Dietary Supplementation of Haskap Berry (Lonicera caerulea L.) Anthocyanins and Probiotics Attenuate Dextran Sulfate Sodium-Induced Colitis: Evidence from an Experimental Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Anthocyanin-Rich Haskap Berry Fraction
2.4. Preparation of AHF Microparticles
2.5. The Mixture of Probiotic Bacteria
2.6. Determination of the Encapsulating Parameters of Microparticles
2.7. Profiling and Quantification of Anthocyanin in Microparticles
2.8. Determination of Physical Characteristics of microparticles
2.8.1. Moisture Content and Water Activity (aW)
2.8.2. Hygroscopicity
2.8.3. Water Solubility Index (WSI)
2.8.4. The Bulk Density (ρbulk)
2.8.5. Powder Morphology and Particle Size
2.9. Fourier-Transform Infrared (FTIR) Spectroscopy
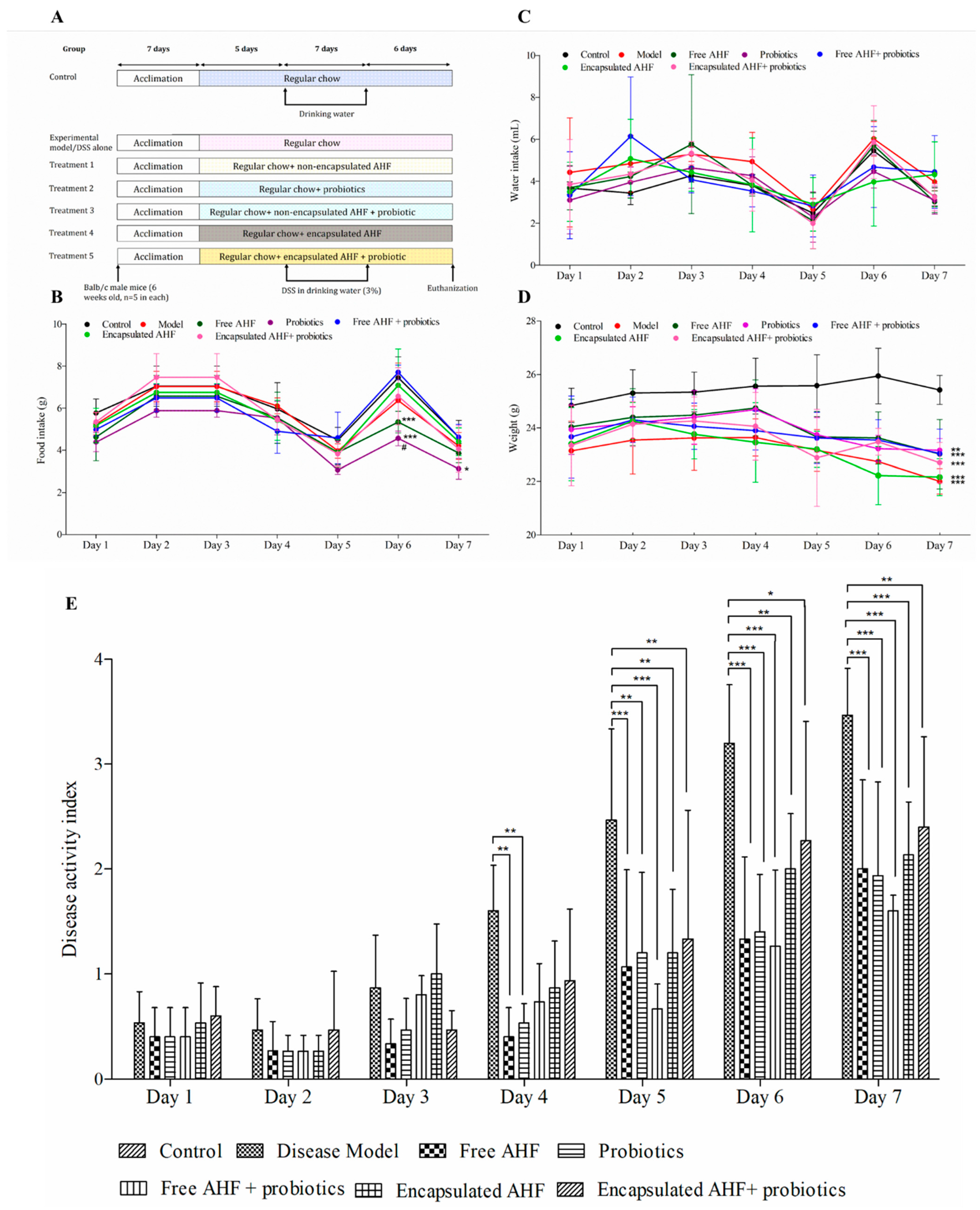
2.10. In Vivo Experimental Design
2.10.1. Organ and Blood Harvesting
2.10.2. Disease Activity Index (DAI)
2.10.3. Hepatotoxicity
2.10.4. Measurement of Protein Expression in Mice Colon Using Western Blot Analysis
2.10.5. Enzyme-Linked Immunosorbent Assays (ELISA)
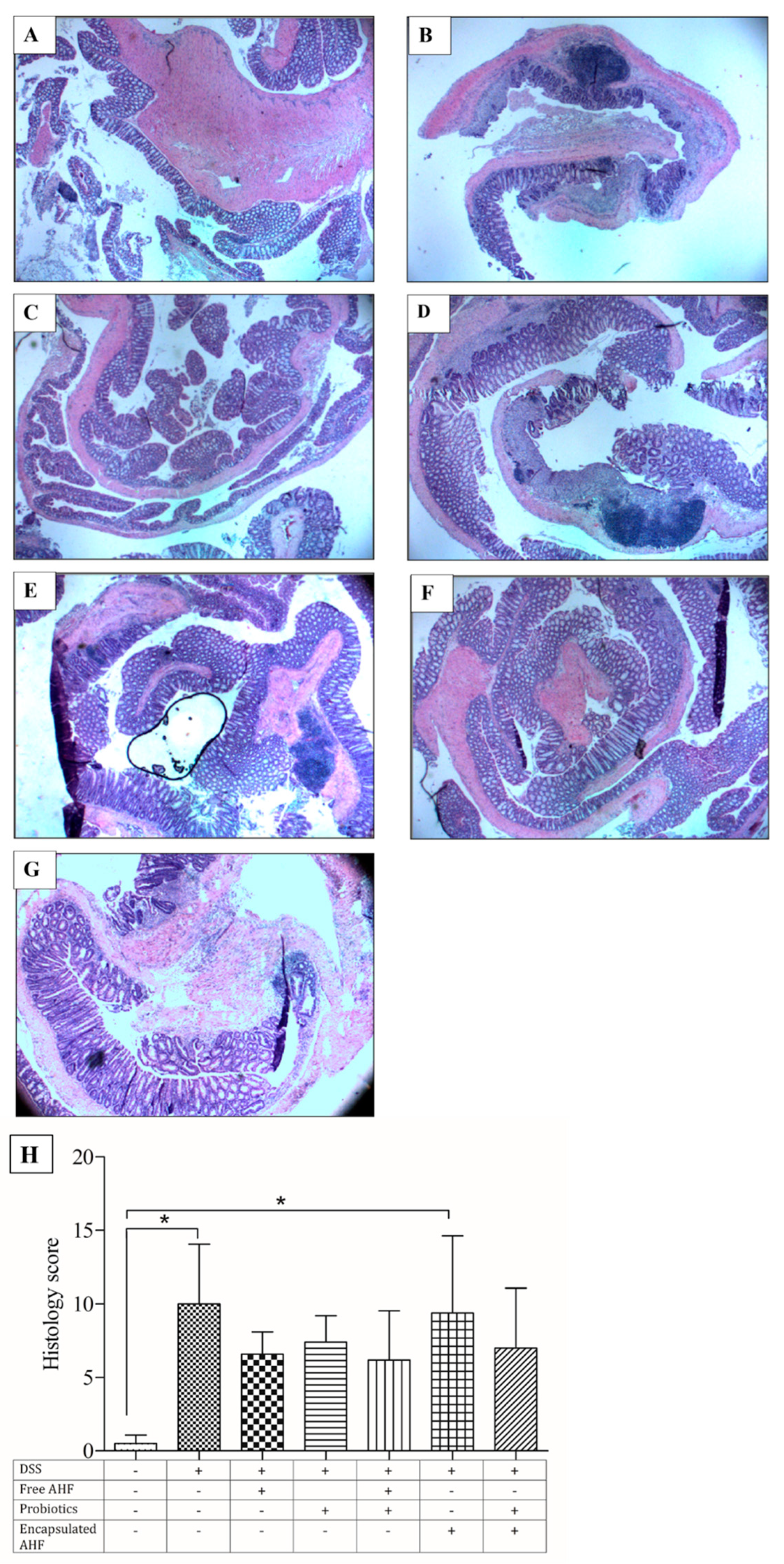
2.10.6. Histological Analysis
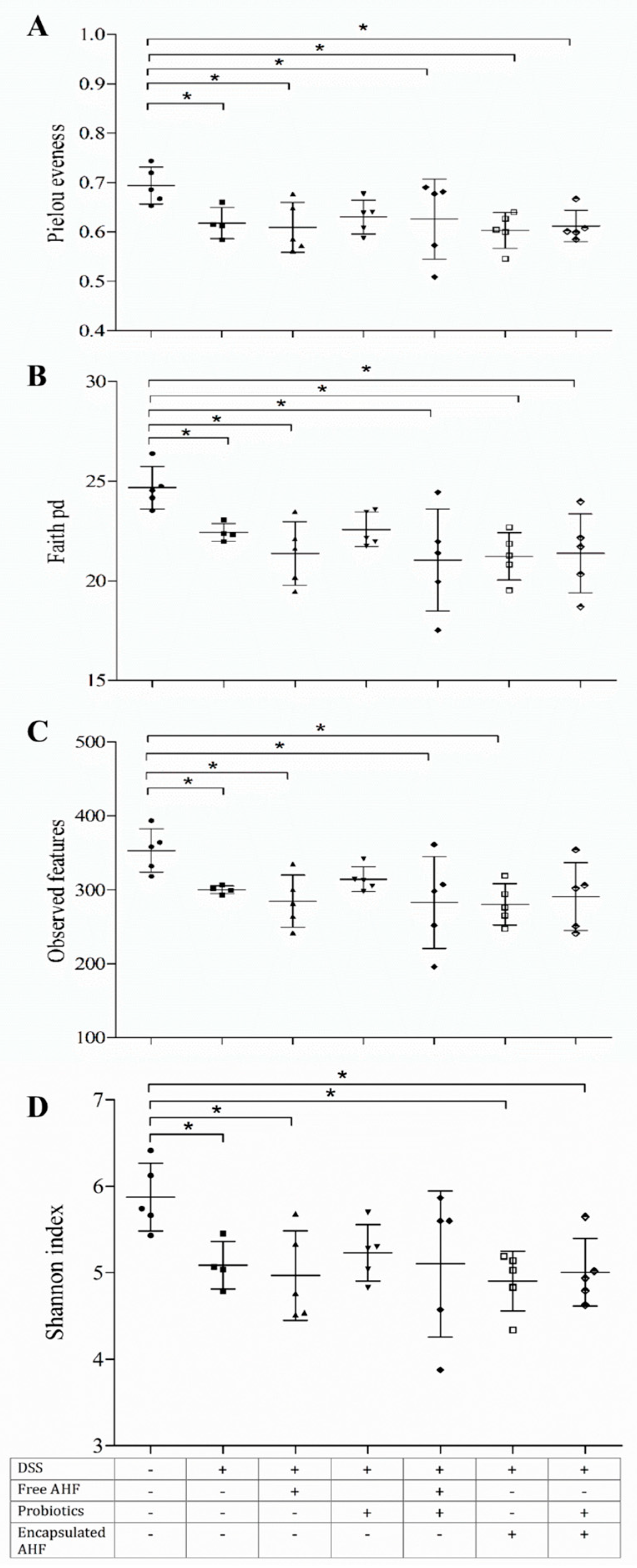
2.10.7. Alpha Diversity of Fecal Microbiota
2.10.8. Statistical Analysis
3. Results
3.1. Assessment of AHF Entrapment in MD and IN Matrix
3.2. Anthocyanin Profiling and Physical Properties of Microparticles
3.3. Assessment of the Encapsulation Productivity
3.4. The Clinical Response of Mice to Dietary Supplementations and DSS Administration
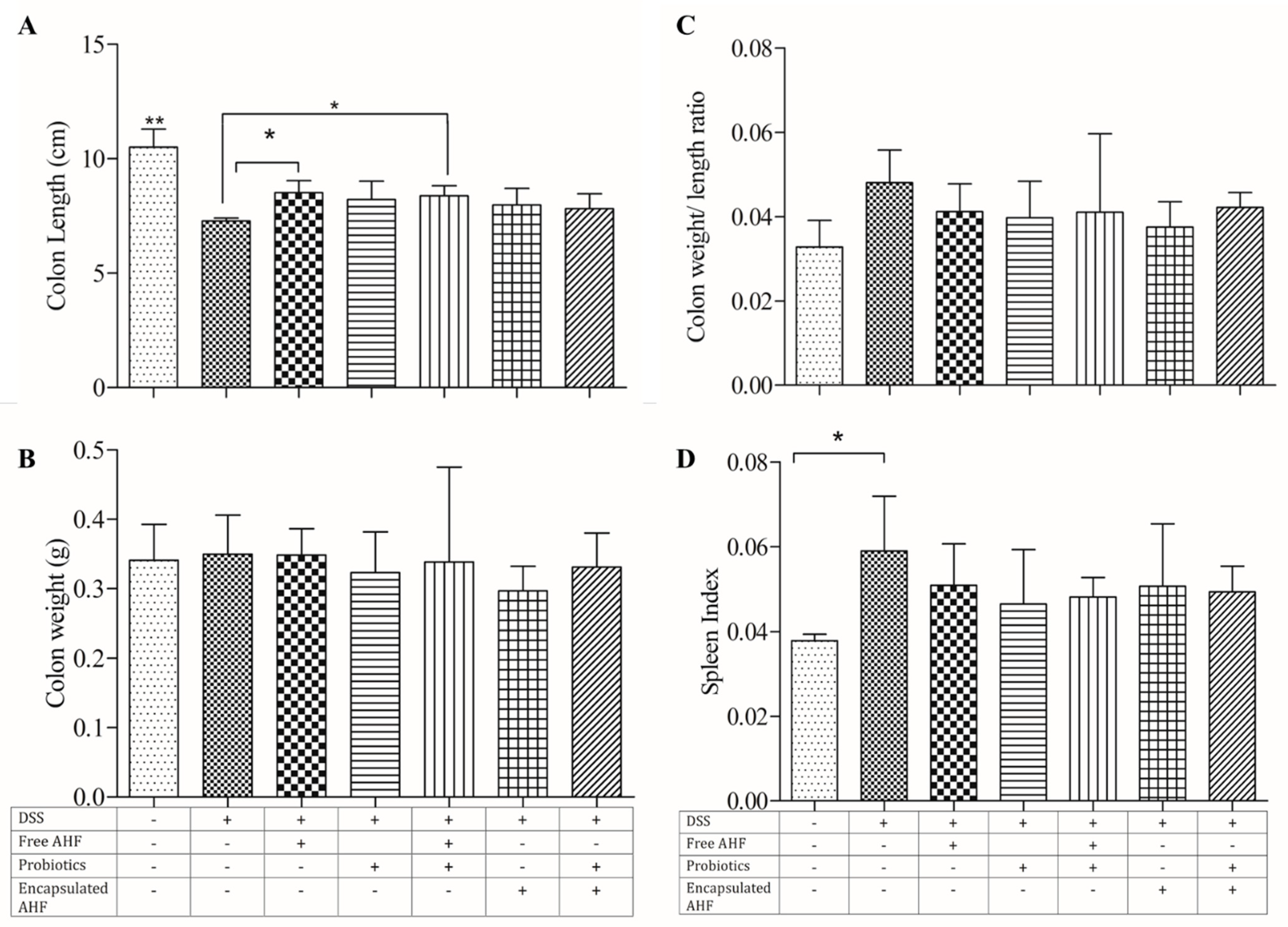
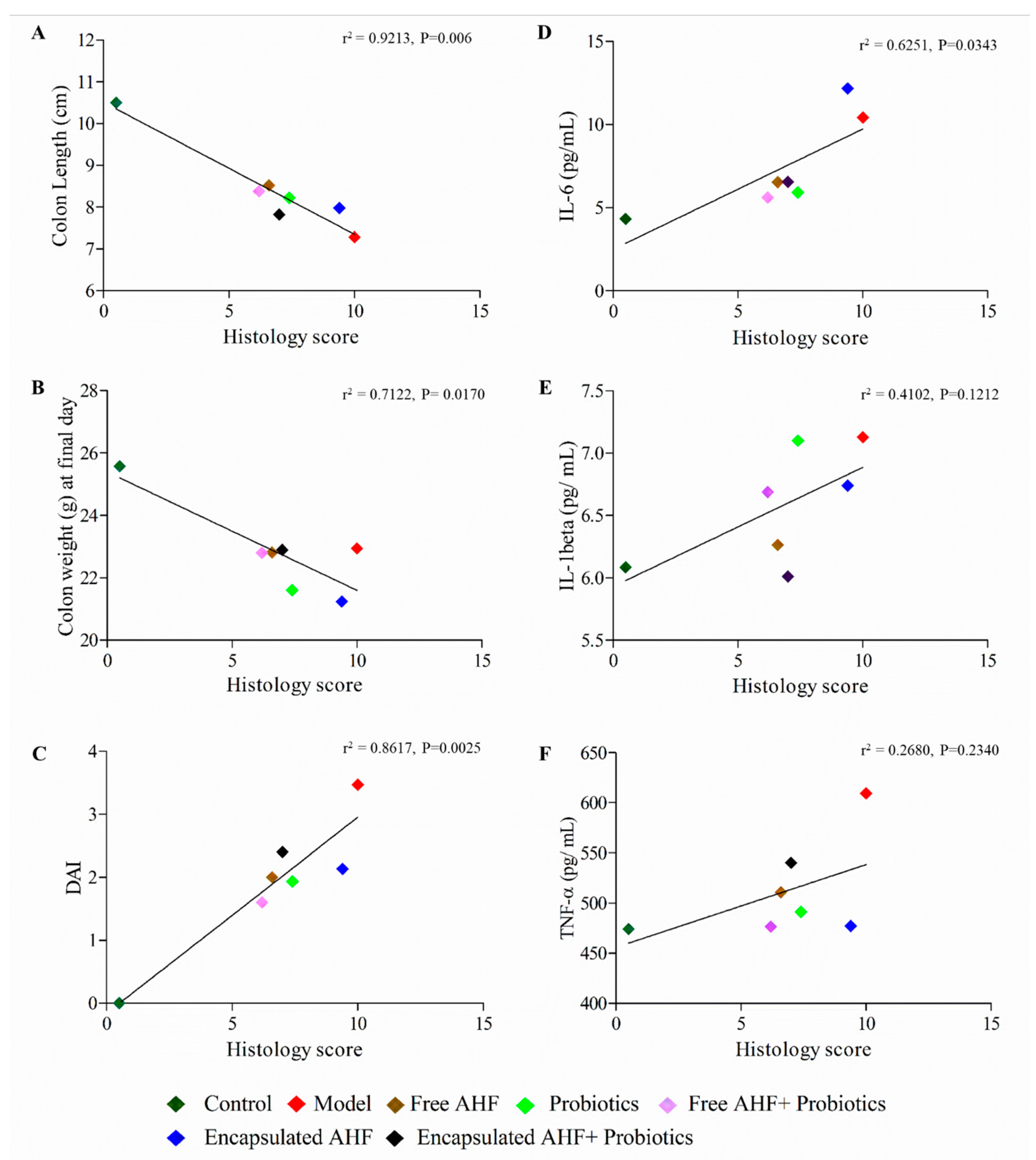
3.5. Dietary Supplementations and Macroscopic Measures of DSS-Induced Colitis
3.6. Dietary Supplementations on Hepatotoxicity and DSS-Induced Colonic Inflammation
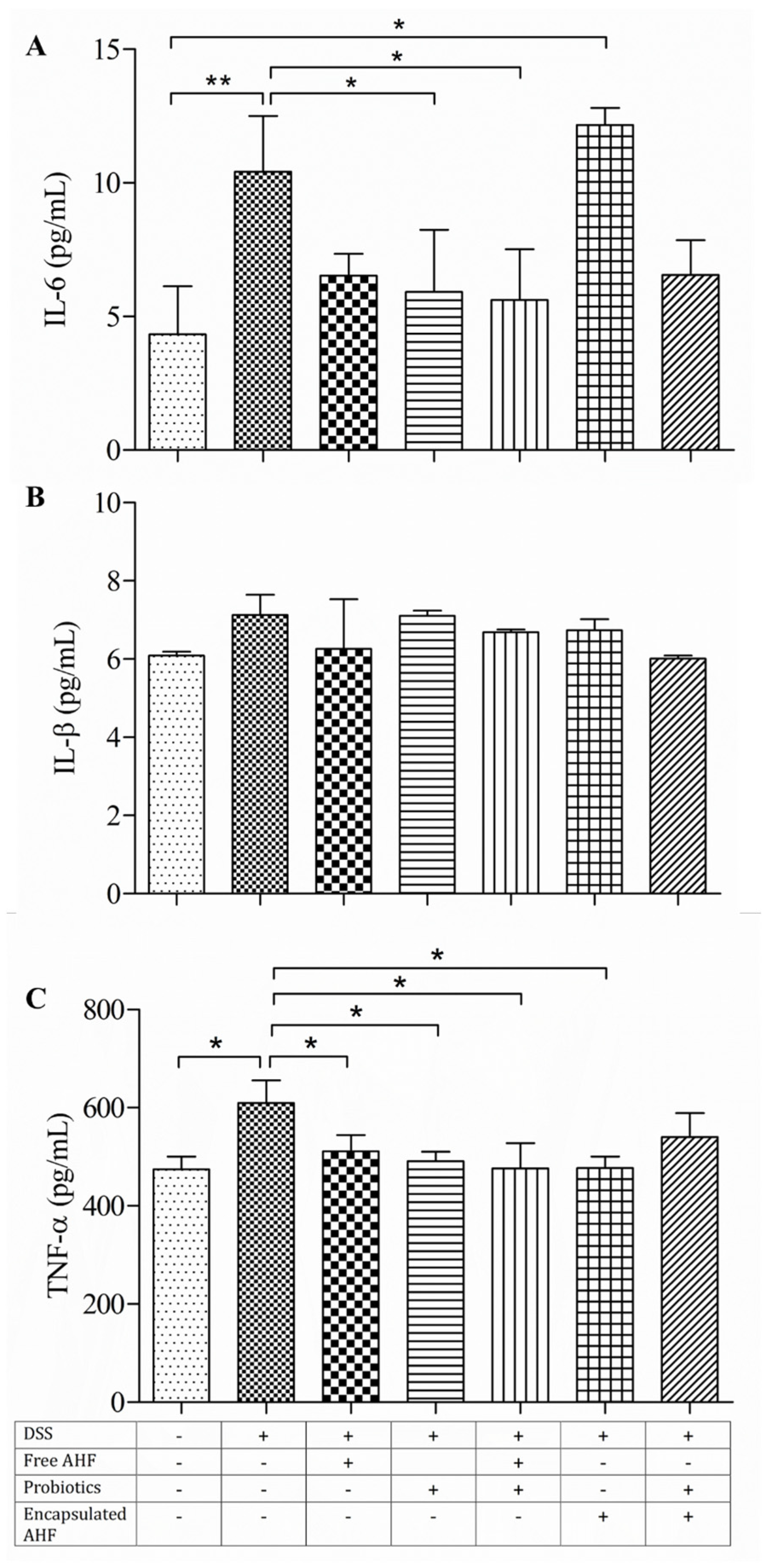
3.7. Dietary Supplementations in Restoring the DSS-Induced Serum Inflammatory Cytokine Expressions
3.8. Dietary Supplementation on Reducing DSS-Induced Colonic Inflammation
3.9. Dietary Supplementation on DSS-Induced Disruption of Gut Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. The Spectrum of Inflammatory Responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zhang, Q.; Yang, J.; Liu, G. Roles of Macrophages on Ulcerative Colitis and Colitis-Associated Colorectal Cancer. Front. Immunol. 2023, 14, 1103617. [Google Scholar] [CrossRef]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef]
