Effects of Litsea cubeba Essential Oil–Chitosan/Corn Starch Composite Films on the Quality and Shelf-Life of Strawberry (Fragaria × ananassa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Strawberry Samples and Chemicals
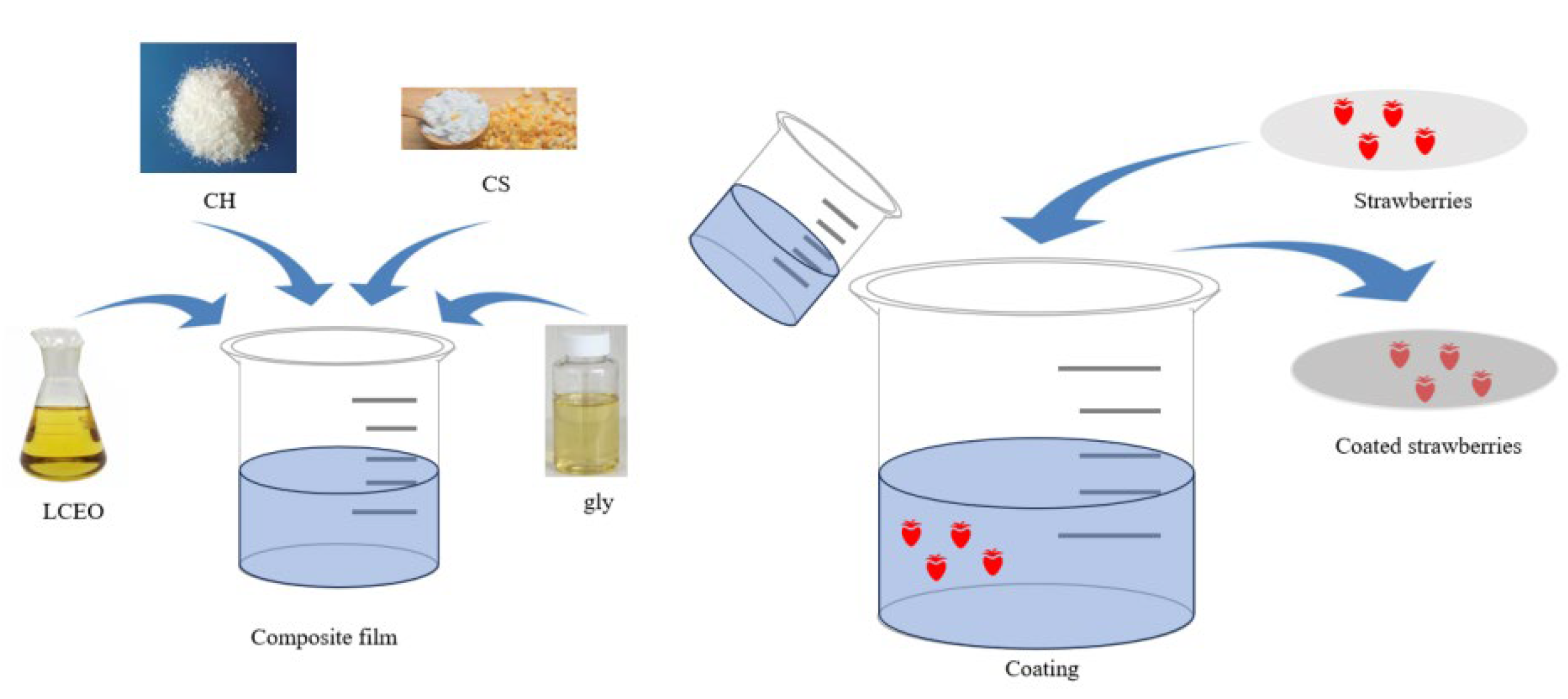
2.2. LCEO/CH/CS/gly Film-Forming Process
2.3. Physical and Mechanical Properties of Films
2.3.1. Thickness
