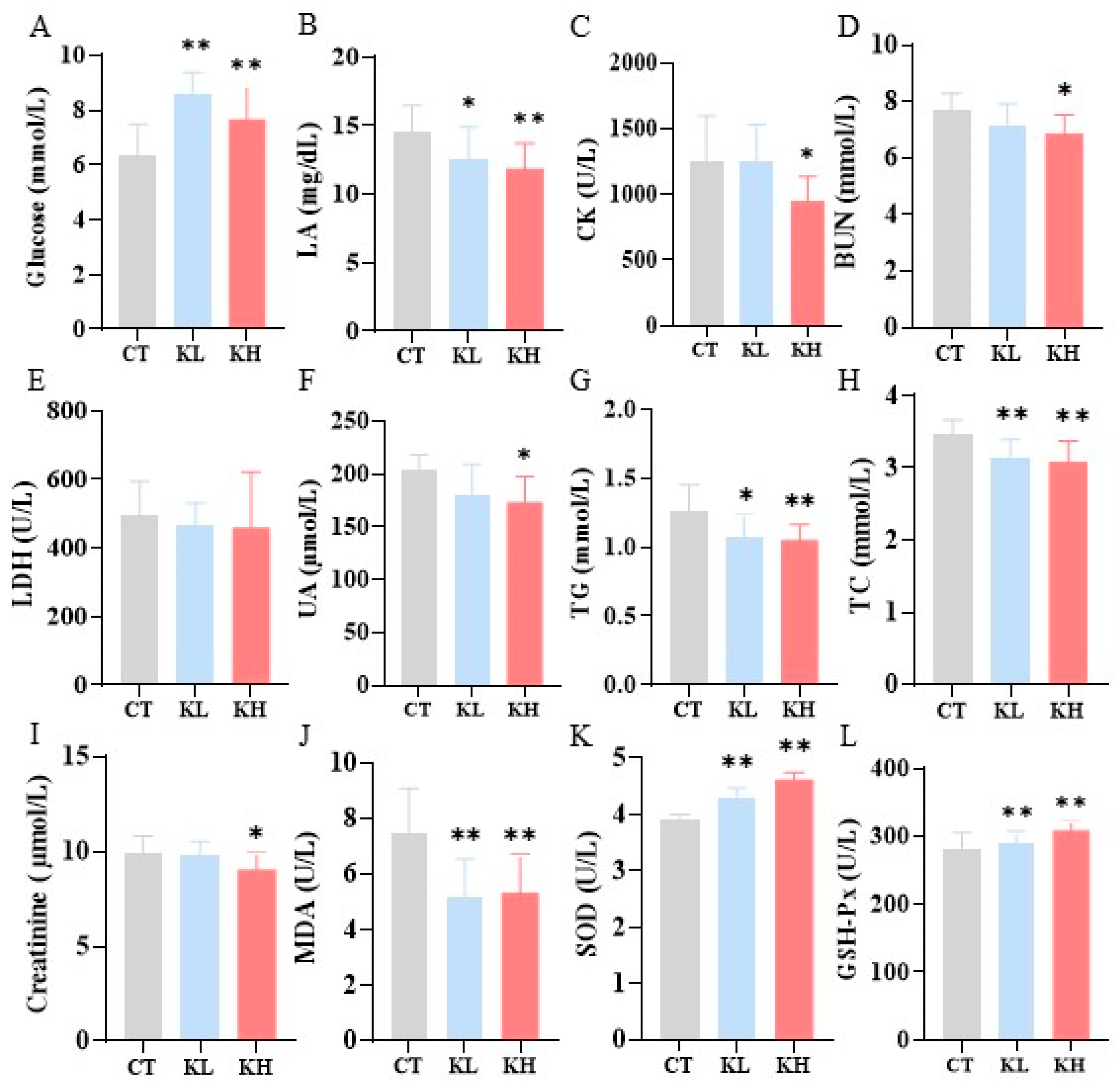
2.6. Blood Biochemical Assessments
2.7. 2D/3D Cells Culture and Treatment
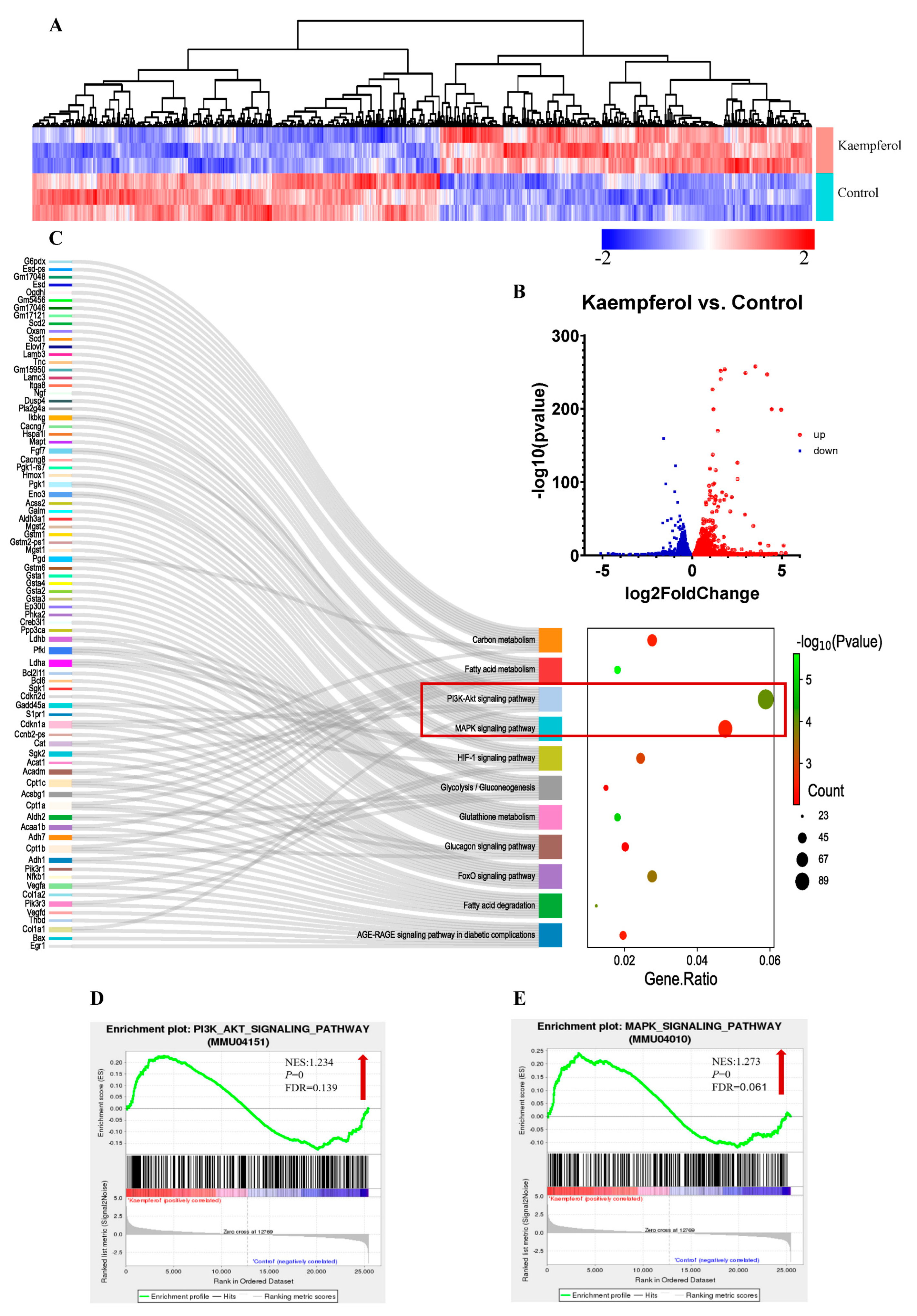
2.8. Transcriptome Sequencing
2.9. Immunofluorescence Staining and Cell High-Content Imaging Analysis
2.9.1. Glucose Uptake
2.9.2. Measurement of ROS
2.9.3. Measurement of Mitochondrial Mass and Mitochondrial Membrane Potential
2.9.4. Protein Synthesis Measurements
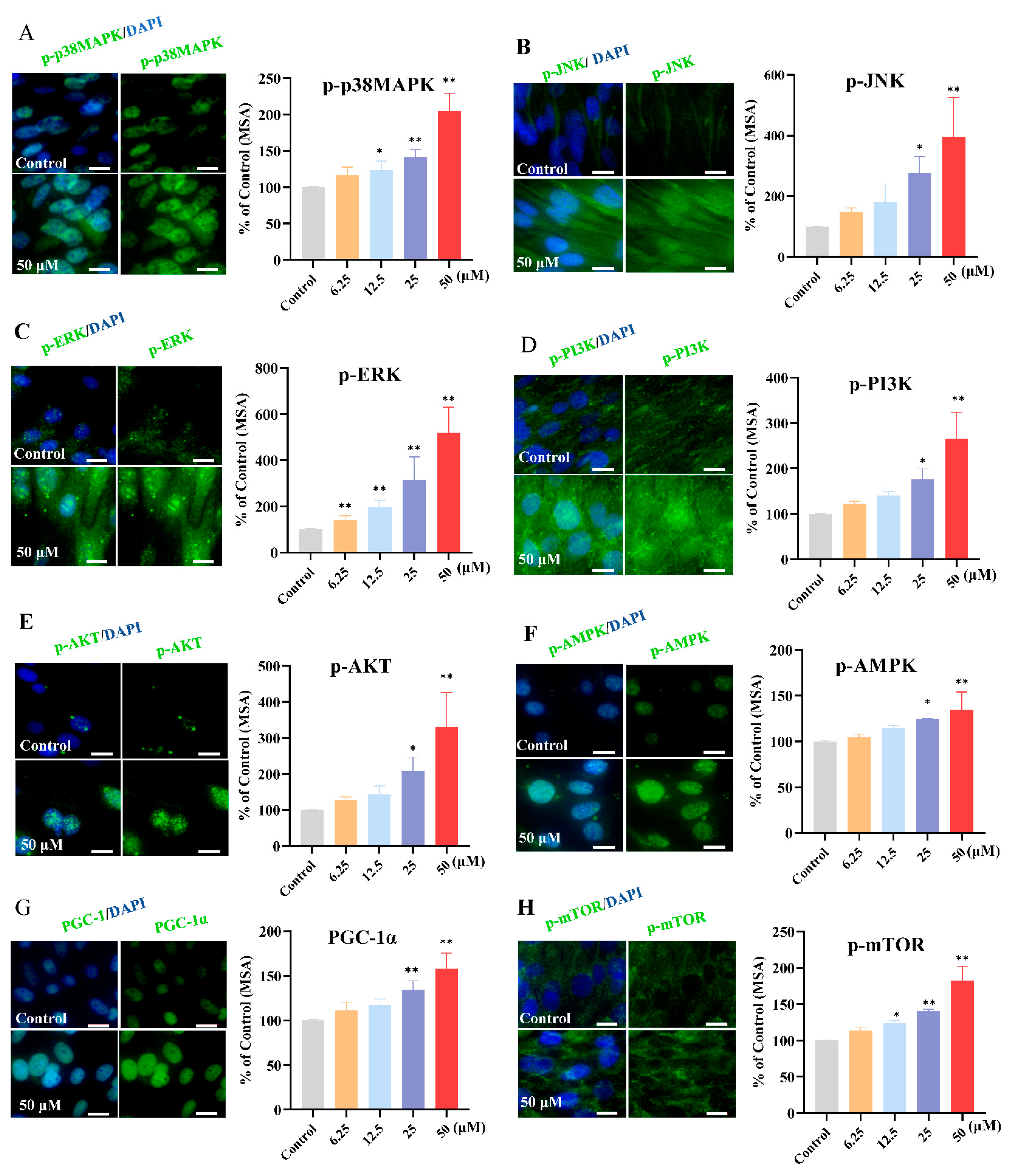
2.9.5. Expression of Key Target Proteins
2.9.6. High-Content Imaging Analysis
2.10. Statistical Analyses
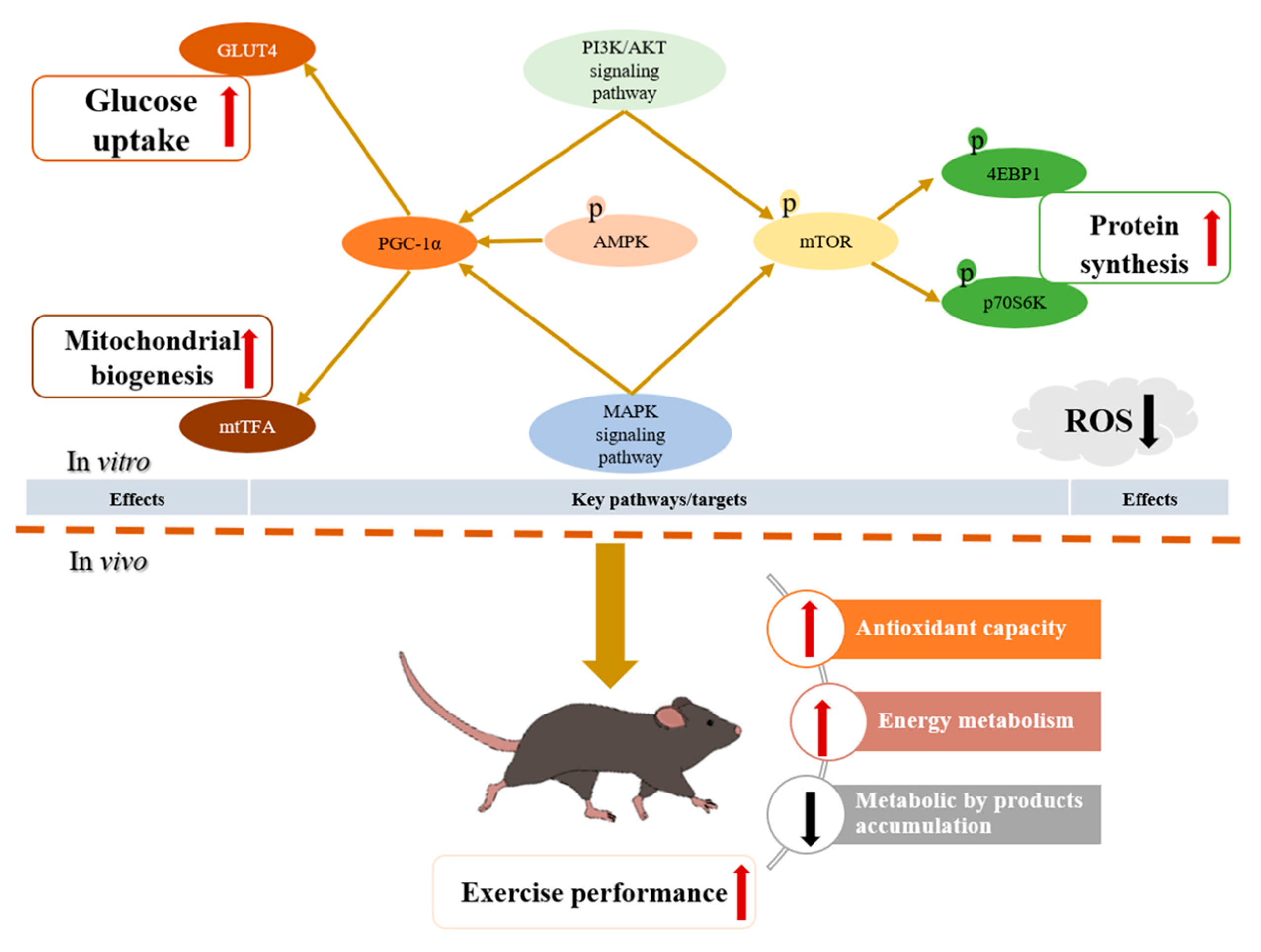
3. Results and Discussion
3.1. Effect of Kaempferol on Grip Strength and Exercise Capacity
3.2. Effect of Kaempferol on the ATP and Glycogen Contents in the Liver and Skeletal Muscle
3.3. Effect of Kaempferol on Biochemical Parameters after Exercise
3.4. Effect of Kaempferol on the Glucose Uptake, Protein Synthesis, and Mitochondrial Biogenesis in Both 2D and 3D C2C12 Myotube Cultures
3.5. Effect of Kaempferol on MAPK and PI3K/AKT Signaling Pathways in C2C12 Myotubes
3.6. Kaempferol Upregulated the Phosphorylation of Key Target Proteins in the MAPK and PI3K/AKT Signaling Pathways
3.7. Effect of Kaempferol on Downstream Maker Related to Glucose Uptake, Protein Synthesis, and Mitochondrial Biogenesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiu, W.C.; Chuang, H.L.; Tang, D.W.; Lee, Z.M.; Wei, L.; Chen, F.A.; Huang, C.C. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, D.; Hu, J.; Tan, B.K.; Zhang, Y.; Lin, S. Natural bioactive peptides to beat exercise-induced fatigue: A review. Food Biosci. 