Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
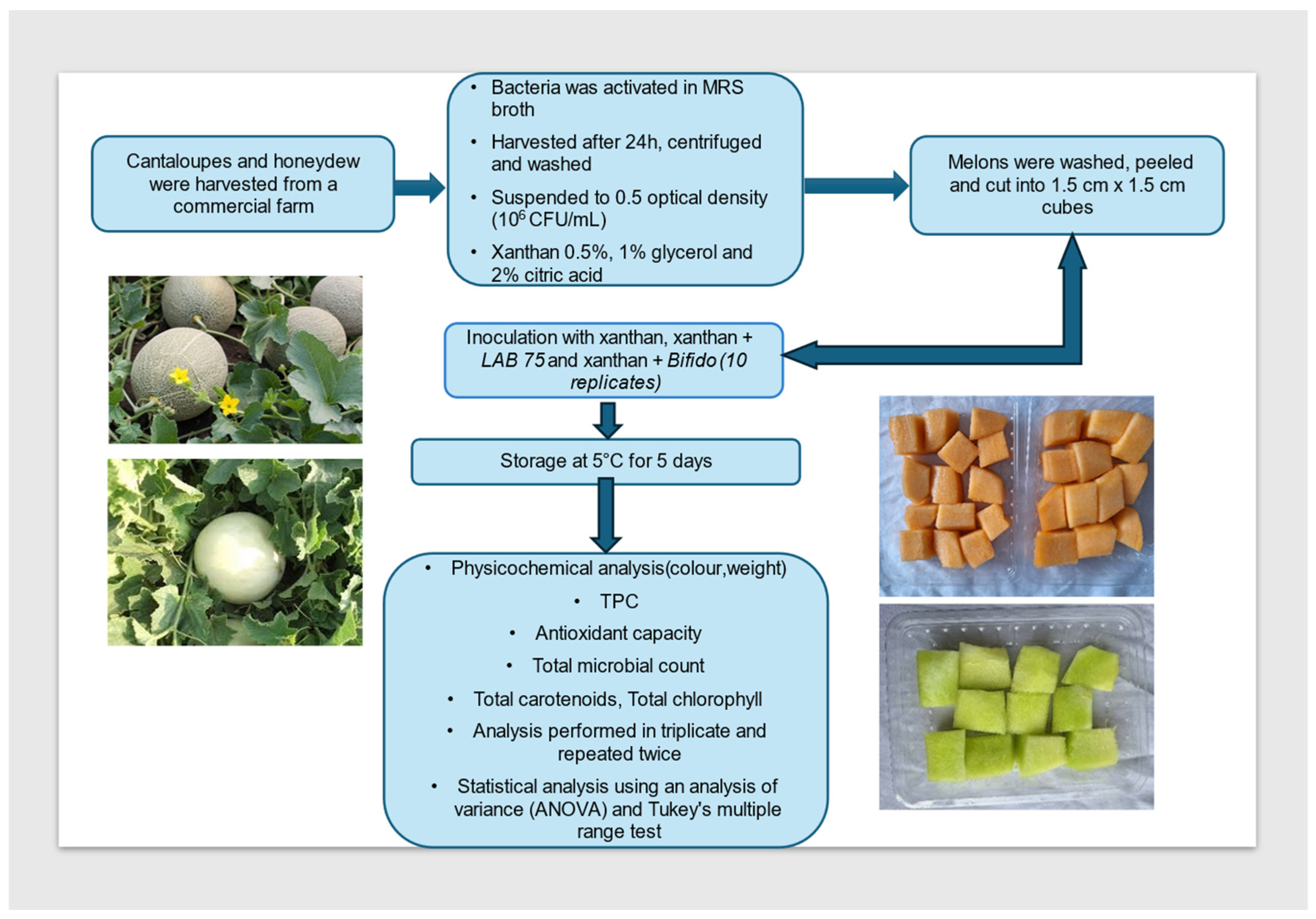
2.2. Melon Fresh Cut
2.3. Probiotic Cultures
2.4. Coating Preparation
2.5. Coating of Melon Fresh Cuts
2.6. Coating Characterization
2.7. Survival of LABs and Microbial Count
2.8. Postharvest Quality
2.8.1. Color Analysis
2.8.2. Sensory Analysis
2.9. Changes in Bioactive Compounds and Antioxidant Activities of Coated Fresh-Cut Melons
2.9.1. Ascorbic Acid
2.9.2. Total Phenolic Content
2.9.3. Total Carotenoid Content
2.9.4. The Total Chlorophyll Content
2.10. Antioxidant Activities
2.10.1. Ferric Reducing Antioxidant Power (FRAP)
2.10.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.10.3. 2,2-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Xanthan Gum-Based Edible Coating
3.1.1. Zeta Potential, Particle Size and Polydispersity Index (PDI) for Pure Xanthan Gum, Xanthan Gum + L. plantarum 75, and Xanthan Gum + Bifidobacterium longum Coating
3.1.2. Rheological Properties of Pure Xanthan Gum, Xanthan Gum + L. plantarum 75, and Xanthan Gum + Bifidobacterium longum Coating
3.1.3. Mechanical Properties (Tensile Strength) of Pure Xanthan Gum, Xanthan Gum + L. plantarum 75, and Xanthan Gum + Bifidobacterium Coating
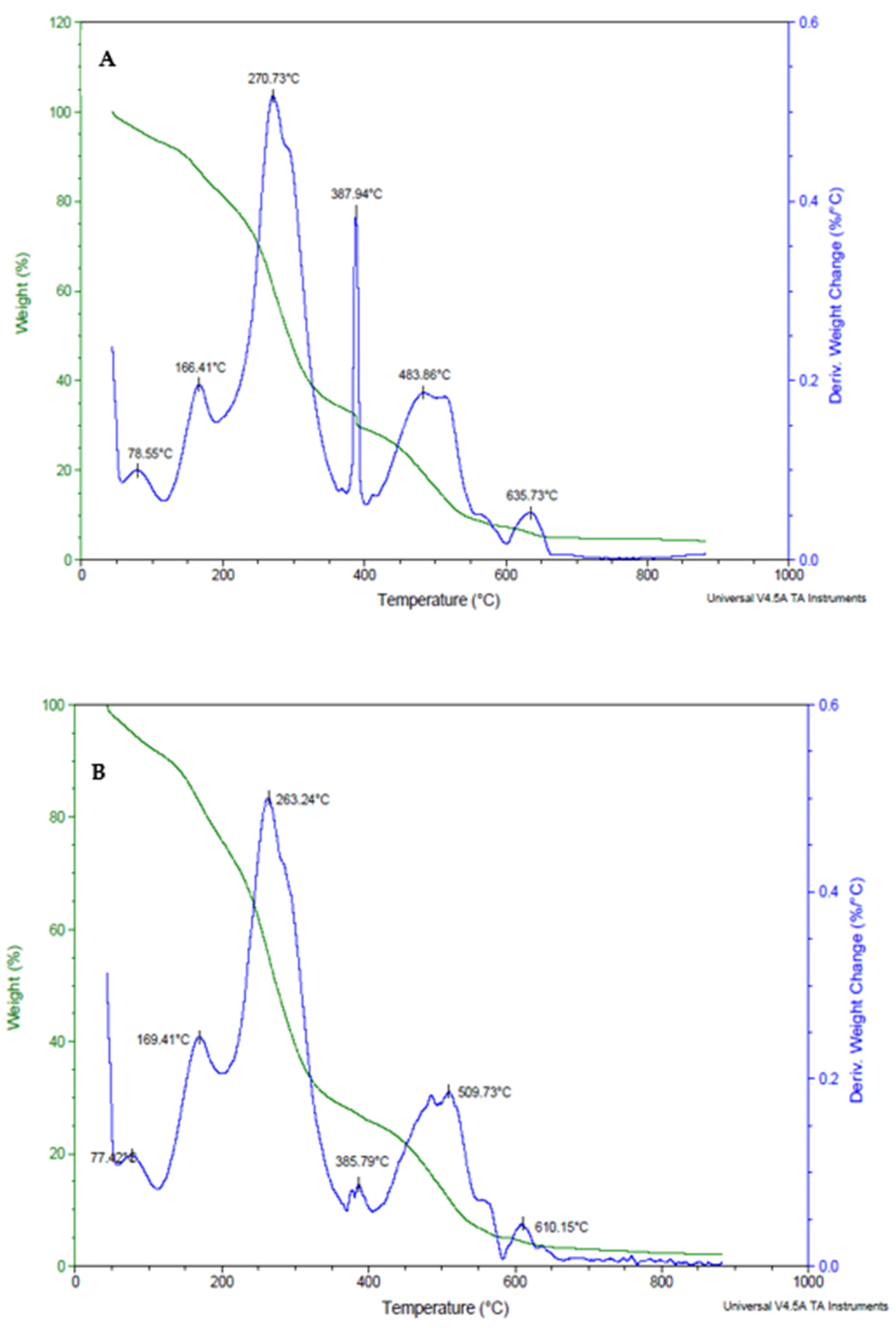
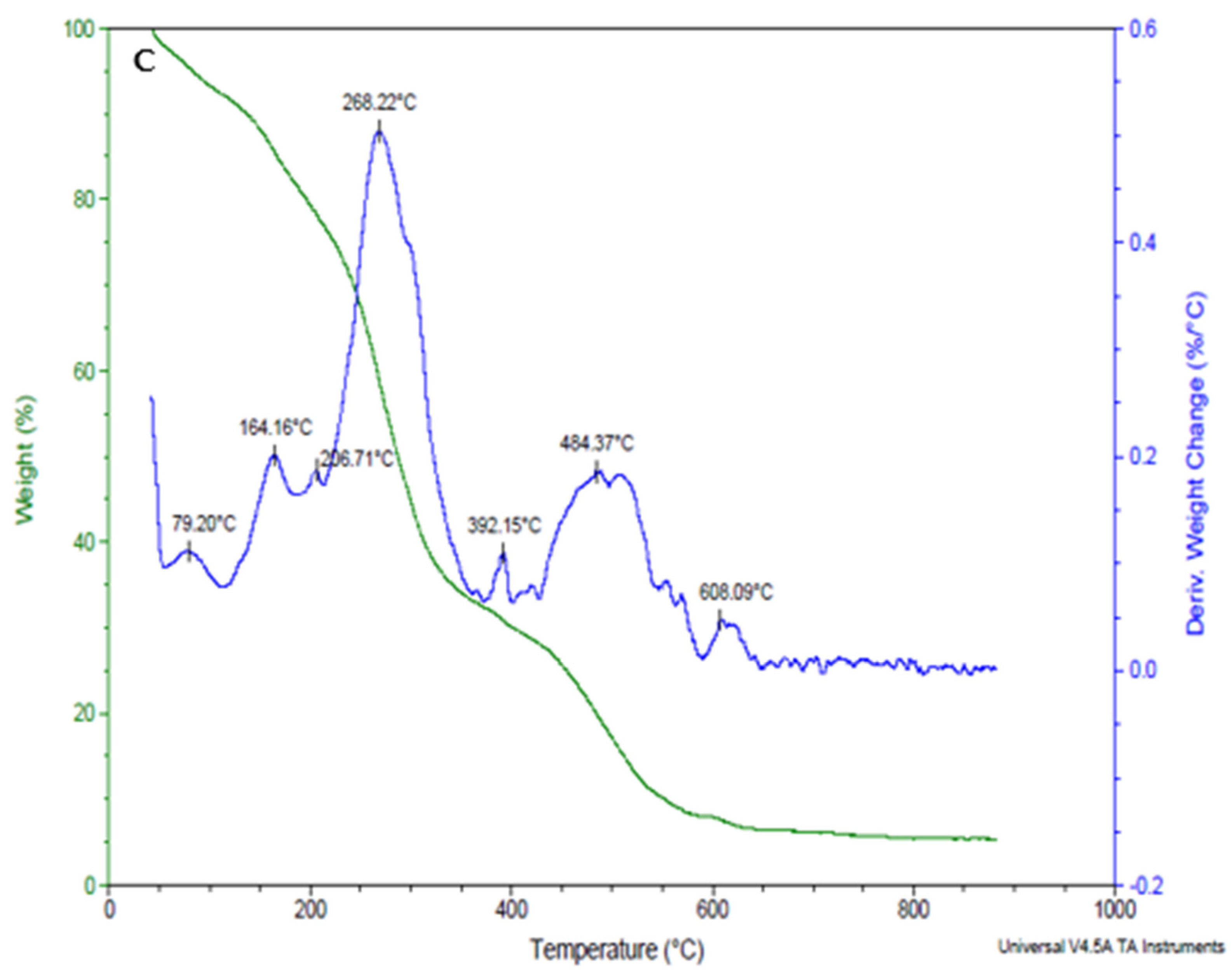
3.1.4. Thermal Stability of Xanthan Gum, Xanthan Gum + L. plantarum 75, and Xanthan Gum + Bifidobacterium longum Coating
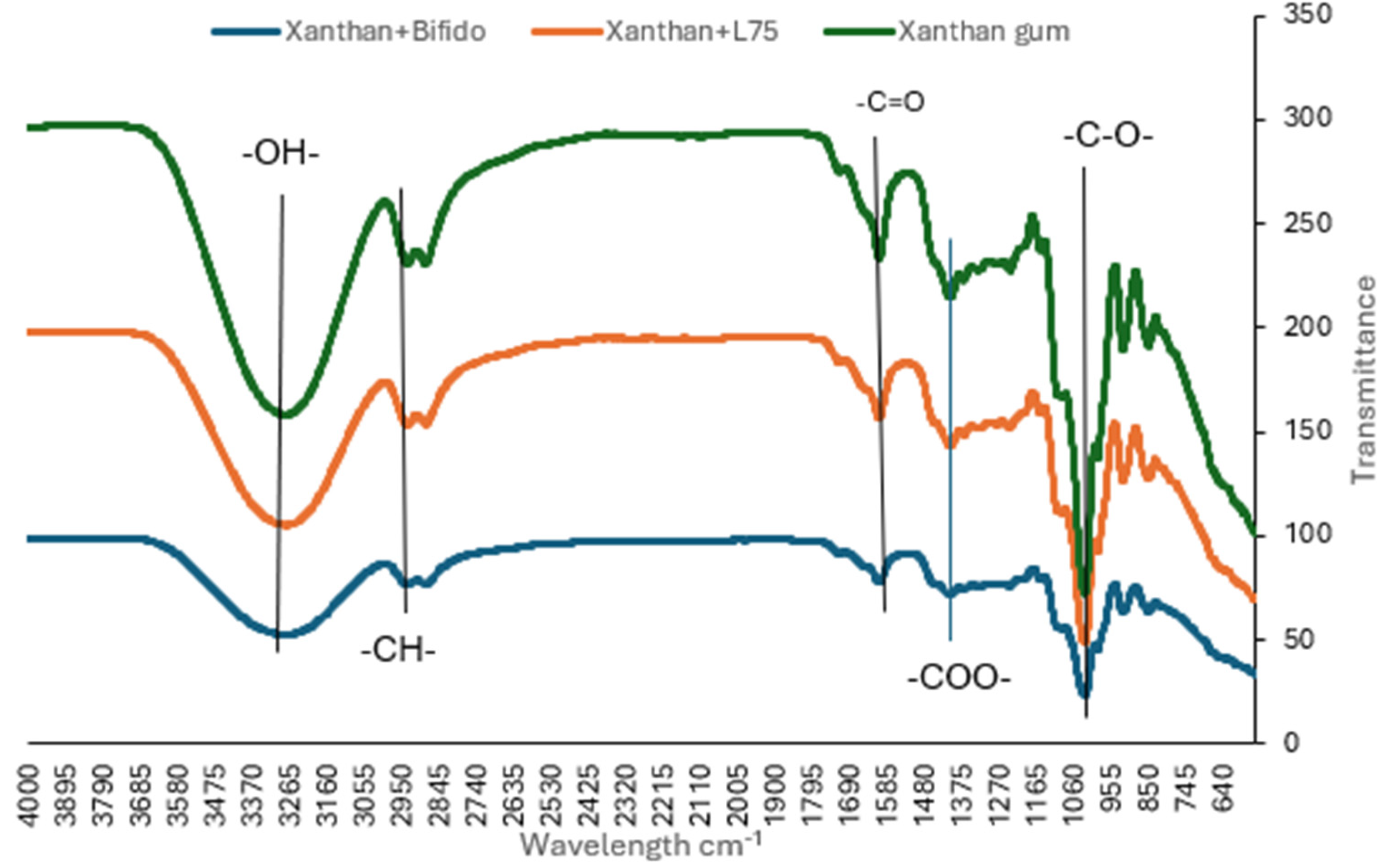
3.1.5. FTIR of Pure Xanthan Gum, Xanthan Gum + L. plantarum 75 and Xanthan Gum + Bifidobacterium longum coating
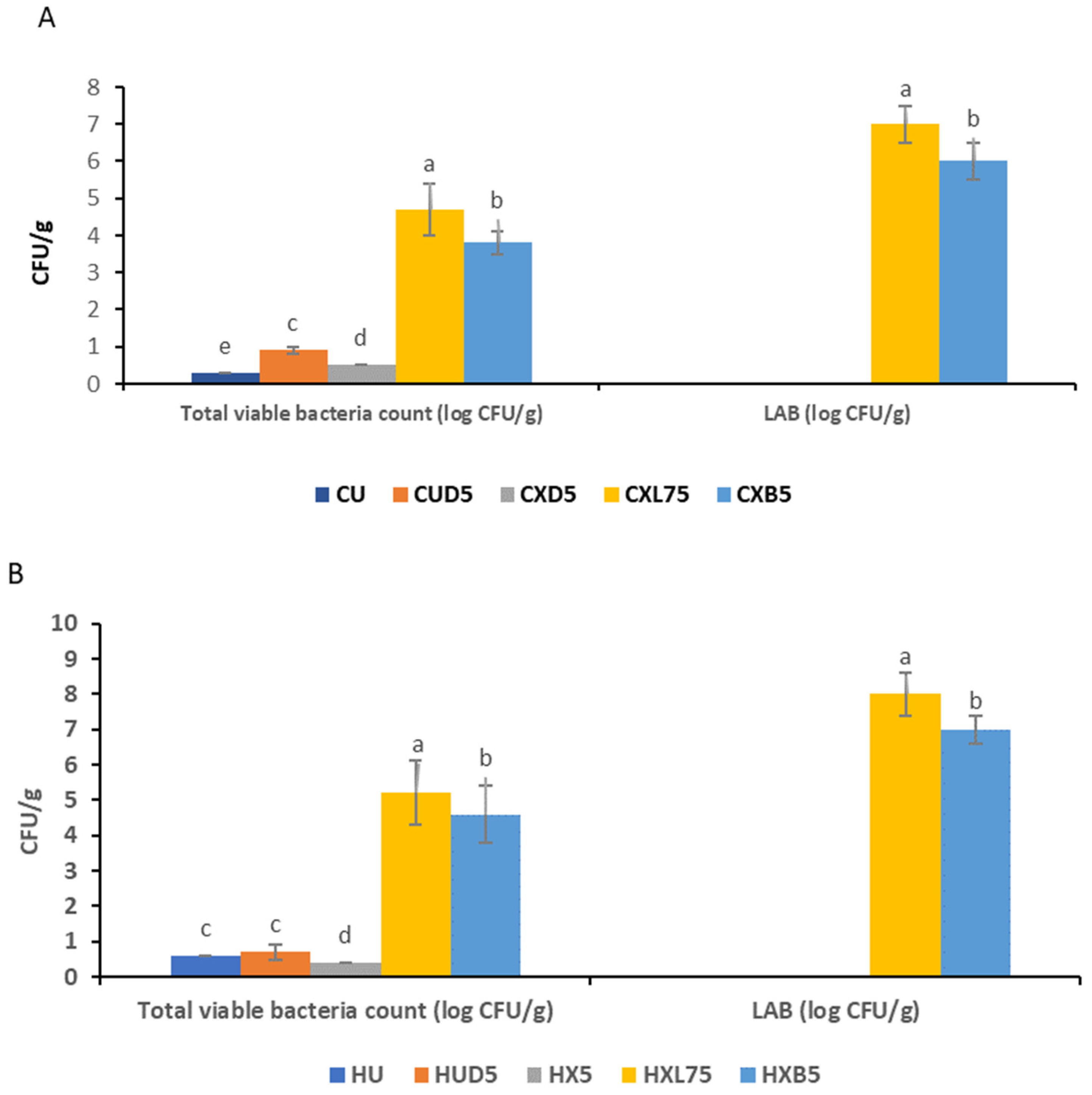
3.2. Survival of Probiotic Bacteria in Edible Coating
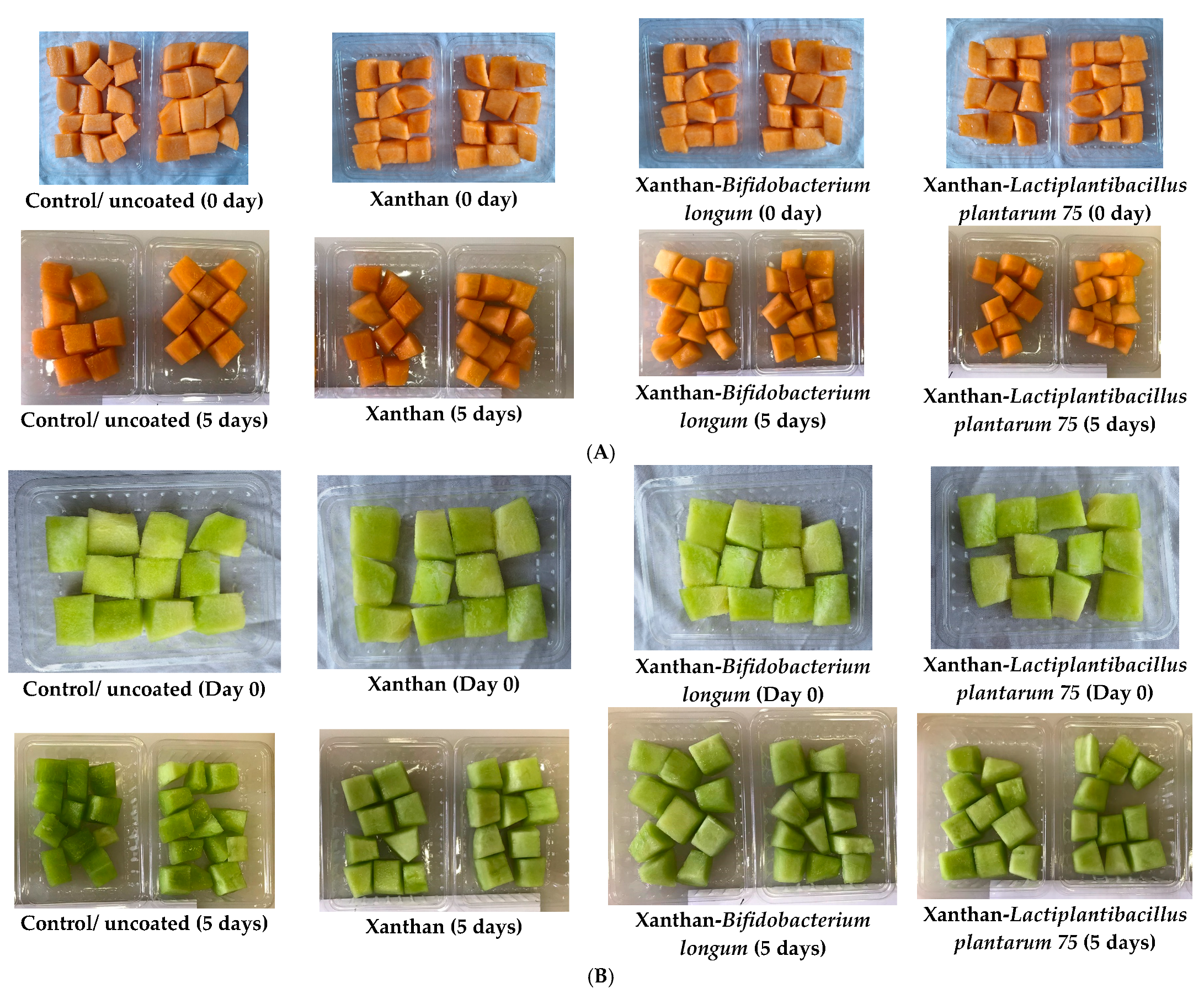
3.3. Probiotic Coatings and Color Properties
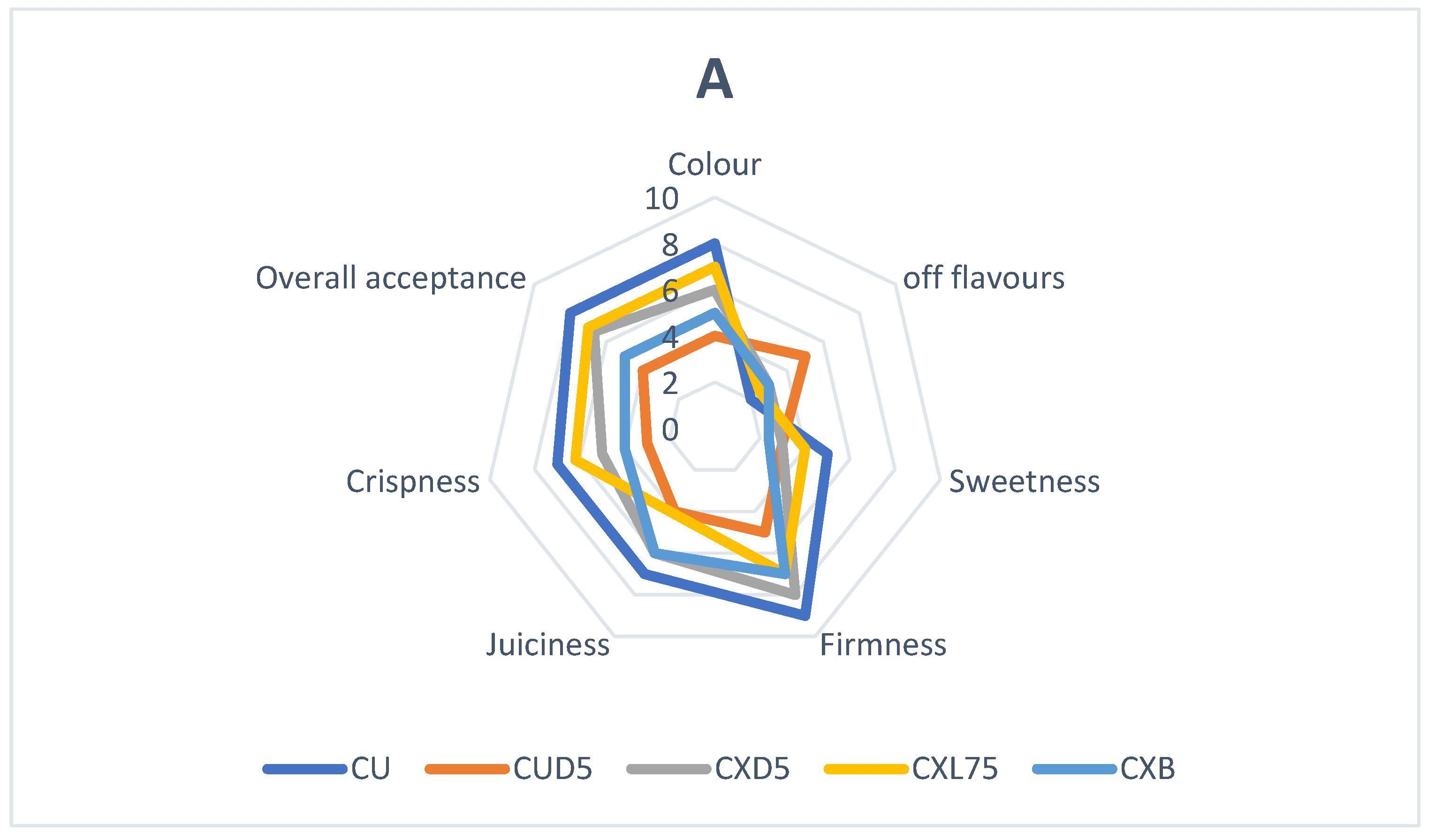
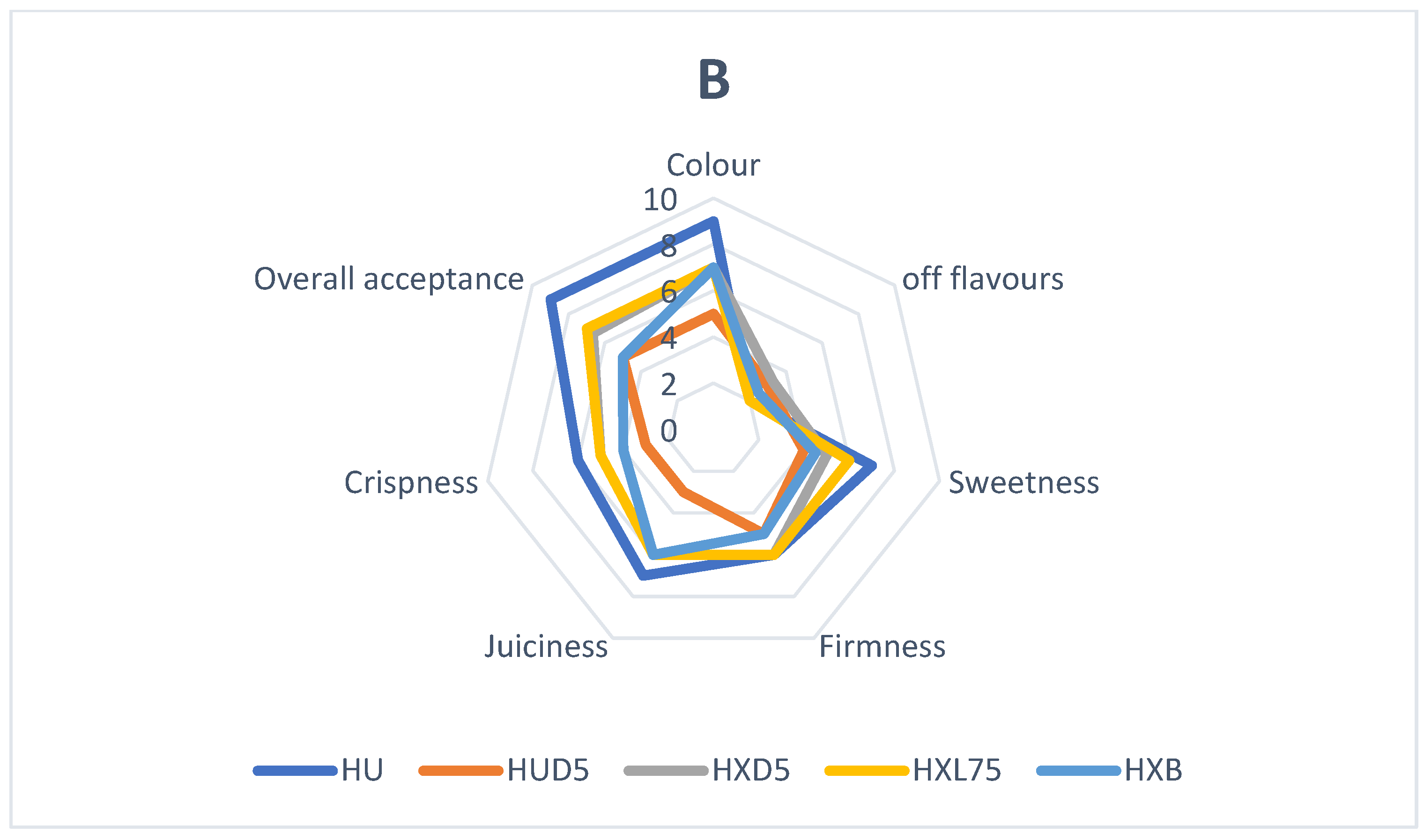
3.4. Probiotic Coatings and Sensory Properties
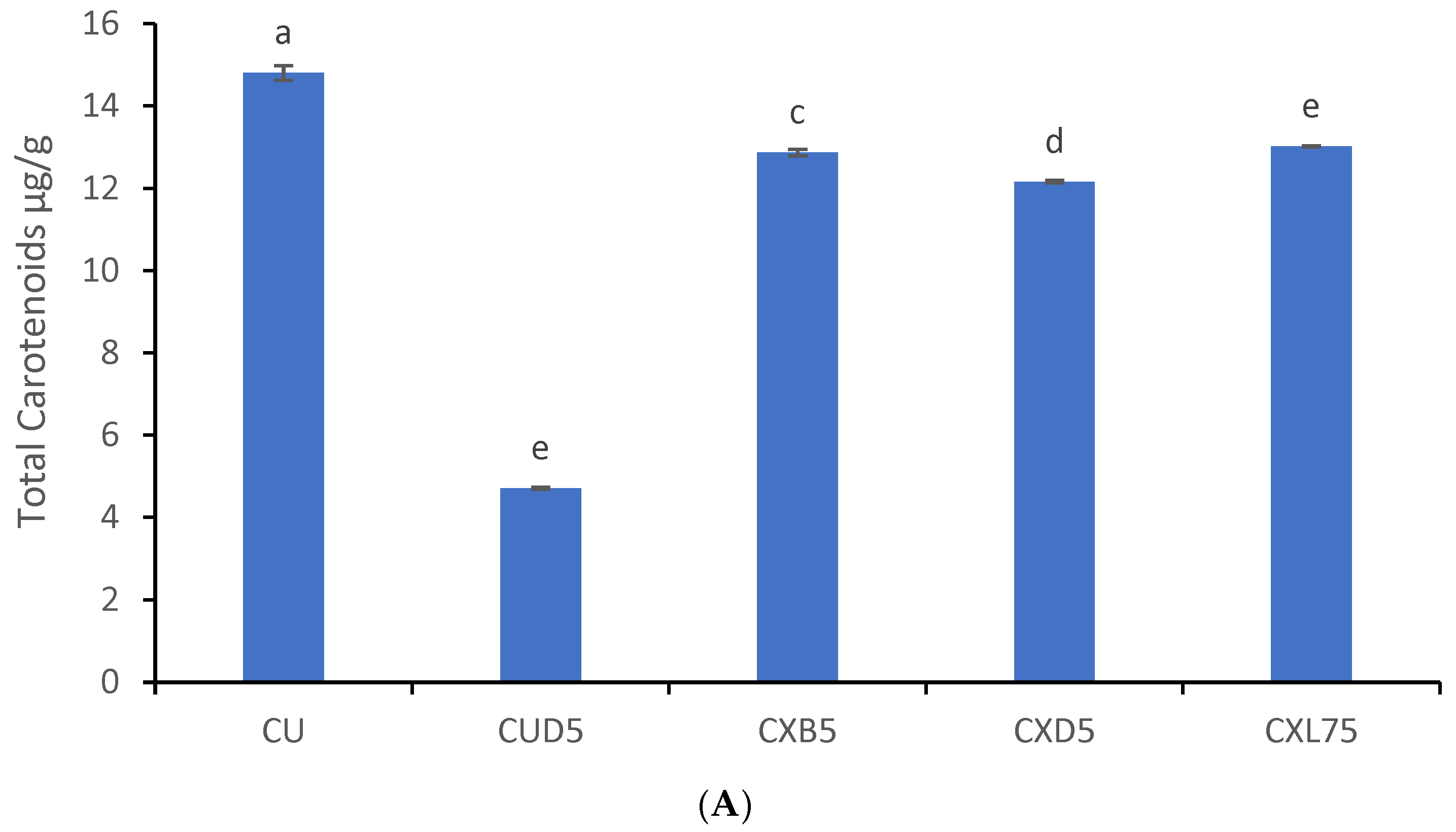
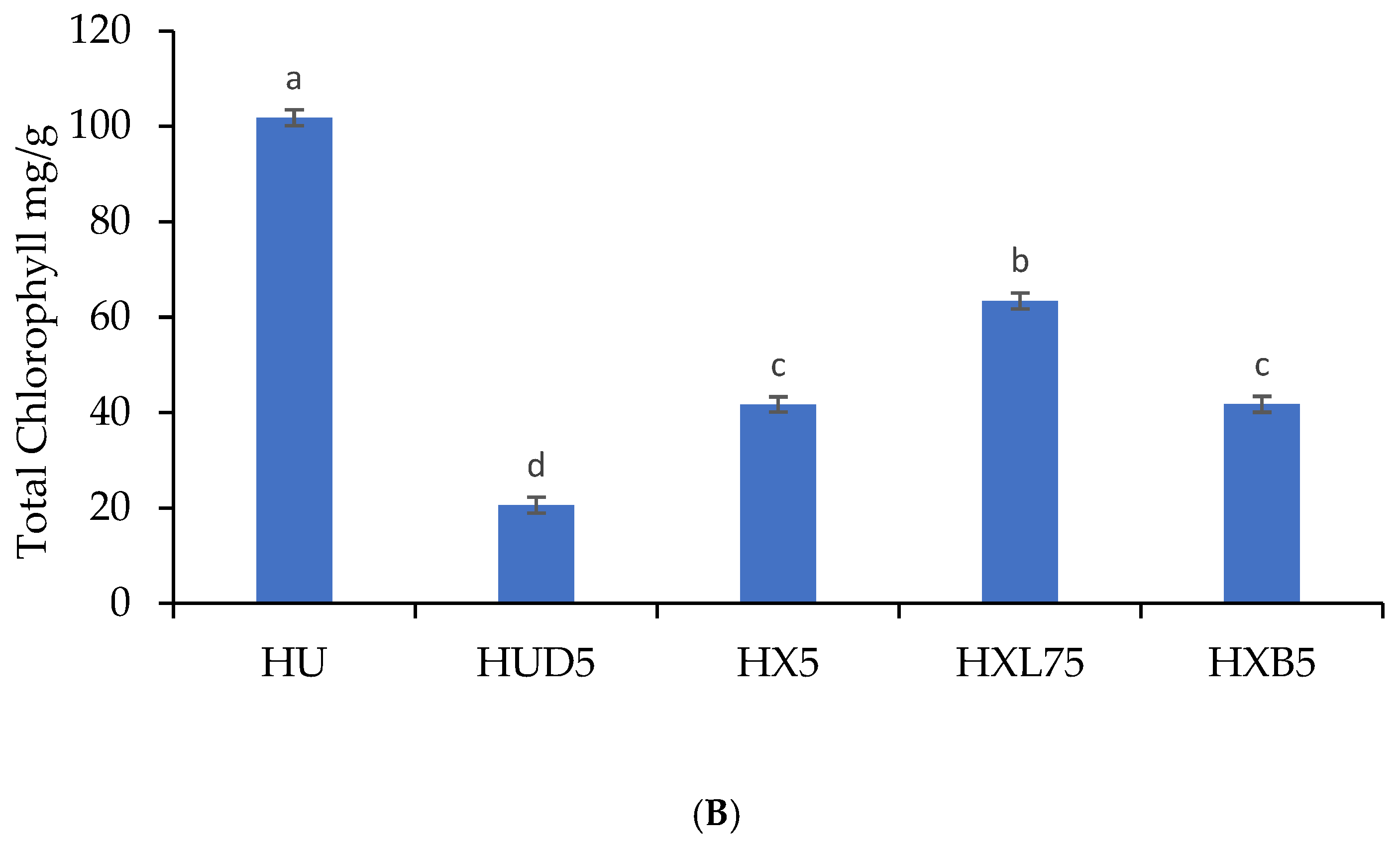
3.5. Probiotic Coatings and Total Carotenoids and Chlorophylls
3.6. Probiotic Coatings and Ascorbic Acid Content
3.7. Probiotic Coatings and Total Phenols
3.8. Probiotic Coatings and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opara, U.L.; Al-Ani, M.R. Antioxidant contents of pre-packed fresh-cut versus whole fruit and vegetables. Br. Food J. 2010, 112, 797–810. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.M.; Brandão, T.R.S. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of Cantaloupe melon. J. Food Meas. Charact. 2017, 12, 292–300. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Ukuku, D.O.; Geveke, D.J.; Chau, L.; Niemira, B.A. Microbial safety and overall quality of cantaloupe fresh-cut pieces prepared from whole fruit after wet steam treatment. Int. J. Food Microbiol. 