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The Potential Health Benefits of Haskap (Lonicera caerulea L.): Role of Cyanidin-3-O-Glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight Junctions: From Molecules to Gastrointestinal Diseases. Tissue Barriers 2023, 3, 11. [Google Scholar] [CrossRef]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red Wine Extract Preserves Tight Junctions in Intestinal Epithelial Cells under Inflammatory Conditions: Implications for Intestinal Inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, M.; Xu, Y.; Xu, H. Tackling Inflammatory Bowel Diseases: Targeting Proinflammatory Cytokines and Lymphocyte Homing. Pharmaceuticals 2022, 15, 1080. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-Glucoside Inhibits NF-KB Signalling in Intestinal Epithelial Cells Exposed to TNF-α and Exerts Protective Effects via Nrf2 Pathway Activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Boehm, M.M.A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.R. Anti-Inflammatory Activity of Haskap Cultivars Is Polyphenols-Dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; De Silva, A.B.K.H.; Mercer, A.; Sarkar, S.; Kienesberger, P.; Langille, M.; Rupasinghe, H.P.V.; Pulinilkunnil, T. Supplementation of Cyanidin-3-O-β-Glucoside-Rich Haskap (Lonicera caerulea L.) Berry Extract Attenuates Hepatic Lipid Dysregulation in Diet-Induced Obese Mice. J. Funct. Foods 2023, 108, 105635. [Google Scholar] [CrossRef]
- Sudheer, S.; Gangwar, P.; Usmani, Z.; Sharma, M.; Sharma, V.K.; Sana, S.S.; Almeida, F.; Dubey, N.K.; Singh, D.P.; Dilbaghi, N.; et al. Shaping the Gut Microbiota by Bioactive Phytochemicals: An Emerging Approach for the Prevention and Treatment of Human Diseases. Biochimie 2022, 193, 38–63. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-Gut Microbiota-Health Axis: A Review. Crit. Rev. Food Sci. Nutr. 2023, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as Inflammatory Modulators and the Role of the Gut Microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, X.; Niu, L.; Chen, X.; Hu, B.; Tang, X. Lonicera caerulea Pomace Alleviates DSS-Induced Colitis via Intestinal Barrier Improvement and