2021, 43, 101298. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural products and skeletal muscle health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wu, Q.; Wang, S.; Kan, J.; Zhang, Z.; Zhang, X.; Zhang, X.; Li, J.; Xu, W.; Du, J.; et al. Pea Peptide Supplementation in Conjunction With Resistance Exercise Promotes Gains in Muscle Mass and Strength. Front. Nutr. 2022, 9, 878229. [Google Scholar] [CrossRef]
- Chen, J.; Wong, H.S.; Leong, P.K.; Leung, H.Y.; Chan, W.M.; Ko, K.M. Ursolic acid induces mitochondrial biogenesis through the activation of AMPK and PGC-1 in C2C12 myotubes: A possible mechanism underlying its beneficial effect on exercise endurance. Food Funct. 2017, 8, 2425–2436. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, C.; Xu, Y.; Jing, Y.; Yuan, Y.; Wang, L.; Wang, S.; Zhu, X.; Gao, P.; Zhang, Y.; et al. Alpha-ketoglutarate promotes skeletal muscle hypertrophy and protein synthesis through Akt/mTOR signaling pathways. Sci. Rep. 2016, 6, 26802. [Google Scholar] [CrossRef] [PubMed]
- Le Plénier, S.; Goron, A.; Sotiropoulos, A.; Archambault, E.; Guihenneuc, C.; Walrand, S.; Salles, J.; Jourdan, M.; Neveux, N.; Cynober, L.; et al. Citrulline directly modulates muscle protein synthesis via the PI3K/MAPK/4E-BP1 pathway in a malnourished state: Evidence from in vivo, ex vivo, and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E27–E36. [Google Scholar] [CrossRef] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.M.; Al-Zougbi, A.; Bodine, S.C.; Adams, C.M. Skeletal Muscle Atrophy: Discovery of Mechanisms and Potential Therapies. Physiology 2019, 34, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Quan, H.; Kang, S.-G.; Huang, K.; Tong, T. Nutraceuticals in the Prevention and Treatment of the Muscle Atrophy. Nutrients 2021, 13, 1914. [Google Scholar] [CrossRef]