2016, 231, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Hanani, Z.A.N. Sensory quality and flavour of alginate coated and repetitive pulsed light treated fresh-cut cantaloupes (Cucumis melo L. Var. Reticulatus Cv. Glamour) during storage. J. Food Sci. Technol. 2019, 56, 2563–2575. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Echeverría, G.; Viñas, I.; López, M.; Abadias, M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT 2018, 87, 581–588. [Google Scholar] [CrossRef]
- Zudaire, L.; Viñas, I.; Plaza, L.; Iglesias, M.B.; Abadias, M.; Aguiló-Aguayo, I. Evaluation of postharvest calcium treatment and biopreservation with Lactobacillus rhamnosus GG on the quality of fresh-cut ‘Conference’ pears. J. Sci. Food Agric. 2018, 98, 4978–4987. [Google Scholar] [CrossRef]
- de Oliveira, K.R.; Fernandes, K.F.D.; de Souza, E.L. Current advances on the development and application of probiotic-loaded edible films and coatings for the bioprotection of fresh and minimally processed fruit and vegetables. Foods 2021, 10, 2207. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.D.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Ashok Pandey, A.P.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Tech. Biotech. 2010, 48, 413–434. [Google Scholar]
- Chen, W.; Yu, L.; Shi, Y. Safety Evaluation of Lactic Acid Bacteria, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 371–409. [Google Scholar] [CrossRef]
- Temiz, N.N.; Özdemir, K.S. Microbiological and physicochemical quality of strawberries (Fragaria × ananassa) coated with Lactobacillus rhamnosus and inulin enriched gelatin films. Postharvest Biol. Technol. 2021, 173, 111433. [Google Scholar] [CrossRef]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. BioMed Res. Int. 2014, 2014, 309183. [Google Scholar] [CrossRef] [PubMed]
- Rößle, C.; Brunton, N.; Gormley, R.T.; Ross, P.R.; Butler, F. Development of potentially synbiotic fresh-cut apple slices. J. Funct. Foods 2010, 2, 245–254. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Silva, M.; Farinha, D.; Sales, H.; Pontes, R.; Nunes, J. Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods 2023, 12, 3308. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyn’ska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioprocess Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Tapia, M.; Rojas-Graü, M.; Rodríguez, F.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and gellan-based edible films for probiotic coatings on fresh-cut fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of bioactive edible coatings loaded with Lactobacillus plantarum on physicochemical properties of fresh strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- Wong, C.H.; Mak, I.E.K.; Li, D. Bilayer edible coating with stabilized Lactobacillus plantarum 299v improved the shelf life and safety quality of fresh-cut apple slices. Food Packag. Shelf Life 2021, 30, 100746. [Google Scholar] [CrossRef]