Gut Microbiota Modulation. Foods 2023, 12, 3329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, N.; Nieto-Veloza, A.; Zhou, L.; Sun, X.; Si, X.; Tian, J.; Lin, Y.; Jiao, X.; Li, B. Lonicera caerulea Polyphenols Inhibit Fat Absorption by Regulating Nrf2-ARE Pathway Mediated Epithelial Barrier Dysfunction and Special Microbiota. Food Sci. Hum. Wellness 2023, 12, 1309–1322. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, Y.S.; Park, E.J.; Lee, H.J. Honeysuckle Berry (Lonicera caerulea L.) Inhibits Lipase Activity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Molecules 2022, 27, 4731. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Wang, X.; Rao, L.; Yan, X.; Gao, R.; Shen, T.; Zhou, Y.; Kong, C.; Zhou, L. Probiotic Cocktail Alleviates Intestinal Inflammation through Improving Gut Microbiota and Metabolites in Colitis Mice. Front. Cell. Infect. Microbiol. 2022, 12, 886061. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Haskap Berries (Lonicera caerulea L.) Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation Optimization of Natural Anthocyanins with Maltodextrin, Gum Arabic and Gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation Kinetics and Color Stability of Spray-Dried Encapsulated Anthocyanins from Hibiscus sabdariffa L. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Method 2005.02 Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines PH Differential Method. In Official Methods of Analysis of AOAC International; AOCA: Washington, DC, USA, 2006; pp. 37–39. ISBN 0-935584-77-3. [Google Scholar]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The Microencapsulation of Maqui (Aristotelia chilensis (Mol.) Stuntz) Juice by Spray-Drying and Freeze-Drying Produces Powders with Similar Anthocyanin Stability and Bioaccessibility. Molecules 2018, 23, 1227. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of Baking on Dietary Fibre and Phenolics of Muffins Incorporated with Apple Skin Powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Khazaei, M.K.; Jafari, S.M.; Ghorbani, M.; Kakhki, H.A. Application of Maltodextrin and Gum Arabic in Microencapsulation of Saffron Petal’s Anthocyanins and Evaluating Their Storage Stability and Color. Carbohydr. Polym. 