2.3.2. Mechanical Properties
2.3.3. Water Vapor Permeability (WVP)
2.3.4. Determination of Water-Solubility (WS)
2.4. Treatment of Strawberries
2.5. Measurement of Physiological Indices of Strawberries
2.5.1. Decay Rate
2.5.2. Firmness
2.5.3. Weight Loss
2.5.4. Reducing Sugars
2.5.5. Ascorbic Acid and Anthocyanin Content
2.5.6. Respiratory Intensity
2.5.7. MDA
2.6. Characterization of the Coating
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of LCEO/CH/CS/gly Film
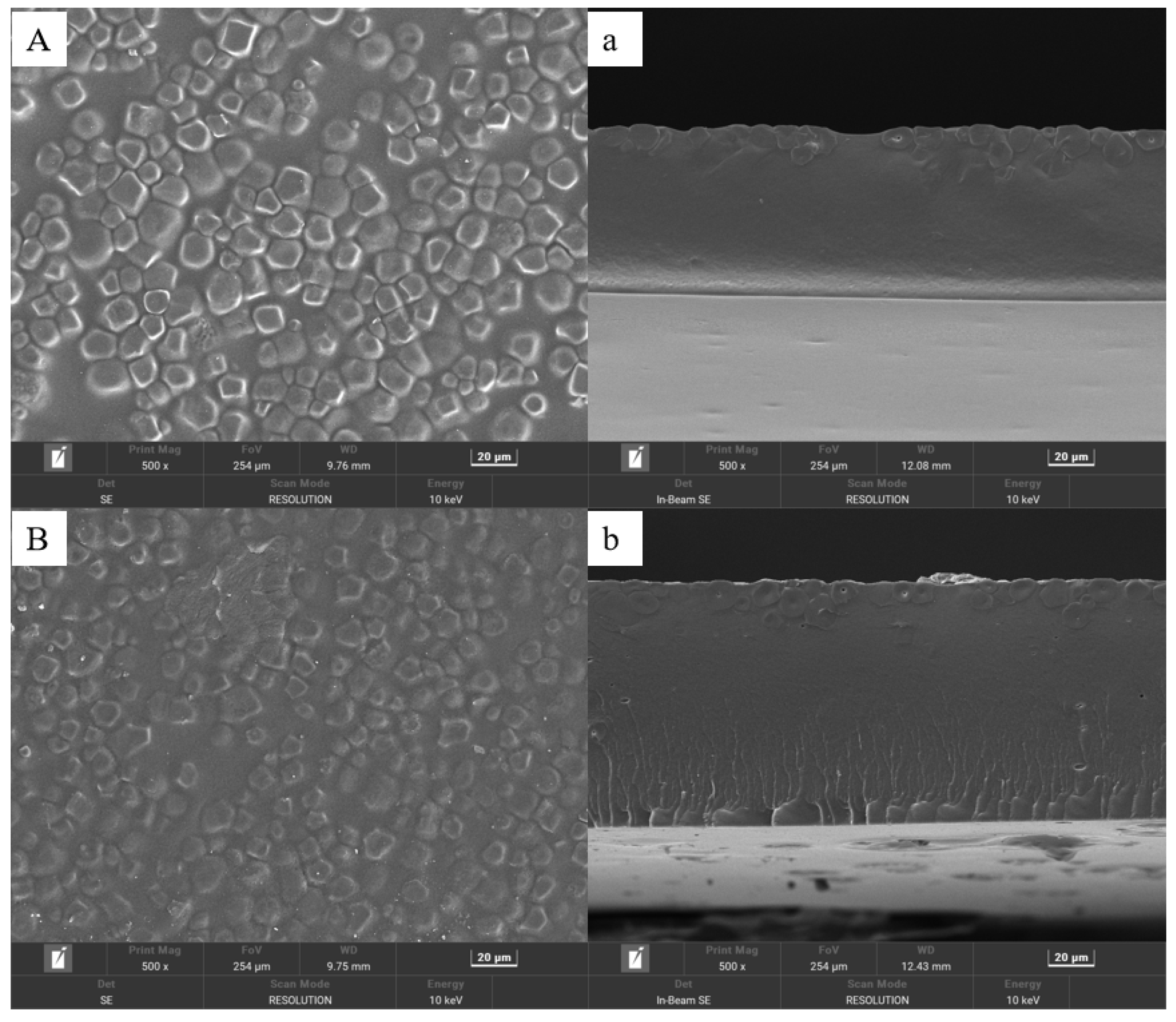
3.1.1. Surface Morphology by SEM
3.1.2. Structural and Thermal Analysis
3.2. Physical and Mechanical Properties of Films
3.3. Application of the Films in the Preservation of Strawberry
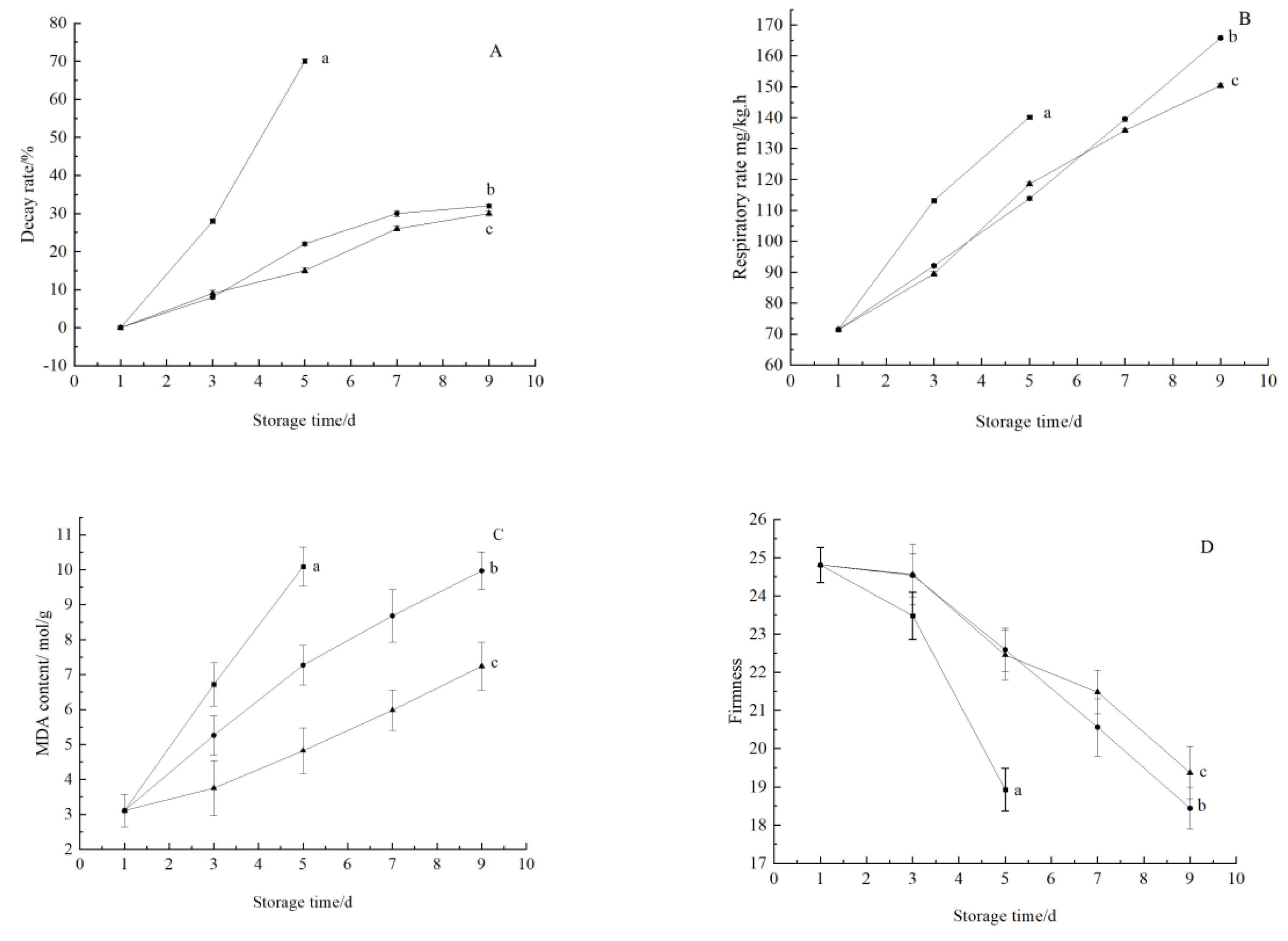
