Phytochemical, Nutritional and Mineral Content of Four Edible Flowers
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Plant Material and Cultivation
2.3. Biochemical Analyses
2.3.1. Soluble Sugars (D-Glucose, D-Fructose, Sucrose) Determination
2.3.2. Organic Nitrogen, Macro- and Microelement Quantification
2.3.3. Secondary Metabolites and Radical Scavenging Activity (DPPH Assay)
2.3.4. Determination of the Vitamin B1, B2, B3, B9, and C Content
2.4. Volatile Organic Compounds Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soluble Sugars, Total Crude Protein and Mineral Composition
3.2. Antioxidant Compounds and Radical Scavenger Activity
3.3. Water-Soluble Vitamins (B1, B2, B3, B9, C)
3.4. Volatile Organic Compound Analyses
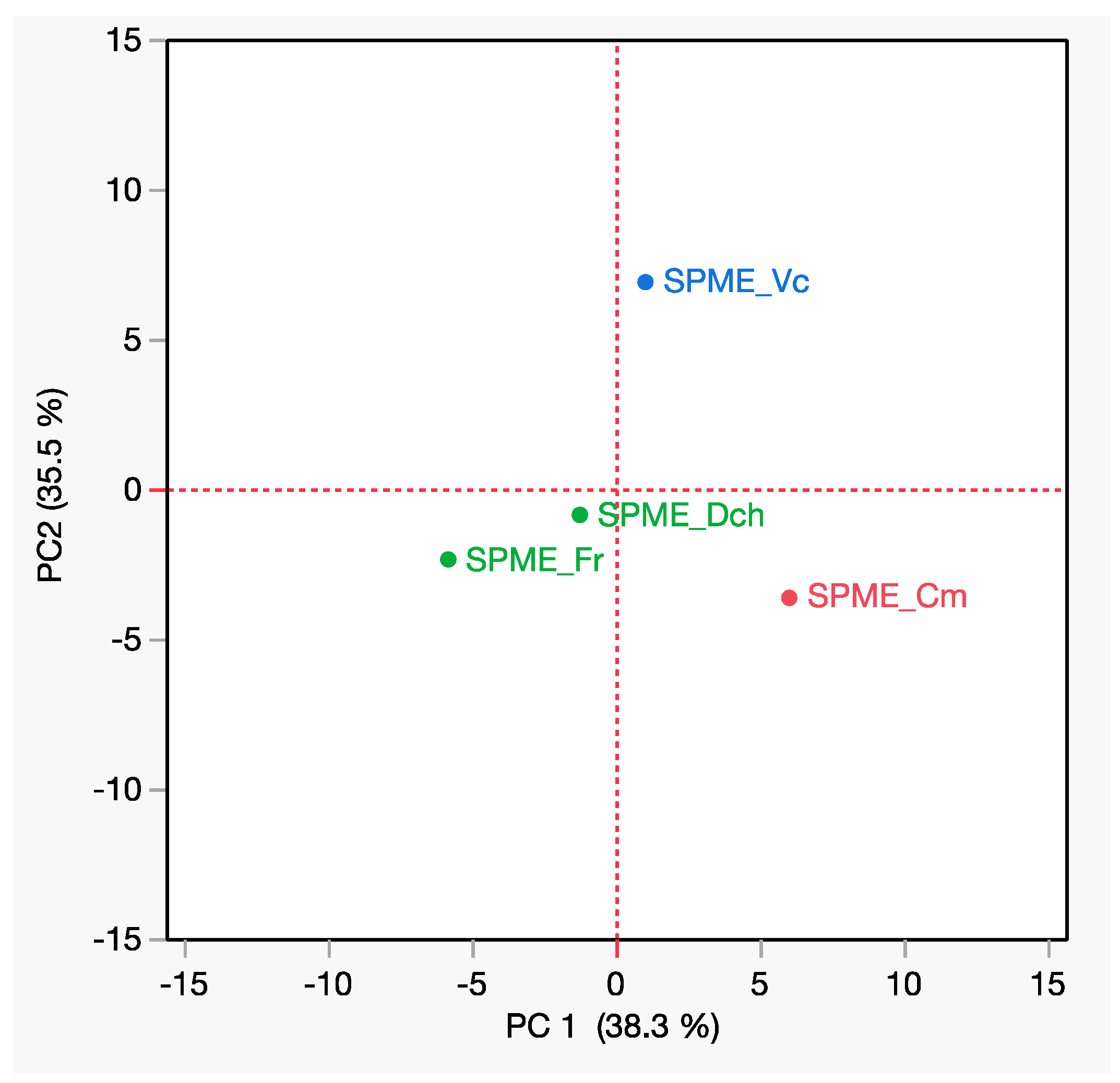
3.5. Multivariate Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amrouche, T.A.; Yang, X.; Capanoglu, E.; Huang, W.; Chen, Q.; Wu, L.; Zhu, Y.; Liu, Y.; Lu, B. Contribution of edible flowers to the Mediterranean diet: Phytonutrients, bioactivity evaluation and applications. Food Front. 2022, 3, 592–630. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The contribution of wild edible plants to the Mediterranean diet: An ethnobotanical case study along the coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Lara-Cortés, E.; Osorio-Díaz, P.; Jiménez-Aparicio, A.; Bautista-Bañios, S. Nutritional content, functional properties and conservation of edible flowers. Rev. Arch. Latinoam. Nutr. 2013, 63, 197–208. [Google Scholar]
- Bohra, M.; Visen, A. Research Anthology on Recent Advancements in Ethnopharmacology and Nutraceuticals. In Nutraceutical Properties in Flowers; Bohra, M., Visen, A., Eds.; IGI Global: Hershey, PA USA, 2022; pp. 1036–1054. [Google Scholar] [CrossRef]
- Santini, A. Nutraceuticals and functional foods: Is it possible and sustainable for bridging health and food? Foods 2022, 11, 1608. [Google Scholar] [CrossRef]
- Prabawati, N.B.; Oktavirina, V.; Palma, M.; Setyaningsih, W. Edible flowers: Antioxidant compounds and their functional properties. Horticulturae 2021, 7, 66. [Google Scholar] [CrossRef]
- Paris, H.S.; Janick, J. Early evidence for the culinary use of squash flowers in Italy. Chron. Hortic. 2005, 45, 20–21. [Google Scholar]
- Halder, S.; Khaled, K.L. Quantitative estimation of mineral content from edible flowers of Allium cepa, Cucurbita maxima and Carica papaya: A comparative study. Int. J. Pharm. Sci. Res. 2022, 13, 2116–2124. [Google Scholar] [CrossRef]
- Paris, H.S. Summer squash. In Vegetables I. Handbook of Plant Breedin; Prohen, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1, pp. 351–379. [Google Scholar] [CrossRef]
- Mulík, S.; Ozuna, C. Mexican edible flowers: Cultural background, traditional culinary uses, and potential health benefits. Inter. J. Gastr. Food Sci. 2020, 21, 100235. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jungran, A.K.; et al. Cucurbita plants: From farm to industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Nutraceutical potential of dietary phytochemicals in edible flowers—A review. J. Food Biochem. 2021, 45, e13642. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Inter. 2020, 129, 108868. [Google Scholar] [CrossRef]