2014, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Caroline Ferrari, C.; Pimentel Marconi Germer, S.; Dutra Alvim, I.; Maurício de Aguirre, J. Storage Stability of Spray-Dried Blackberry Powder Produced with Maltodextrin or Gum Arabic. Dry. Technol. 2013, 31, 470–478. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, L.X.; Yan, X.; Guo, C.; Xiong, Y. Effects of Root and Stem Extracts of Asparagus cochinchinensis on Biochemical Indicators Related to Aging in the Brain and Liver of Mice. Am. J. Chin. Med. 2012, 39, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J. Le Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate-(DSS-) Induced Ulcerative Colitis in Kunming (Km) Mice. Oxid. Med. Cell. Longev. 2020, 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome Helper: A Custom and Streamlined Workflow for Microbiome Research. mSystems 2017, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-Containing Purple Potatoes Ameliorate DSS-Induced Colitis in Mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Nair, S.V.G.; Robinson, R.A. Chemopreventive Properties of Fruit Phenolic Compounds and Their Possible Mode of Actions. Stud. Nat. Prod. Chem. 2014, 42, 229–266. [Google Scholar]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Kaushalya, K.G.D.; Rupasinghe, H.P.V. Health Benefits of Microencapsulated Dietary Polyphenols: A Review. Food Rev. Int. 2023, 39, 1–24. [Google Scholar] [CrossRef]
- Tadesse Teferra, F. Direct and Indirect Actions of Inulin as Prebiotic Polysaccharide: A Review. CPQ Nutr. 2019, 3, 1–15. [Google Scholar]
- Che Man, Y.B.; Irwandi, J.; Abdullah, W.J.W. Effect of Different Types of Maltodextrin and Drying Methods on Physico-Chemical and Sensory Properties of Encapsulated Durian Flavour. J. Sci. Food Agric. 1999, 79, 1075–1080. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Fabrication and Characterization of Food-Grade Fibers from Mixtures of Maltodextrin and Whey Protein Isolate Using Needleless Electrospinning. J. Appl. Polym. Sci. 2018, 135, 46328. [Google Scholar] [CrossRef]