- Speranza, B.; Campaniello, D.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Viability of Lactobacillus plantarum on Fresh-Cut Chitosan and Alginate-Coated Apple and Melon Pieces. Front. Microbiol. 2018, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- FDA (Food and Drug Administration). Title 21 Food and Drugs Section 172: Food Additives Permitted for Direct Addition to Food for Human Consumption. Code of Federal Regulations (2013). Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.695 (accessed on 7 February 2024).
- Russo, P.; Peña, N.; de Chiara, M.L.V.; Amodio, M.L.; Colelli, G.; Spano, G. Probiotic lactic acid bacteria for the production of multifunctional fresh-cut cantaloupe. Food Res. Int. 2015, 77, 762–772. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.R. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Freitas, I.R.; Cortez-Vega, W.R.; Pizato, S.; Prentice-Hernández, C.; Borges, C.D. Xanthan gum as a carrier of preservative agents and calcium chloride applied on fresh-cut apple. J. Food Saf. 2013, 33, 229–238. [Google Scholar] [CrossRef]
- Guroo, I.; Gull, A.; Wani, S.M.; Wani, S.A.; Al-Huqail, A.A.; Alhaji, J.H. Influence of different types of polysaccharide-based coatings on the storage stability of fresh-cut kiwi fruit: Assessing the physicochemical, antioxidant and phytochemical properties. Foods 2021, 10, 2806. [Google Scholar] [CrossRef]
- Lecholocholo, N.; Shoko, T.; Manhivi, V.E.; Maboko, M.M.; Akinola, S.A.; Sivakumar, D. Influence of different rootstocks on quality and volatile constituents of cantaloupe and honeydew melons (Cucumis melo L) grown in high tunnels. Food Chem. 2022, 393, 133388. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray technology applications of xanthan gum-based edible coatings for fresh-cut lotus root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef]
- Osondu, H.A.A.; Akinola, S.A.; Shoko, T.; Pillai, S.K.; Sivakumar, D. Coating properties, resistance response, molecular mechanisms and anthracnose decay reduction in green skin avocado fruit (‘Fuerte’) coated with chitosan hydrochloride loaded with functional compounds. Postharvest Biol. Technol. 2021, 186, 111812. [Google Scholar] [CrossRef]