- Bacher, M.; Sweets, M.B. Cooking with Flowers: Sweet and Savory Recipes with Rose Petals, Lilacs, Lavender, and Other Edible Flowers; Quirk Books: Philadelphia, PA, USA, 2013; ISBN 9781594746253. [Google Scholar]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, nutritional and sensory characteristics of six ornamental edible flowers species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Kandylis, P. Phytochemicals and antioxidant properties of edible flowers. Appl. Sci. 2022, 12, 9937. [Google Scholar] [CrossRef]
- Lim, T.K. Fuchsia × hybrida. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 548–551. [Google Scholar] [CrossRef]
- Webby, R.; Bloor, S. Pigments in the blue pollen and bee pollen of Fuchsia excorticata. Z. Naturforschung C 2000, 55, 503–505. [Google Scholar] [CrossRef]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Marchioni, I.; Colla, L.; Pistelli, L.; Ruffoni, B.; Tinivella, F.; Minuto, G. Different growing conditions can modulate metabolites content during post-harvest of Viola cornuta L. edible flowers. Adv. Hortic. Sci. 2020, 34, 61–69. [Google Scholar] [CrossRef]
- Gonçalves, A.F.K.; Friedrich, R.B.; Boligon, A.A.; Piana, M.; Beck, R.C.R.; Athayde, M.L. Antioxidant capacity, total phenolic contents and HPLC determination of rutin in Viola tricolor L. flowers. Free Radic. Antioxid. 2012, 2, 32–37. [Google Scholar] [CrossRef]
- da Silva, L.A.; Fischer, S.Z.; Zambiazi, R.C. Proximal composition, bioactive compounds content and color preference of Viola × wittrockiana flowers. Int. J. Gastron. Food Sci. 2020, 22, 100236. [Google Scholar] [CrossRef]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic analysis of four edible flowers from Agastache genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Tobias, R.B.; Boyer, C.D.; Shannon, J.C. Alterations in carbohydrate intermediates in the endosperm of starch-deficient maize (Zea mays L.) genotypes. Plant Physiol. 1992, 99, 146–152. [Google Scholar] [CrossRef]
- Jones, J.R.; Benton, J.; Wolf, B.; Mills, H.A. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro International: Athens, GA, USA, 1991; ISBN 101878148001. [Google Scholar]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Carmassi, G.; Serra, G. Spatial and temporal distribution of mineral nutrients and sugars throughout the lifespan of Hibiscus rosa-sinensis L. flower. Open Life Sci. 2011, 6, 365–375. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, E.L. Phosphorus. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Plaza, B.M.; Carmassi, G.; Diara, C.; Pardossi, A.; Lao, M.T.; Jiménez-Becker, S. Effects of fertigation with untreated and treated leachates from municipal solid waste on the microelement status and biometric parameters of Viola × wittrockiana. Agronomy 2021, 11, 186. [Google Scholar] [CrossRef]