- Petkova, N.; Hambarlyiska, I.; Tumbarski, Y.; Vrancheva, R.; Raeva, M.; Ivanov, I. Phytochemical Composition and Antimicrobial Properties of Burdock (Arctium lappa L.) Roots Extracts. Biointerface Res. Appl. Chem. 2022, 12, 2826–2842. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica oleraceae (Red Cabbage) as Time-Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- da Silva, H.R.; de Assis, D.d.C.; Prada, A.L.; Silva, J.O.C.; de Sousa, M.B.; Ferreira, A.M.; Amado, J.R.R.; Carvalho, H.d.O.; dos Santos, A.V.T.d.L.T.; Carvalho, J.C.T. Obtaining and Characterization of Anthocyanins from Euterpe oleracea (Açaí) Dry Extract for Nutraceutical and Food Preparations. Rev. Bras. Farmacogn. 2019, 29, 677–685. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Smrdel, P.; Bogataj, M.; Zega, A.; Planinšek, O.; Mrhar, A. Shape Optimization and Characterization of Polysaccharide Beads Prepared by Ionotropic Gelation. J. Microencapsul. 2008, 25, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/Microencapsulation of Anthocyanins; a Systematic Review and Meta-Analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.J.; Lockhart, J.S.; Estaki, M.; Quin, C.; Hirota, S.A.; Alston, L.; Buret, A.G.; Hancock, T.M.; Petri, B.; Gibson, D.L.; et al. Effects of Azithromycin on Behavior, Pathologic Signs, and Changes in Cytokines, Chemokines, and Neutrophil Migration in C57BL/6 Mice Exposed to Dextran Sulfate Sodium. Comp. Med. 2019, 69, 4. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104(1), 15–25. [Google Scholar] [CrossRef]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and Spatial Analysis of Clinical and Molecular Parameters in Dextran Sodium Sulfate Induced Colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Peng, W.; Wang, Z.; Liu, X.; Dai, W.; Mei, Q.; Hu, X. Polysaccharides from Garlic Protect against Liver Injury in DSS-Induced Inflammatory Bowel Disease of Mice via Suppressing Pyroptosis and Oxidative Damage. Oxid. Med. Cell. Longev. 2022, 2022, 2042163. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-Induced Colitis Is Associated with Adipose Tissue Dysfunction and Disrupted Hepatic Lipid Metabolism Leading to Hepatosteatosis and Dyslipidemia in Mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; McClain, C.; Feng, W. DSS-Induced Intestinal Damage Exacerbates Liver Injury by Acute Alcohol Exposure in Mice: Role of Intestinal Hypoxia-Inducible Factor 1α. FASEB J. 2017, 31, 1–994. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Wang, Z.-X.; Huang, L.-J.; Yang, R.-X.; Zou, Z.-Y.; Ge, W.-S.; Ren, T.-Y.; Fan, J.-G. Premorbid Steatohepatitis Increases the Seriousness of Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. J. Clin. Transl. Hepatol. 2022, 10, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J. Anthocyanins Delay Ageing-Related Degenerative Changes in the Liver. Plant Foods Hum. Nutr. 2017, 72, 425–431. [Google Scholar] [CrossRef]