- Seke, F.; Manhivi, V.E.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. In Vitro Release of Anthocyanins from Microencapsulated Natal Plum (Carissa macrocarpa) Phenolic Extract in Alginate/Psyllium Mucilage Beads. Foods 2022, 11, 2550. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alfonso, J.; Cuadri, A.; Berta, M.; Stading, M. Relation between concentration and shear-extensional rheology properties of xanthan and guar gum solutions. Carbohydr. Polym. 2018, 181, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.d.M.; Carneiro, L.C.; Bianchini, D.; Dias, A.R.G.; Zavareze, E.d.R.; Prentice, C.; Moreira, A.d.S. Structural, thermal, physical, mechanical, and barrier properties of chitosan films with the addition of xanthan gum. J. Food Sci. 2017, 82, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote leaf (Sechium edule) and Pineapple (Ananas comosus) Smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.K.d.O.C.; Gomes, C.d.C.; Amaral, M.L.Q.d.A.; de Medeiros, L.D.G.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Maciel, B.L.L.; Morais, A.H.d.A.; Passos, T.S. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.). Food Chem. 2018, 270, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.M.M.; Lee, S.M.; Boyden, L.; Guinard, J. Sensory Properties and Consumer Acceptance of Cantaloupe Melon Cultivars. J. Food Sci. 2019, 84, 2278–2288. [Google Scholar] [CrossRef]
- Mphaphuli, T.; Manhivi, V.E.; Slabbert, R.; Sultanbawa, Y.; Sivakumar, D. Enrichment of mango fruit leathers with natal plum (Carissa macrocarpa) improves their phytochemical content and antioxidant properties. Foods 2020, 9, 431. [Google Scholar] [CrossRef]
- Seke, F.; Manhivi, V.E.; Shoko, T.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Extraction optimisation, hydrolysis, antioxidant properties and bioaccessibility of phenolic compounds in Natal plum fruit (Carissa Macrocarpa). Food Biosci. 2021, 44, 101425. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Managa, M.G.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of moist cooking blanching on colour, phenolic metabolites and glucosinolate content in Chinese cabbage (Brassica rapa L. subsp. chinensis). Foods 2019, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Ndou, A.; Tinyani, P.P.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stages. Food Chem. 2019, 293, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- S Kulkarni, N.; Ranpise, N.S.; Rathore, D.S.; Dhole, S.N. Characterization of Self-Microemulsifying Dosage Form: Special Emphasis on Zeta Potential Measurement. Int. J. Pharmac. Bio. Arch. 2019, 10, 172–179. [Google Scholar]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Faria, S.; Petkowicz, C.L.d.O.; de Morais, S.A.L.; Terrones, M.G.H.; de Resende, M.M.; de França, F.P.; Cardoso, V.L. Characterization of xanthan gum produced from sugar cane broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef]
- Miranda, A.L.; Costa, S.S.; Assis, D.d.J.; de Jesus, C.S.; Gil Guimarães, A.; Druzian, J.I. Influence of strain and fermentation time on the production, composition, and properties of xanthan gum. J. Appl. Polym. Sci. 2020, 137, 48557. [Google Scholar] [CrossRef]