- Marchioni, I.; Pistelli, L.; Ferri, B.; Cioni, P.; Copetta, A.; Pistelli, L.; Ruffoni, B. Preliminary studies on edible saffron bio-residues during different post-harvest storages. Bulg. Chem. Commun 2019, 51, 131–136. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-Carotene) of Okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 831357. [Google Scholar] [CrossRef]
- Aslam, J.; Mohajir, M.; Khan, S.; Khan, A. HPLC analysis of water-soluble vitamins (B1, B2, B3, B5, B6) in in vitro and ex vitro germinated chickpea (Cicer arietinum L.). Afr. J. Biotechnol. 2008, 7, 2310–2314. [Google Scholar]
- Marzougui, N.; Guasmi, F.; Mounira, M.; Boubaya, A.; Mrabet, A.; Elfalleh, W.; Ferchichi, A.; Beji, M. Assessment of Tunisian Trigonella foenum graecum diversity using seed vitamin B6, B1, B9 and C contents. J. Food Agric. Envir. 2009, 7, 56–61. [Google Scholar]
- Kampfenkel, K.; Montagu, M.V.; Inzé, D. Extraction and determination of ascorbate e dehydroascorbate from plant tissue. Anal. Chem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Degl’innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. Var. Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Chen, N.H.; Wei, S. Factors influencing consumers’ attitudes towards the consumption of edible flowers. Food Qual. Prefer. 2017, 56, 93–100. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Lee, A.A.; Owyang, C. Sugars, sweet taste receptors, and brain responses. In Molecular Nutrition: Carbohydrates; Academic Press: Cambridge, MA, USA, 2019; pp. 265–283. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological value of various edible flower species. Acta Sci. Pol. Hortorum Cultus 2016, 15, 109–119. [Google Scholar]
- Stefaniak, A.; Grzeszczuk, M. Nutritional and Biological Value of Five Edible Flower Species. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 128–134. [Google Scholar] [CrossRef]
- Bieżanowska-Kopeć, R.; Ambroszczyk, A.M.; Piątkowska, E.; Leszczyńska, T. Nutritional value and antioxidant activity of fresh pumpkin flowers (Cucurbita sp.) grown in Poland. Appl. Sci. 2022, 12, 6673. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nut. 2007, 62, 133–138. [Google Scholar] [CrossRef]
- Toro-Vélez, K.; Chávez-Jáuregui, R.; Wessel-Beaver, L.; Brunner, B. Production and postharvest assessment of tropical pumpkin flowers harvested for consumption. HortTechnology 2022, 32, 199–212. [Google Scholar] [CrossRef]
- Ghosh, P.; Rana, S.S. Physicochemical, nutritional, bioactive compounds and fatty acid profiling of pumpkin flower (Cucurbita maxima), as a potential functional food. SN Appl. Sci. 2021, 3, 216. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical contents and medical importance of Dianthus caryophyllus—A review. IOSR J. Pharm. 2017, 7, 61–71. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H.; Hassan, S.; Gul, F. Evaluation of Viola betonicifolia for its nutrition value. Pak. J. Pharm. Sci. 2012, 25, 1–6. [Google Scholar]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterization of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015, 16, 805–822. [Google Scholar] [CrossRef]