- Daniel, C.; Poiret, S.; Goudercourt, D.; Dennin, V.; Leyer, G.; Pot, B. Selecting Lactic Acid Bacteria for Their Safety and Functionality by Use of a Mouse Colitis Model. Appl. Environ. Microbiol. 2006, 72, 5799–5805. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Yuan, H.; Xu, Y.; Liu, R.M.; Luo, Y.; Xiao, J.H. Protective Effects of Ligularia fischeri Root Extracts against Ulcerative Colitis in Mice through Activation of Bcl-2/Bax Signalings. Phytomedicine 2022, 99, 154006. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple Peel Polyphenols Reduce Mitochondrial Dysfunction in Mice with DSS-Induced Ulcerative Colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef]
- Tan, C.; Wang, M.; Kong, Y.; Wan, M.; Deng, H.; Tong, Y.; Lyu, C.; Meng, X. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of High Hydrostatic Pressure Treated Cyanidin-3-Glucoside and Blueberry Pectin Complexes on Dextran Sodium Sulfate-Induced Ulcerative Colitis Mice. Food Funct. 2022, 13, 4384–4398. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the Pathogenesis of Inflammatory Bowel Disease and Colon Cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β Mediates Chronic Intestinal Inflammation by Promoting the Accumulation of IL-17A Secreting Innate Lymphoid Cells and CD4+ Th17 Cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xue, Y.; Du, M.; Zhu, M.J. Preventive Effects of Goji Berry on Dextran-Sulfate-Sodium-Induced Colitis in Mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, L.; Sun, Q.; Mo, Q.G.; Sun, W.C.; Wang, Y.W. Maqui Berry Exhibited Therapeutic Effects against DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Food Funct. 2019, 10, 6655–6665. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jeon, Y.D.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Myung, H.; Kim, J.S.; Kim, H.J.; Bang, K.S.; et al. Aronia Berry Extract Ameliorates the Severity of Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. J. Med. Food 2017, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, H.; Xiang, C.; Mu, J.; Zhao, X. Preventive Effect of Lactobacillus Acidophilus XY27 on DSS-Induced Ulcerative Colitis in Mice. Drug Des. Devel. Ther. 2020, 14, 5645–5657. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Jansen, T.; Jacobs, L.; Bonder, M.J.; Kurilshikov, A.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.J. Dietary Red Raspberries Attenuate Dextran Sulfate Sodium-Induced Acute Colitis. J. Nutr. Biochem. 2018, 51, 40–46. [Google Scholar] [CrossRef]
- Čužić, S.; Antolić, M.; Ognjenović, A.; Stupin-Polančec, D.; Petrinić Grba, A.; Hrvačić, B.; Dominis Kramarić, M.; Musladin, S.; Požgaj, L.; Zlatar, I.; et al. Claudins: Beyond Tight Junctions in Human IBD and Murine Models. Front. Pharmacol. 2021, 12, 2968. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Asif, A.R. Transcriptional Regulators of Claudins in Epithelial Tight Junctions. Mediators Inflamm. 2015, 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Oh-oka, K.; Kono, H.; Ishimaru, K.; Miyake, K.; Kubota, T.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Expressions of Tight Junction Proteins Occludin and Claudin-1 Are under the Circadian Control in the Mouse Large Intestine: Implications in Intestinal Permeability and Susceptibility to Colitis. PLoS ONE 2014, 9, e98016. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.M.; Gaudichon, C.; Leclerc, M.; Blachier, F.; Lan, A. Mucosal Healing Progression after Acute Colitis in Mice. World J. Gastroenterol. 2019, 25, 3572. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X.; et al. Cyanidin-3-O-Glucoside and Its Phenolic Metabolites Ameliorate Intestinal Diseases via Modulating Intestinal Mucosal Immune System: Potential Mechanisms and Therapeutic Strategies. Crit. Rev. Food Sci. Nutr. 2021, 63, 1629–1647. [Google Scholar] [CrossRef]
- Nadolski, N.L.W.; Prawitz, R.; Winker, O.; Walton, K.L.W. Effects of Prebiotic Inulin Pre-Treatment on Susceptibility to DSS-Induced Colitis in Mice. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
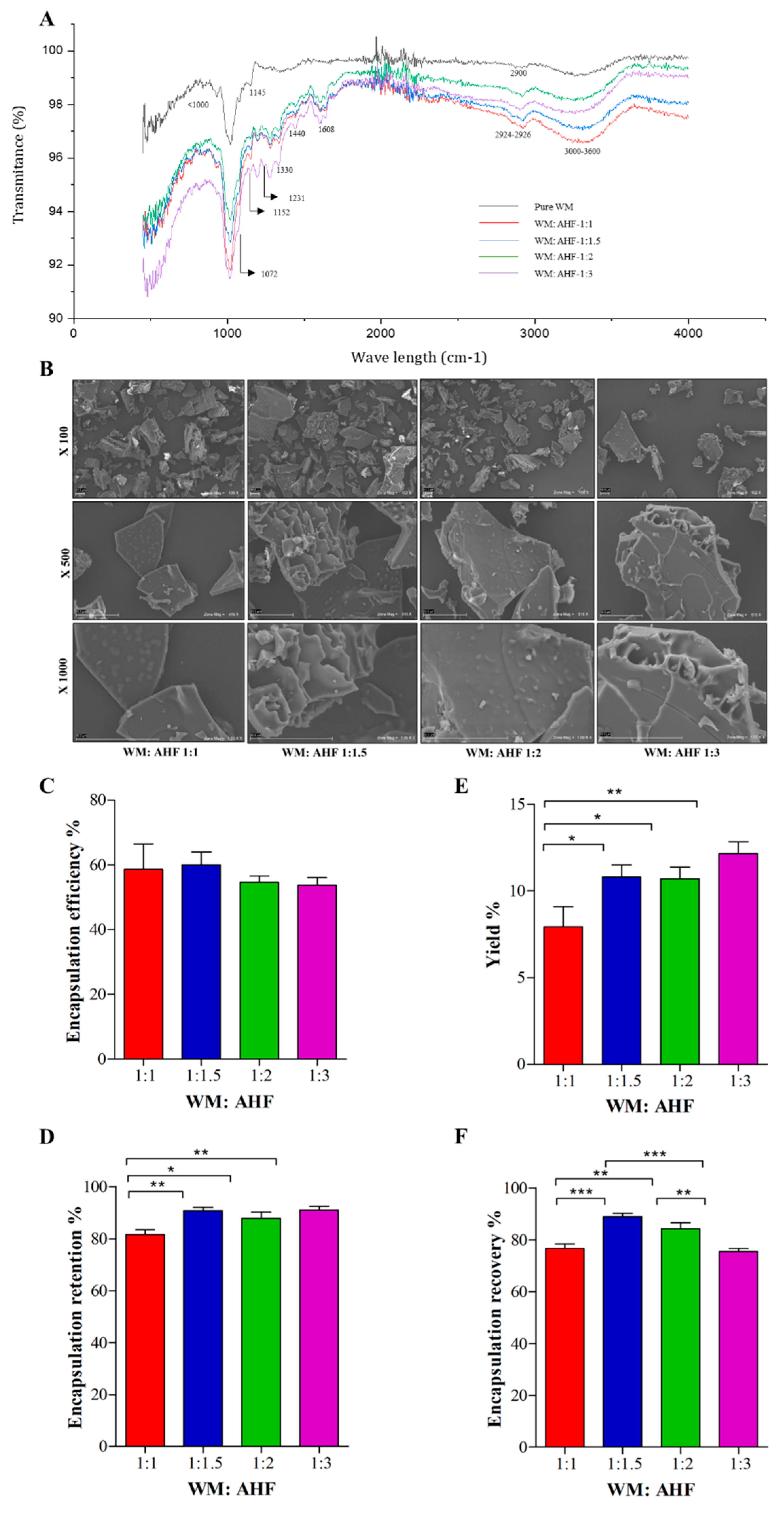
| Anthocyanin | AHF-Incorporated Microparticles | AHF Powder | |||
|---|---|---|---|---|---|
| WM: AHF 1:1 | WM: AHF 1:1.5 | WM: AHF 1:2 | WM: AHF 1:3 | ||
| Cyanidine-3-rutinoside | 1.2 ± 0.06 a | 1.3 ± 0.02 a | 2.5 ± 0.16 a | 16.7 ± 0.22 c | 19.2 ± 1.08 c |