- Watson, C.J.; Stone, G.L.; Overbeek, D.L.; Chiba, T.; Burns, M.M. Performance-enhancing drugs and the Olympics. J. Intern. Med. 2022, 291, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Yu, K.Q.; Liu, Y.Y.; Ouyang, M.Z.; Yan, M.H.; Luo, R.; Zhao, X.S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Kim, J.S.; Jung, C.H.; Ahn, J.; Hur, J.; Ha, T.Y. Coffee consumption promotes skeletal muscle hypertrophy and myoblast differentiation. Food Funct. 2018, 9, 1102–1111. [Google Scholar] [CrossRef]
- Bach, H.V.; Kim, J.; Myung, S.K.; Cho, Y.A. Efficacy of Ginseng Supplements on Fatigue and Physical Performance: A Meta-analysis. J. Korean Med. Sci. 2016, 31, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, Y.; Zhang, Y.; Lang, J.; Chen, Y.; Ling, W. Estimated daily flavonoid and stilbene intake from fruits, vegetables, and nuts and associations with lipid profiles in Chinese adults. J. Acad. Nutr. Diet. 2013, 113, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Wang, C.; Ren, C. Intakes of total and individual flavonoids by US adults. Int. J. Food Sci. Nutr. 2014, 65, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.W.; Wang, M.; Arroo, R.R.J.; Zou, L.; et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.T.; Luo, J.; Liu, D. Kaempferol improves glucose uptake in skeletal muscle via an AMPK-dependent mechanism. Food Sci. Hum. Wellness 2023, 12, 2087–2094. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, J.; El-Seedi, H.R.; Woźniak, K.S.; Daglia, M.; Little, P.J.; Weng, J.; Xu, S. Kaempferol and atherosclerosis: From mechanism to medicine. Crit. Rev. Food Sci. Nutr. 2022, 64, 2157–2175. [Google Scholar] [CrossRef]