- Menegazzi, G.d.S.; Ribeiro, E.S.; de Farias, B.S.; Luz, G.d.Q.d.; Oliveira, G.M.; Junior, T.R.S.C.; Pinto, L.A.d.A.; Diaz, P.S. Microencapsulation of Lacticaseibacillus casei CSL3 using cheese whey, fructo-oligosaccharide and xanthan gum by spray drying. Food Biosci. 2023, 56, 103348. [Google Scholar] [CrossRef]
- Dário, A.F.; Hortêncio, L.M.; Sierakowski, M.R.; Neto, J.C.Q.; Petri, D.F. The effect of calcium salts on the viscosity and adsorption behavior of xanthan. Carbohydr. Polym. 2011, 84, 669–676. [Google Scholar] [CrossRef]
- Chagas, B.S.; Machado, D.L.P.; Haag, R.B.; De Souza, C.R.; Lucas, E.F. Evaluation of hydrophobically associated polyacrylamide-containing aqueous fluids and their potential use in petroleum recovery. J. Appl. Polym. Sci. 2004, 91, 3686–3692. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; So, J.-H.; Yang, S.-M. Rheological Evidence for the Silica-Mediated Gelation of Xanthan Gum. J. Colloid Interface Sci. 1999, 216, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, N.; Brinck, O.; Sontag-Strohm, T. Viscosity of β-glucan from oat products at the intestinal phase of the gastrointestinal model. Food Hydrocoll. 2020, 100, 105422. [Google Scholar] [CrossRef]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Release of encapsulated bioactives influenced by alginate viscosity under in-vitro gastrointestinal model. Int. J. Biol. Macromol. 2021, 170, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Hazirah, M.N.; Isa, M.; Sarbon, N. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Davachi, S.M.; Pottackal, N.; Torabi, H.; Abbaspourrad, A. Development and characterization of probiotic mucilage based edible films for the preservation of fruits and vegetables. Sci. Rep. 2021, 11, 16608. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Fang, Y.; Yao, K. Water vapor barrier and mechanical properties of konjac glucomannan–chitosan–soy protein isolate edible films. Food Bioprod. Process. 2009, 87, 7–10. [Google Scholar] [CrossRef]
- Lawal, O.; Adebowale, K.; Ogunsanwo, B.; Barba, L.; Ilo, N. Oxidized and acid thinned starch derivatives of hybrid maize: Functional characteristics, wide-angle X-ray diffractometry and thermal properties. Int. J. Biol. Macromol. 2005, 35, 71–79. [Google Scholar] [CrossRef]
- Soares, R.; Lima, A.; Oliveira, R.; Pires, A.; Soldi, V. Thermal degradation of biodegradable edible films based on xanthan and starches from different sources. Polym. Degrad. Stab. 2005, 90, 449–454. [Google Scholar] [CrossRef]