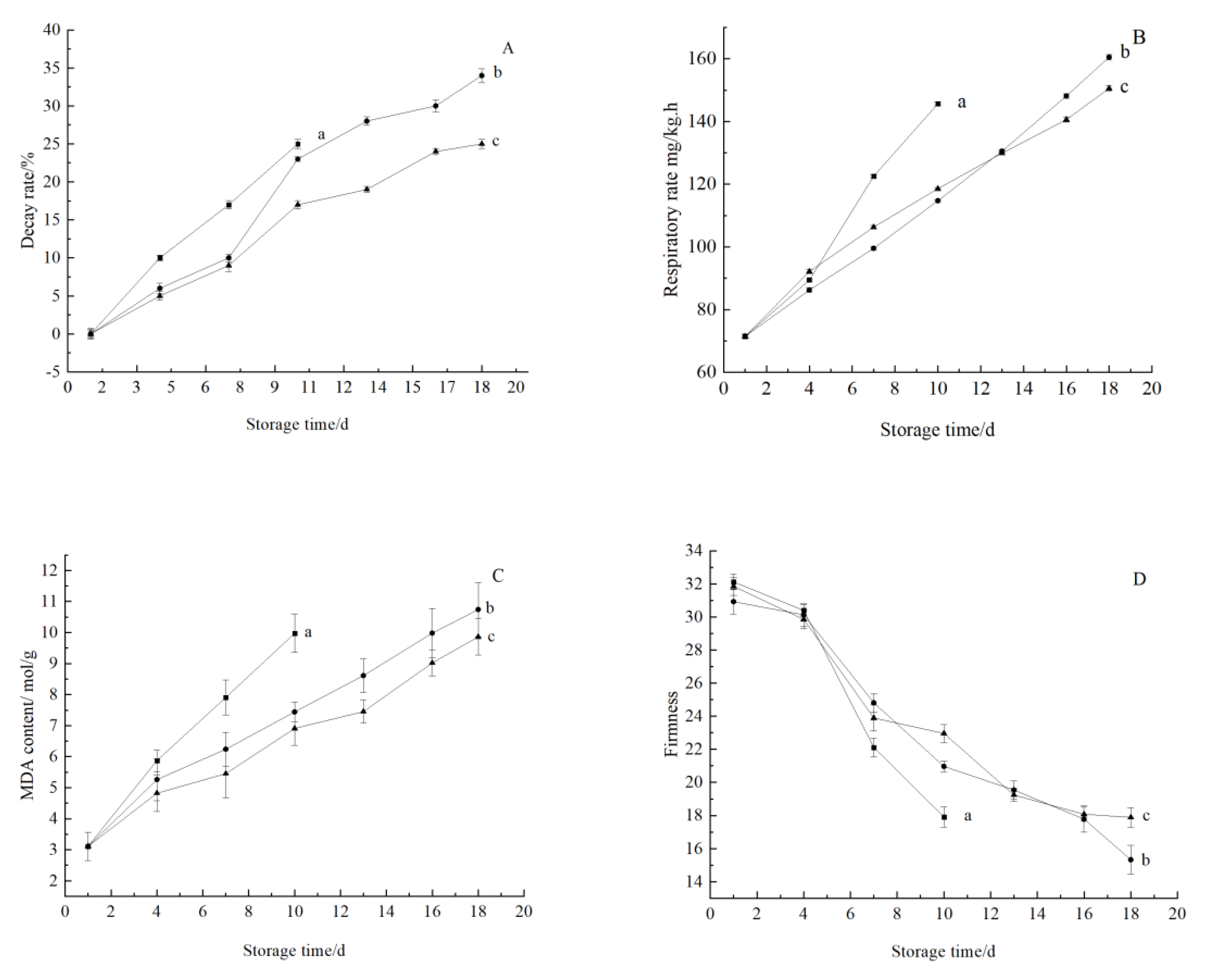
3.3.1. Change in Indexes Related to Maturity and Aging
Change in Decay Rate
Changes in Respiratory Intensity
Changes in MDA Concentration
Changes in Firmness
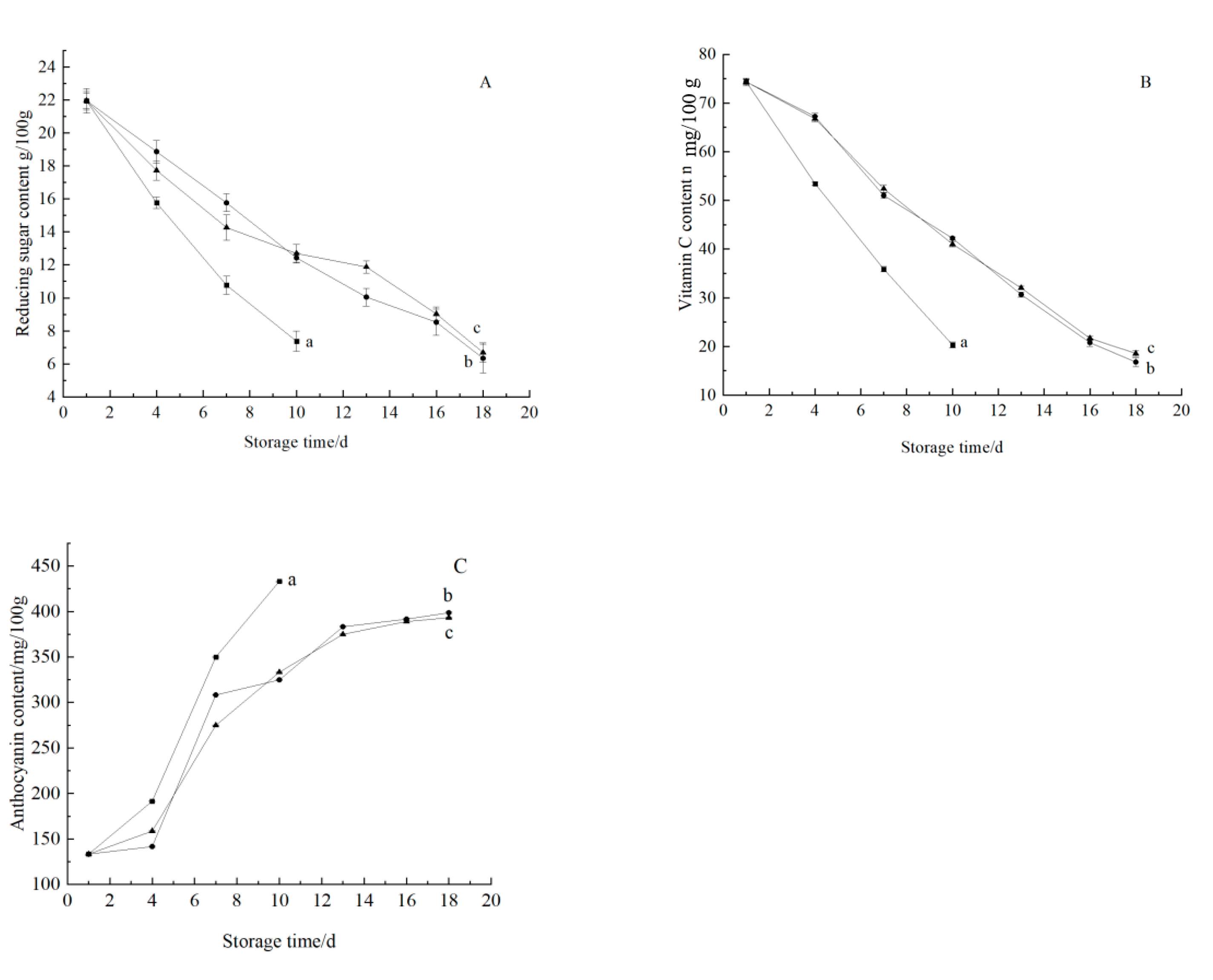
3.3.2. Change in Nutritional Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalbandi, H.; Seiiedlou, S. Sensitivity analysis of the precooling process of strawberry: Effect of package designing parameters and the moisture loss. Food Sci. Nutr. 2020, 8, 2458–2471. [Google Scholar] [CrossRef] [PubMed]
- Global Strawberry Market Continues to Expand, Report Finds. 2018. Available online: https://www.freshfruitportal.com/news/2018/06/04/global-strawberry-market-continues-to-expand-report-finds/ (accessed on 1 January 2020).