- Salehi, F. Recent applications of powdered fruits and vegetables as novel ingredients in biscuits: A review. Nutrire 2020, 45, 1. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Araújo, S.; Matos, C.; Correia, E.; Antunes, M.C. Evaluation of phytochemicals content, antioxidant activity and mineral composition of selected edible flowers. Qual Assur. Saf. Crops Foods 2019, 11, 471–478. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.F.; Li, D.Y.; Chen, Y.C.; Zhao, L.J.; Liu, X.G.; Guo, Y.F.; Shen, J.; Lin, X.; Deng, J.; et al. The good, the bad, and the ugly of calcium supplementation: A review of calcium intake on human health. Clin. Interv. Aging 2018, 13, 2443. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Dietary change scenarios and implications for environmental, nutrition, human health and economic dimensions of food sustainability. Nutrients 2019, 11, 856. [Google Scholar] [CrossRef]
- Publication Office of European Union Regulation (EU). No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Off. J. Eur. Union 2011, 54, 18–63. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2011:304:FULL&from=EN (accessed on 8 August 2022).
- Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Brito, C.; Rodrigues, A.C.; de Carvalho, C.C.; Mortimer, M.M.; Moreno, M.J.; Brito, R.M.; et al. Edible flowers of Helichrysum italicum: Composition, nutritive value, and bioactivities. Food Res. Inter. 2022, 157, 111399. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Benítez, A.; Corell, M.; Hernanz, D.; Mapelli-Brahm, P.; Stinco, C.; Coyago-Cruz, E. Screening for innovative sources of carotenoids and phenolic antioxidants among flowers. Foods 2021, 10, 2625. [Google Scholar] [CrossRef] [PubMed]
- Chensom, S.; Okumura, H.; Mishima, T. Primary screening of antioxidant activity, total polyphenol content, carotenoid content, and nutritional composition of 13 edible flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wildflowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Gonçalves, F.; Gonçalves, J.C.; Ferrão, A.C.; Correia, P.; Guiné, R.P.F. Evaluation of phenolic compounds and antioxidant activity in some edible flowers. Open Agric. 2020, 5, 857–870. [Google Scholar] [CrossRef]
- Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O. Edible flowers—Antioxidant activity and impact on cell viability. Open Life Sci. 2013, 8, 1023–1031. [Google Scholar] [CrossRef]
- Khatib, M.; Pouzet, C.; Lafitte, C.; Chervin, J.; Bonzon-Ponnet, V.; Jauneau, A.; Esquerré-Tugayé, M.T. Phenolic profile of a Parma violet unveiled by chemical and fluorescence imaging. AoB Plants 2021, 13, plab041. [Google Scholar] [CrossRef]