| Cyanidine-3-glucoside | 17.0 ± 1.23 a* | 18.9 ± 0.59 a* | 38.7 ± 0.84 b* | 277.9 ± 10.86 c* | 281.5 ± 16.82 c* |
| Cyanidine-3,5-diglucoside | 1.2 ± 0.09 a | 1.6 ± 0.05 a | 3.3 ± 0.05 a | 23.7 ± 1.36 c | 27.2 ± 1.45 c |
| Peonidine-3-glucoside | 0.8 ± 0.04 a | 0.7 ± 0.05 a | 2.5 ± 0.04 a | 29.4 ± 0.43 c | 26.2 ± 1.90 c |
| Wall Material: AHF Ratio | Moisture% | aW | Solubility% | Bulk Density (g/cm−3) | Hygroscopicity (g/100 g) | Particle Size (µm) | ||
|---|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | ||||||
| 1:1 | 7.3 ± 0.07 b | 0.6 ± 0.00 a | 95.8 ± 08 b | 0.3 ± 0.01 b | 21.7267 ± 0.94 a | 57.5 | 281 | 2711 |
| 1:1.5 | 6.9 ± 0.07 c | 0.6 ± 0.00 a | 97.9 ± 0.61 a | 0.2 ± 0.01 a | 21.2031 ± 0.22 a | 47.8 | 188 | 830 |
| 1:2 | 7.5 ± 0.07 b | 0.6 ± 0.00 a | 93.9 ± 0.09 c | 0.2 ± 0.01 a | 21.4202 ± 0.05 a | 37.5 | 138 | 445 |
| 1:3 | 8.4 ± 0.00 a | 0.6 ± 0.00 a | 95.6 ± 0.13 b | 0.2 ± 0.01 a | 21.7623 ± 0.20 a | 53.5 | 186 | 965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmawansa, K.V.S.; Stadnyk, A.W.; Rupasinghe, H.P.V. Dietary Supplementation of Haskap Berry (Lonicera caerulea L.) Anthocyanins and Probiotics Attenuate Dextran Sulfate Sodium-Induced Colitis: Evidence from an Experimental Animal Model. Foods 2024, 13, 1987. https://doi.org/10.3390/foods13131987
Dharmawansa KVS, Stadnyk AW, Rupasinghe HPV. Dietary Supplementation of Haskap Berry (Lonicera caerulea L.) Anthocyanins and Probiotics Attenuate Dextran Sulfate Sodium-Induced Colitis: Evidence from an Experimental Animal Model. Foods. 2024; 13(13):1987. https://doi.org/10.3390/foods13131987
Chicago/Turabian StyleDharmawansa, K. V. Surangi, Andrew W. Stadnyk, and H. P. Vasantha Rupasinghe. 2024. "Dietary Supplementation of Haskap Berry (Lonicera caerulea L.) Anthocyanins and Probiotics Attenuate Dextran Sulfate Sodium-Induced Colitis: Evidence from an Experimental Animal Model" Foods 13, no. 13: 1987. https://doi.org/10.3390/foods13131987
APA StyleDharmawansa, K. V. S., Stadnyk, A. W., & Rupasinghe, H. P. V. (2024). Dietary Supplementation of Haskap Berry (Lonicera caerulea L.) Anthocyanins and Probiotics Attenuate Dextran Sulfate Sodium-Induced Colitis: Evidence from an Experimental Animal Model. Foods, 13(13), 1987. https://doi.org/10.3390/foods13131987