- Sicari, V.; Loizzo, M.R.; Pellicano, T.M.; Giuffre, A.M.; Poiana, M. Evaluation of aloe arborescens gel as new coating to maintain the organoleptic and functional properties of strawberry (Fragaria × ananassa cv. Cadonga) fruits. Int. J. Food Sci. Technol. 2020, 55, 861–870. [Google Scholar] [CrossRef]
- Del Carmen Martinez-Gonzalez, M.; Bautista-Banos, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Rio-Garcia, J.C.; de Lorena Ramos-Garcia, M. Effect of nanostructured chitosan/propolis coatings on the quality and antioxidant capacity of strawberries during storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Du, Y.; Yang, F.; Yu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Xie, Y. Fabrication of novel self-healing edible coating for fruits preservation and its performance maintenance mechanism. Food Chem. 2021, 351, 129284. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.E.; Llalla, O.; Garcia-Risco, M.R.; Fornari, T. Comparison between essential oils and supercritical extracts into chitosan- based edible coatings on strawberry quality during cold storage. J. Supercrit. Fluids 2021, 171, 105198. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Neeraj; Petkoska, A.T.; Al-Hilifi, S.A.; Fawole, O.A. Effect of chitosan-pullulan composite edible coating functionalized with pomegranate peel extract on the shelf life of mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Petkoska, A.T.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan edible films enhanced with pomegranate peel extract: Study on physical, biological, thermal, and barrier properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Gavara, R.; Hernández-Muñoz, P. Chitosan films as pH-responsive sustained release systems of naturally occurring antifungal volatile compounds. Carbohydr. Polym. 2022, 283, 119137. [Google Scholar] [CrossRef]
- Xia, S.; Lin, H.; Zhu, P.; Wang, P.; Liao, S.; Chen, S.; Wang, Z.; Fan, G. Inhibitory effects of Litsea cubeba oil and its active components on Aspergillus flavus. J. Food Qual. 2020, 2020, 8843251. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli o157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.; Ye, R.; Liu, A.; Xiao, J.; Liu, Y.; Zhao, Y. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Kingwascharapong, P.; Tanaka, F.; Tanaka, F. Effect of edible coatings developed from chitosan incorporated with tea seed oil on japanese pear. Sci. Hortic. 2021, 288, 110314. [Google Scholar] [CrossRef]
- Wang, S.; Jing, Y. Effects of formation and penetration properties of biodegradable montmorillonite/chitosan nanocomposite film on the barrier of package paper. Appl. Clay Sci. 2017, 138, 74–80. [Google Scholar] [CrossRef]
- Li, D.; Ye, Q.; Jiang, L.; Luo, Z. Effects of nano-tio2-ldpe packaging on postharvest quality and antioxidant capacity of strawberry (Fragaria ananassa duch.) stored at refrigeration temperature. J. Sci. Food Agric. 2017, 97, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-cmc electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef]
- Rana, S.; Metha, D.; Bansal, V.; Shivhare, U.S.; Yadav, S.K. Atmospheric cold plasma (acp) treatment improved in-package shelf-life of strawberry fruit. J. Food Sci. Technol 2020, 57, 102–112. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Madushan, R.; Vidanarachchi, J.K.; Prasanna, P.H.P.; Werellagama, S.; Priyashantha, H. Use of natural plant extracts as a novel microbiological quality indicator in raw milk: An alternative for resazurin dye reduction method. LWT-Food Sci. Technol. 2021, 144, 111221. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Tsai, C.-H.; Wu, W.-T.; Chen, F.-Z.; Chiang, P.-J. Numerical investigation into thermal effects of pre-cooling zone in vitrification-based cryopreservation process. Cryobiology 2015, 70, 32–37. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Yu, L.; Hu, Y.W.; Zhu, Z.C.; Zhuang, C.J.; Zhao, Y.Y.; Zhong, Y. Electrostatic spraying of chitosan coating with different deacetylation degree for strawberry preservation. Int. J. Biol. Macromol. 2019, 139, 1232–1238. [Google Scholar] [CrossRef]