- Socha, R.; Kałwik, J.; Juszczak, L. Phenolic profile and antioxidant activity of the selected edible flowers grown in Poland. Acta Univ. Cibiniensis Ser. E Food Technol. 2021, 25, 185–200. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Urrutia-Hernández, T.A.; López Del Castillo-Lozano, M.; Chavéz-Servia, J.L.; Verdalet-Guzmán, I. Physicochemical parameters and antioxidant compounds in edible squash (Cucurbita pepo) flower stored under controlled atmospheres. J. Food Qual. 2013, 36, 302–308. [Google Scholar] [CrossRef]
- Morittu, V.M.; Musco, N.; Mastellone, V.; Bonesi, M.; Britti, D.; Infascelli, F.; Loizzo, M.R.; Tundis, R.; Sicari, V.; Tudisco, R.; et al. In vitro and in vivo studies of Cucurbita pepo L. flowers: Chemical profile and bioactivity. Nat. Prod. Res. 2019, 35, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Nadot, S.; Carrive, L. The colourful life of flowers. Bot. Lett. 2020, 168, 120–130. [Google Scholar] [CrossRef]
- Farzad, M.; Griesbach, R.; Hammond, J.; Weiss, M.R.; Elmendorf, H.G. Differential expression of three key anthocyanin biosynthetic genes in a color-changing flower, Viola cornuta cv. Yesterday, Today and Tomorrow. Plant Sci. 2003, 165, 1333–1342. [Google Scholar] [CrossRef]
- Lim, T.K. Viola × wittrockiana. In Edible Medicinal and Non Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 818–821. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitao, K.; Watanabe, T.; Yamagaki, T. Imaging mass spectrometry analysis of flavonoids in blue viola petals and their enclosure effects on violanin during color expression. Anal. Chem. 2019, 91, 896–902. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Li, T.; Zhao, Y.; Zhou, Y.; Shi, Y.; Peng, T.; Song, X.; Zhu, Z.; Wang, J. Comparative Transcriptome Analysis of Anthocyanin Biosynthesis in Pansy (Viola × wittrockiana Gams.). Agronomy 2022, 12, 919. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Baranska, M.; Schulz, H.; Heiselmayer, P.; Musso, M. Discrimination of carotenoid and flavonoid content in petals of pansy cultivars (Viola × wittrockiana) by FT-Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 1240–1247. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Baptista, P.; Pereira, J.A.; Saraiva, J.A.; Casal, S.I. Nutritional and nutraceutical composition of pansies (Viola × wittrockiana) during flowering. J. Food Sci. 2019, 84, 490–498. [Google Scholar] [CrossRef]
- Said, H.M. Water-soluble vitamins. In Nutrition for the Primary Care Provider; Bier, D., Ed.; Karger: Basel, Switzerland, 2015; pp. 30–37. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Mariotti, F. Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier Academic Press: London, UK, 2017; ISBN 9780128039687. [Google Scholar]
- Publication Office of European Union Regulation (EU). No 1168/2011 of the European Parliament and of the Council of 25 October 2011 Amending Council Regulation (EC). No 2007/2004 Establishing a European Agency for the Management of Operational Cooperation at the External Borders of the Member States of the European Union. Off. J. Eur. Union 2011, 54, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2011:304:FULL&from=EN (accessed on 8 August 2022).