- Chen, J.; Xuan, Y.-H.; Luo, M.-X.; Ni, X.-G.; Ling, L.-Q.; Hu, S.-J.; Chen, J.-Q.; Xu, J.-Y.; Jiang, L.-Y.; Si, W.-Z.; et al. Kaempferol alleviates acute alcoholic liver injury in mice by regulating intestinal tight junction proteins and butyrate receptors and transporters. Toxicology 2020, 429, 152338. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, F.; Wu, Q.; Luo, Y.; Guo, T.; Han, S.; Huang, M.; Hu, Z.; Bai, J.; Luo, F.; et al. Dietary Supplementation of Octacosanol Improves Exercise-Induced Fatigue and Its Molecular Mechanism. J. Agric. Food Chem. 2021, 69, 7603–7618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, W.; Wang, N.; Jiang, W.; Cheng, Y.; Guo, Y.; Yao, W.; Hu, B.; Du, P.; Qian, H. Anti-fatigue effect of Lepidium meyenii Walp. (Maca) on preventing mitochondria-mediated muscle damage and oxidative stress in vivo and vitro. Food Funct. 2021, 12, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.X.; He, W.G.; Cai, H.J.; Jiang, J.H.; Yang, Y.Y.; Dan, Y.R.; Luo, H.H.; Du, Y.; Chen, L.; He, B.C. Analysis and Validation of Hub Genes in Blood Monocytes of Postmenopausal Osteoporosis Patients. Front. Endocrinol. 2021, 12, 815245. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, X.; Li, Y.; Geng, X.; Jia, X.; Zhang, L.; Yang, H. Arachidonic Acid Metabolism Controls Macrophage Alternative Activation Through Regulating Oxidative Phosphorylation in PPARgamma Dependent Manner. Front. Immunol. 