- Zhao, S.S.; Jia, R.Y.; Yang, J.; Dai, L.; Ji, N.; Xiong, L.; Sun, Q.J. Development of chitosan/tannic acid/corn starch multifunctional bilayer smart films as pH-responsive actuators and for fruit preservation. Int. J. Biol. Macromol. 2022, 205, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Guan, C.; Zhou, C.; Pan, Q.Y.; He, Z.Y.; Wang, C.; Liu, Y.H.; Song, S.H.; Yu, L.J.; Qu, Y.H.; et al. Amphiphilic chitosan/carboxymethyl gellan gum composite films enriched with mustard essential oil for mango preservation. Carbohydr. Polym. 2023, 300, 120290. [Google Scholar] [CrossRef]
- Khruengsai, S.; Phoopanasaeng, P.; Sripahco, T.; Soykeabkaew, N.; Pripdeevech, P. Application of chitosan films incorporated with Zanthoxylum limonella essential oil for extending shelf life of pork. Int. J. Biol. Macromol. 2024, 129703, in press. [Google Scholar] [CrossRef] [PubMed]
- Go, E.J.; Song, K.B. Effect of java citronella essential oil addition on the physicochemical properties of Gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Abarca, R.L.; Rodriguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, B.T.T.; Nguyen, D.V.; Phung, T.K. Development and characterization of antibacterial packaging film based on poly (vinyl alcohol)/agarose enriched with cinnamon oil. J. Food Saf. 2023, 43, e13081. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Chen, J.; Lin, L.; Wan, C. Possible fungicidal effect of citral on kiwifruit pathogens and their mechanisms of actions. Physiol. Mol. Plant Pathol. 2021, 114, 101631. [Google Scholar] [CrossRef]
- Thielmann, J.; Theobald, M.; Wutz, A.; Krolo, T.; Buergy, A.; Niederhofer, J.; Welle, F.; Muranyi, P. Litsea cubeba fruit essential oil and its major constituent citral as volatile agents in an antimicrobial packaging material. Food Microbiol. 2021, 96, 103725. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, W.; Fu, B.; Fu, M.; Ren, Q.; Yang, F.; Qin, C. Physical, antibacterial and antioxidant properties of chitosan films containing hardleaf oatchestnut starch and Litsea cubeba oil. Int. J. Biol. Macromol. 2018, 118, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.; Linke, M.; Geyer, M.; Mahajan, P.V. Shelf life prediction model for strawberry based on respiration and transpiration processes. Food Packag. Shelf Life 2020, 25, 100525. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Zhang, D.; Dong, Y.; Meng, Q.; Zhu, S.; Zhang, L. Strigolactone maintains strawberry quality by regulating phenylpropanoid, no, and h2s metabolism during storage. Postharvest Biol. Technol. 2021, 178, 111546. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; An, X.; Zheng, M.; Lu, Z.; Lu, F.; Zhang, C. Preparation of ferulic acid-grafted chitosan using recombinant bacterial laccase and its application in mango preservation. RSC Adv. 2018, 8, 6759–6767. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, K.A.; Fawole, O.A. Effects of chitosan coatings fused with medicinal plant extracts on postharvest quality and storage stability of purple passion fruit (Passiflora edulis var. Ester). Food Qual. Saf. 2022, 6, fyac016. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Q.; Zhou, Y. Strawberry leaf extract treatment alleviates cognitive impairment by activating nrf2/ho-1 signaling in rats with streptozotocin-induced diabetes. Front. Aging Neurosci. 2020, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Seal, T.; Chaudhuri, K.; Pillai, B.; Chakrabarti, S.; Mondal, T.; Auddy, B. Evaluation of antioxidant activities, toxicity studies and the DNA damage protective effect of various solvent extracts of Litsea cubeba fruits. Heliyon 2020, 6, e03637. [Google Scholar] [CrossRef]