- Lim, T.K. Cucurbita pepo. In Edible Medicinal and Non Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; pp. 755–763. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Rodrigues, S.; Cárcel, J.A.; García-Pérez, J.V. Ultrasound-assisted air-drying of apple (Malus domestica L.) and its effects on the vitamin of the dried product. Food Bioprocess Technol. 2015, 8, 1503–1511. [Google Scholar] [CrossRef]
- Velvizhi, M.; Sujatha, T.; Mohankumar, J.B. Effect of cooking on the phytonutrient content of selected edible flowers. Food Sci. Indian J. Res. Food Sci Nutri. 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Assi, O.Y.; Coulibaly, A.; Kouakou, P.; Konan, Y.N.G.; Chatigre, O.; Biego, H.G. Vitamins contents in edible parts of some mucilaginous food plants from Côte d’Ivoire. J. Adv. Biol. Biotechnol. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Sayeed, R.; Thakur, M.; Gani, A. Celosia cristata Linn. flowers as a new source of nutraceuticals-A study on nutritional composition, chemical characterization, and in vitro antioxidant capacity. Heliyon 2020, 6, e05792. [Google Scholar] [CrossRef]
- Debnath, B.; Manna, K. Ethnomedicinal information and High-Performance Liquid Chromatography analysis of water-soluble vitamins (C, B1, B3, B6, folic acid) and fat-soluble vitamins (A, D3, E) of three consumable parts of Musa paradisiaca: Cultivated in Tripura, India. Food Sci. Eng. 2021, 2, 31–37. [Google Scholar] [CrossRef]
- Luvonga, W.A.; Njoroge, M.S.; Makokha, A.; Ngunjiri, P.W. Chemical characterisation of Hibiscus sabdariffa (Roselle) calyces and evaluation of its functional potential in the food industry. Sci. Conf. Proc. 2012, 1, 631–638. [Google Scholar]
- Patel, M.; Naik, S.N. Flowers of Madhuca indica JF Gmel.: Present status and future perspectives. Indian J. Nat. Prod. Res. 2010, 1, 438–443. [Google Scholar]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Fratianni, F. Health attributes of ten Mediterranean edible flowers: Anti-proliferative and enzyme-inhibitory properties. Trends Phytochem. Res. 2019, 3, 251–260. [Google Scholar]
- Landi, M.; Ruffoni, B.; Combournac, L.; Guidi, L. Nutraceutical value of edible flowers upon cold storage. Ital. J. Food Sci. 2018, 30, 336–347. [Google Scholar] [CrossRef]
- Fernandes, L.; Saraiva, J.A.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Post-harvest technologies applied to edible flowers: A review: Edible flowers preservation. Food Rev. Int. 2019, 35, 132–154. [Google Scholar] [CrossRef]
- Mena Granero, A.; Egea Gonzalez, F.J.; Guerra Sanz, J.M.; Martínez Vidal, J.L. Analysis of biogenic volatile organic compounds in zucchini flowers: Identification of scent sources. J. Chem Ecol. 2005, 31, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Musse, M.A. The Effect of Experimentally Induced Drought on the Production of Volatile Organic Compounds in Squash (Cucurbita pepo) Flowers. Master Thesis, University of California San Diego, La Jolla, CA, USA, 2019. [Google Scholar]
- Muhlemann, J.K.; Kaplan, I. Biosynthesis, function, and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Dhenge, R.; Rinaldi, M.; Ganino, T.; Santi, S.; Ferrarese, I.; Dall’Acqua, S. Variations of polyphenols, sugars, carotenoids, and volatile constituents in pumpkin (Cucurbita moschata) during high pressure processing: A kinetic study. Innov. Food Sci. Emerg. Technol. 2022, 78, 103005. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, Y.; Luo, J.; Wang, W.; Wu, H.; Liu, X.; Zhao, G.; Gong, H.; Zheng, X.; Zhong, Y. Volatiles and transcriptome profiling revealed the formation of ‘Taro-like’ aroma in the leaf of pumpkin (Cucurbita moschata). Agronomy 2022, 12, 2641. [Google Scholar] [CrossRef]
- Jürgens, A.; Witt, T.; Gottsberger, G. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem. Syst. Ecol. 2003, 31, 345–357. [Google Scholar] [CrossRef]