- Pawlicka, A.; Tavares, F.C.; Dörr, D.S.; Cholant, C.M.; Ely, F.; Santos, M.J.L.; Avellaneda, C.O. Dielectric behavior and FTIR studies of xanthan gum-based solid polymer electrolytes. Electrochim. Acta 2019, 305, 232–239. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Ye, J.; Yang, Y.; Huang, Y.; Zhang, X.; Xiao, M. Effect of gellan gum and xanthan gum synergistic interactions and plasticizers on physical properties of plant-based enteric polymer films. Polymers 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; Gama, F.M.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M. Bacterial cellulose/cashew gum films as probiotic carriers. LWT 2020, 130, 109699. [Google Scholar] [CrossRef]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P.; Henridubernet, S.; Guéguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. Influence of storage temperature and packaging on bacteria and yeast viability in a plant-based fermented food. Foods 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Garcia, S. Influence of Linseed Mucilage Incorporated into an Alginate-Base Edible Coating Containing Probiotic Bacteria on Shelf-Life of Fresh-Cut Yacon (Smallanthus sonchifolius). Food Bioprocess Technol. 2018, 11, 1605–1614. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef]
- Thang, T.D.; Quyen, L.T.H.; Hang, H.T.T.; Luan, N.T.; KimThuy, D.T.; Lieu, D.M. Survival Survey of Lactobacillus acidophilus In Additional Probiotic Bread. Turk. J. Agric. Food Sci. Technol. 2019, 7, 588–592. [Google Scholar] [CrossRef]
- Talwalkar, A.; Kailasapathy, K.; Peiris, P.; Arumugaswamy, R. Application of RBGR—A simple way for screening of oxygen tolerance in probiotic bacteria. Int. J. Food Microbiol. 2001, 71, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated Probiotics: Potential Techniques and Coating Materials for Non-Dairy Food Applications. Appl. Sci. 2022, 12, 10005. [Google Scholar] [CrossRef]
- Gidlöf, K.; Anikin, A.; Lingonblad, M.; Wallin, A. Looking is buying. How visual attention and choice are affected by consumer preferences and properties of the supermarket shelf. Appetite 2017, 116, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bambace, M.F.; Alvarez, M.V.; Moreira, M.d.R. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.M.; Calandrelli, R.; Laratta, B. Influence of Ripening on Polyphenolic Content, Degradative, and Browning Enzymes in Cantaloupe Varieties (C. Melo L.). Horticulturae 2021, 7, 421. [Google Scholar] [CrossRef]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2019, 118, 108758. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Jacxsens, L.; Devlieghere, F.; Ragaert, P.; Vanneste, E.; Debevere, J. Relation between microbiological quality, metabolite production and sensory quality of equilibrium modified atmosphere packaged fresh-cut produce. Int. J. Food Microbiol. 