- Leerach, N.; Yakaew, S.; Phimnuan, P.; Soimee, W.; Nakyai, W.; Luangbudnark, W.; Viyoch, J. Effect of thai banana (Musa aa group) in reducing accumulation of oxidation end products in uvb-irradiated mouse skin. J. Photochem. Photobiol. B-Biol. 2017, 168, 50–58. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT-Food Sci. Technol. 2021, 140, 110809. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Luo, F.; He, H.; Yuan, H.; Gao, J.; Zeng, X.; Huang, C. Use of controlled atmospheres to maintain postharvest quality and improve storage stability of a novel red-fleshed kiwifruit (Actinidiachinensis Planch. var. rufopulpa cf liang et rh huang cf liang et ar ferguson). J. Food Process. Preserv. 2015, 39, 907–914. [Google Scholar] [CrossRef]
- Matar, C.; Salou, T.; Helias, A.; Penicaud, C.; Gaucel, S.; Gontard, N.; Guilbert, S.; Guillard, V. Benefit of modified atmosphere packaging on the overall environmental impact of packed strawberries. Postharvest Biol. Technol. 2021, 177, 111521. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, M.; Bhandari, B.; Guo, Z. Combined effects of microporous packaging and nano-chitosan coating on quality and shelf-life of fresh-cut eggplant. Food Biosci. 2021, 43, 101302. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.-Y.; Jeong, E.J.; Cha, S.W.; Kim, B.G.; Kerr, W.L.; Choi, S.-G. Effect of vacuum-grinding on the stability of anthocyanins, ascorbic acid, and oxidative enzyme activity of strawberry. LWT-Food Sci. Technol. 2021, 136, 110304. [Google Scholar] [CrossRef]
- Zha, J.; Koffas, M.A.G. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017, 2, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, E.; Chaurand, P.; Raghavan, V. Visualizing the distribution of strawberry plant metabolites at different maturity stages by maldi-tof imaging mass spectrometry. Food Chem. 2021, 345, 128838. [Google Scholar] [CrossRef]
- Dziedzic, E.; Blaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopec, A. Effect of modified (map) and controlled atmosphere (ca) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Wang, L.; Gu, J.; Peng, X.; Zhao, J. Effects of Litsea cubeba Essential Oil–Chitosan/Corn Starch Composite Films on the Quality and Shelf-Life of Strawberry (Fragaria × ananassa). Foods 2024, 13, 599. https://doi.org/10.3390/foods13040599
Fu H, Wang L, Gu J, Peng X, Zhao J. Effects of Litsea cubeba Essential Oil–Chitosan/Corn Starch Composite Films on the Quality and Shelf-Life of Strawberry (Fragaria × ananassa). Foods. 2024; 13(4):599. https://doi.org/10.3390/foods13040599
Chicago/Turabian StyleFu, Hongjun, Liyuan Wang, Jiahui Gu, Xianglian Peng, and Jian Zhao. 2024. "Effects of Litsea cubeba Essential Oil–Chitosan/Corn Starch Composite Films on the Quality and Shelf-Life of Strawberry (Fragaria × ananassa)" Foods 13, no. 4: 599. https://doi.org/10.3390/foods13040599
APA StyleFu, H., Wang, L., Gu, J., Peng, X., & Zhao, J. (2024). Effects of Litsea cubeba Essential Oil–Chitosan/Corn Starch Composite Films on the Quality and Shelf-Life of Strawberry (Fragaria × ananassa). Foods, 13(4), 599. https://doi.org/10.3390/foods13040599