2021, 12, 618501. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Fellows Maxwell, K.; Wahls, T.; Browne, R.W.; Rubenstein, L.; Bisht, B.; Chenard, C.A.; Snetselaar, L.; Weinstock-Guttman, B.; Ramanathan, M. Lipid profile is associated with decreased fatigue in individuals with progressive multiple sclerosis following a diet-based intervention: Results from a pilot study. PLoS ONE 2019, 14, e0218075. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Orhan, C.; Erten, F.; Er, B.; Acharya, M.; Morde, A.A.; Padigaru, M.; Sahin, K. Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats. Antioxidants 2021, 10, 1692. [Google Scholar] [CrossRef]
- Song, H.; Tian, X.; Liu, D.; Liu, M.; Liu, Y.; Liu, J.; Mei, Z.; Yan, C.; Han, Y. CREG1 improves the capacity of the skeletal muscle response to exercise endurance via modulation of mitophagy. Autophagy 2021, 17, 4102–4118. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, F.; Wen, L.; Ouyang, M.; Wang, Y.; Wang, Q.; Luo, L.; Jian, Z. Osteocalcin Induces Proliferation via Positive Activation of the PI3K/Akt, P38 MAPK Pathways and Promotes Differentiation through Activation of the GPRC6A-ERK1/2 Pathway in C2C12 Myoblast Cells. Cell. Physiol. Biochem. 2017, 43, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-Y.; Wu, S.-J.; Chang, Y.-C.; Ng, L.-T.; Chang, S.-J. Stimulation of GLUT4 Glucose Uptake by Anthocyanin-Rich Extract from Black Rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK Signaling in C2C12 Cells. Metabolites 2022, 12, 856. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Abdullah, N.; Thowfeik, F.S.; Altorki, N.K.; McGraw, T.E. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. eLife 2017, 6, e26896. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, C.; Giguère, V. Transcriptional control of energy metabolism by nuclear receptors. Nat. Rev. Mol. Cell Biol. 2022, 23, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2019, 60, 515–528. [Google Scholar] [CrossRef]
- Zhou, X.; Trinh-Minh, T.; Tran-Manh, C.; Gießl, A.; Bergmann, C.; Györfi, A.H.; Schett, G.; Distler, J.H.W. Impaired Mitochondrial Transcription Factor A Expression Promotes Mitochondrial Damage to Drive Fibroblast Activation and Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2022, 74, 871–881. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Zhang, C.; Yang, J.; Tian, Y.; You, L.; Yang, H.; Li, Y.; Liu, H.; Pan, D.; Liu, Z. Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways. Foods 2024, 13, 1068. https://doi.org/10.3390/foods13071068
Ji X, Zhang C, Yang J, Tian Y, You L, Yang H, Li Y, Liu H, Pan D, Liu Z. Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways. Foods. 2024; 13(7):1068. https://doi.org/10.3390/foods13071068
Chicago/Turabian StyleJi, Xiaoning, Chaozheng Zhang, Jing Yang, Yaru Tian, Lijuan You, Hui Yang, Yongning Li, Haibo Liu, Deng Pan, and Zhaoping Liu. 2024. "Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways" Foods 13, no. 7: 1068. https://doi.org/10.3390/foods13071068
APA StyleJi, X., Zhang, C., Yang, J., Tian, Y., You, L., Yang, H., Li, Y., Liu, H., Pan, D., & Liu, Z. (2024). Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways. Foods, 13(7), 1068. https://doi.org/10.3390/foods13071068