2003, 83, 263–280. [Google Scholar] [CrossRef]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality Changes and Nutrient Retention in Fresh-Cut versus Whole Fruits during Storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; Volume 71. [Google Scholar]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Osorio, S.; Fernie, A.R. Biochemistry of fruit ripening. In The Molecular Biology and Biochemistry of Fruit Ripening, 1st ed.; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 1–19. [Google Scholar] [CrossRef]
- Amal, S.A.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Horti. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT 2008, 41, 1862–1870. [Google Scholar] [CrossRef]
- Valerio, F.; Volpe, M.G.; Santagata, G.; Boscaino, F.; Barbarisi, C.; Di Biase, M.; Bavaro, A.R.; Lonigro, S.L.; Lavermicocca, P. The viability of probiotic Lactobacillus paracasei IMPC2.1 coating on apple slices during dehydration and simulated gastro-intestinal digestion. Food Biosci. 2020, 34, 100533. [Google Scholar] [CrossRef]
- Zibaei-Rad, A.; Rahmati-Joneidabad, M.; Behbahani, B.A.; Taki, M. Assessing the protection mechanisms on Enterobacter aerogenes ATCC 13048 by potentially probiotic strain Lacticaseibacillus casei XN18: An experimental and modeling study. Microb. Pathog. 2023, 181, 106177. [Google Scholar] [CrossRef] [PubMed]
- Zibaei-Rad, A.; Rahmati-Joneidabad, M.; Behbahani, B.A.; Taki, M. Probiotic-loaded seed mucilage-based edible coatings for fresh pistachio fruit preservation: An experimental and modeling study. Sci. Rep. 2024, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Manhivi, V.E.; Slabbert, R.M.; Sivakumar, D. Co-ingestion of natal plums (Carissa macrocarpa) and marula nuts (Sclerocarya birrea) in a snack bar and its effect on phenolic compounds and bioactivities. Molecules 2022, 27, 310. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]

| Sample Name | Z-Ave (nm) | PdI | ZP mV |
|---|---|---|---|
| Xanthan gum | 420.4 | 1 | −50.5 |
| Xanthan + B. longum | 498.1 | 1 | −39.7 |
| Xanthan + L. plantarum 75 | 641.5 | 1 | −51.4 |
| Coating Formulation | Thickness (mm) | Tensile Strength (MPa) | Maximum Tensile Strain (%) |
| Xanthan gum | 0.38 a ± 0.04 | 1 a ± 0.4 | 79.39 a ± 10.3 |
| Xanthan gum+ L. plantarum 75 | 0.42 b ±0.05 | 0.74 b ± 0.2 | 42.0 c ± 18.9 |
| Xanthan gum+ B. longum | 0.42 b ± 0.04 | 0.83 c ± 0.4 | 67.1 b ± 12.4 |
| L* | a* | b* | C* | hº | |
|---|---|---|---|---|---|
| Cantaloupe | |||||
| CU | 45.60 ± 1.01 a | 9.72 ± 1.56 d | 29.00 ± 1.97 a | 30.59 ± 2.34 a | n/a |
| CUD5 | 29.25 ± 6.80 d | 14.52 ± 1.74 a | 15.86 ± 5.91 d | 28.90 ± 3.20 bc | n/a |
| CXD5 | 35.39 ± 1.81 c | 14.13 ± 0.59 ab | 17.82 ± 0.12 c | 26.23 ± 0.04 c | n/a |
| CXL75 | 42.52 ± 2.60 b | 12.88 ± 2.87 c | 20.42 ± 2.94 b | 31.24 ± 0.30 a | n/a |
| CXB5 | 39.72 ± 2.25 c | 14.38 ± 0.36 a | 17.34 ± 3.16 c | 25.97 ± 2.99 c | n/a |
| LSD | 1.073 | 2.763 | 2.078 | 7.800 | |
| Honeydew | |||||
| HU | 46.23 ± 2.95 a | −2.40 ± 0.95 c | 11.65 ± 2.05 c | 20.61 ± 2.18 bc | 114.55 ± 1.75 a |
| HUD5 | 19.57 ± 0.81 d | −0.46 ± 0.21 b | 7.42 ± 0.63 d | 15.96 ± 0.64 d | 113.86 ± 0.53 a |
| HX5 | 25.59 ± 1.12 c | −9.04 ± 0.86 d | 19.10 ± 1.34 a | 21.14 ± 1.57 b | 115.34 ± 0.48 a |
| HXL75 | 29.72 ± 2.25 b | 1.38 ± 0.36 a | 17.34 ± 3.16 b | 25.97 ± 2.99 a | 114.42 ± 0.95 a |
| HXB5 | 28.57 ± 5.27 b | −1.26 ± 0.51 b | 8.40 ± 1.26 d | 17.18 ± 1.35 c | 114.99 ± 0.72 a |
| LSD | 2.211 | 4.717 | 1.946 | 1.289 | ns |
| AA (mg/100 g FW) | TPC (mg/100 g GAE FW) | FRAP (mM TE/100 g FW) | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | |
|---|---|---|---|---|---|
| Cantaloupe | |||||
| CU | 24.57 ± 1.57 a | 30.63 ± 0.039 a | 2.03 ± 0.002 a | 22.27 ± 0.53 d | 3.71 ± 0.07 c |
| CUD5 | 14.86 ± 1.28 e | 21.51 ± 0.001 e | 1.44 ± 0.002 e | 53.73 ± 0.06 a | 6.80 ± 0.20 a |
| CXB5 | 19.43 ± 1.28 c | 26.28 ± 0.006 d | 1.80 ± 0.001b | 41.37 ± 0.10 b | 4.29 ± 0.11 b |
| CXD5 | 17.71 ± 1.28 d | 27.05 ± 0.015 b | 1.61 ± 0.004 d | 41.32 ± 0.49 b | 4.40 ± 0.09 b |
| CXL75 | 21.71 ± 1.57 b | 26.66 ± 0.016 c | 1.78 ± 0.001 c | 35.44 ± 0.39 c | 3.75 ± 0.0 c |
| LSD | 1.317 | 0.00591 | 0.004043 | 0.2389 | 0.0298 |
| Honeydew | |||||
| HU | 21.71 ± 1.57 a | 27.35 ± 0.034 a | 2.43 ± 0.01 a | 27.56 ± 0.06 c | 3.03 ± 0.14 c |
| HUD5 | 12.00 ± 1.28 e | 13.46 ± 0.020 e | 1.40 ± 0.01 d | 66.26 ± 0.09 a | 5.35 ± 0.06 a |
| HXB5 | 18.29 ± 1.57 c | 20.72 ± 0.002 d | 1.94 ± 0.00 b | 44.57 ± 0.00 b | 4.60 ± 0.002 b |
| HXD5 | 13.14 ± 1.57 e | 21.00 ± 0.013 b | 1.84 ± 0.002 c | 44.73 ± 0.04 b | 4.62 ± 0.002 b |
| HXL75 | 21.14 ± 1.57 b | 20.92 ± 0.00 c | 1.93 ± 0.001 b | 44.40 ± 0.00 b | 4.61 ± 0.001 b |
| LSD | 1.862 | 0.00836 | 0.005717 | 0.3378 | 0.0422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikhala, T.; Seke, F.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons. Foods 2024, 13, 940. https://doi.org/10.3390/foods13060940
Chikhala T, Seke F, Slabbert RM, Sultanbawa Y, Sivakumar D. Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons. Foods. 2024; 13(6):940. https://doi.org/10.3390/foods13060940
Chicago/Turabian StyleChikhala, Tshudufhadzo, Faith Seke, Retha M. Slabbert, Yasmina Sultanbawa, and Dharini Sivakumar. 2024. "Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons" Foods 13, no. 6: 940. https://doi.org/10.3390/foods13060940
APA StyleChikhala, T., Seke, F., Slabbert, R. M., Sultanbawa, Y., & Sivakumar, D. (2024). Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons. Foods, 13(6), 940. https://doi.org/10.3390/foods13060940