- Aliyazicioglu, R.; Demir, S.; Badem, M.; Sener, S.O.; Korkmaz, N.; Demi, E.A.; Ozgen, U.; Karaoglu, S.A.; Aliyazicioglu, Y. Antioxidant, antigenotoxic, antimicrobial activities and phytochemical analysis of Dianthus carmelitarum. Rec. Nat. Prod. 2017, 11, 270–284. [Google Scholar]
- Mecca, M.; Racioppi, R.; Romano, V.A.; Viggiani, L.; Lorenz, R.; D’Auria, M. Volatile organic compounds from Orchis species found in Basilicata (Southern Italy). Compounds 2021, 1, 83–93. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, L.; Tan, H.; Cheng, S.; Dong, F.; Yang, Z. Biochemical pathway of benzyl nitrile derived from l-phenylalanine in tea (Camellia sinensis) and its formation in response to postharvest stresses. J. Agric. Food Chem. 2020, 68, 1397–1404. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Zhang, D.; Wang, G.; Cao, F. Flowering stage and daytime affect scent emission of Malus ioensis “Prairie Rose”. Molecules 2019, 24, 2356. [Google Scholar] [CrossRef]
- Yan, T.; Lin, J.; Zhu, J.; Ye, N.; Huang, J.; Wang, P.; Jin, S.; Zheng, D.; Yang, J. Aroma analysis of Fuyun 6 and Jinguanyin black tea in the Fu’an area based on E-nose and GC–MS. Eur. Food Res. Technol. 2022, 248, 947–961. [Google Scholar] [CrossRef]
- Beghè, D.; Cirlini, M.; Beneventi, E.; Miroslav, Č.; Tatjana, P.; Ganino, T.; Petruccelli, R.; Dall’Asta, C. Volatile profile of Italian and Montenegrine pomegranate juices for geographical origin classification. Eur. Food Res. Technol. 2021, 247, 211–220. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Inter. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Dong, N.; Liu, S.; Yi, Z.; Zhang, Y. Chemical features of pericarpium Citri reticulatae and pericarpium Citri reticulatae Viride revealed by GC–MS metabolomics analysis. Food Chem. 2015, 186, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Besada, C.; Salvador, A.; Sdiri, S.; Gil, R.; Granell, A. A combination of physiological and chemometrics analyses reveals the main associations between quality and ripening traits and volatiles in two loquat cultivars. Metabolomics 2013, 9, 324–336. [Google Scholar] [CrossRef]
- Karagül-Yüceer, Y.; Drake, M.; Cadwallader, K.R. Aroma-active components of nonfat dry milk. J. Agric. Food Chem. 2001, 49, 2948–2953. [Google Scholar] [CrossRef]
- Van Holle, A.; Muylle, H.; Haesaert, G.; Naudts, D.; De Keukeleire, D.; Roldán-Ruiz, I.; Van Landschoot, A. Relevance of hop terroir for beer flavour. J. Inst. Brewing 2021, 127, 238–247. [Google Scholar] [CrossRef]
- Oyama-Okubo, N.; Tsuji, T. Analysis of floral scent compounds and classification by scent quality in tulip cultivars. J. Jpn Soc. Hortic. Sci. 2013, 82, 344–353. [Google Scholar] [CrossRef]
- Wang, L.F.; Lee, J.Y.; Chung, J.O.; Baik, J.H.; So, S.; Park, S.K. Discrimination of teas with different degrees of fermentation by SPME–GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Garzoli, S. GC-MS and SPME-GC/MS analysis and bioactive potential evaluation of essential oils from two Viola species belonging to the V. calcarata Complex. Separations 2022, 9, 39. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Y.; Jiang, Y. Determination of volatile components in different parts of Viola inconspicus in Giuzhou. Guizhou Agric. Sci. 2017, 45, 14–17. [Google Scholar]



| Species/Varieties | ||||
|---|---|---|---|---|
| C. moschata | D. chinensis | F. regia | V. cornuta | |
| D-glucose (mg/g FW) | 3.73 ± 0.35 b | 5.78 ± 0.10 a | 4.73 ± 0.45 ab | 1.26 ± 0.10 c |
| D-fructose (mg/g FW) | 2.44 ± 0.14 c | 4.74 ± 0.13 a | 3.77 ± 0.28 b | 0.62 ± 0.05 d |
| Sucrose (mg/g FW) | ND c | 1.36 ± 0.04 a | ND c | 0.33 ± 0.02 b |
| Species/Varieties | ||||
|---|---|---|---|---|
| C. moschata | D. chinensis | F. regia | V. cornuta | |
| Organic nitrogen (mg/g DW) | 23.15 ± 1.82 a | 15.50 ± 0.03 b | 9.75 ± 0.43 c | 20.07 ± 0.02 ab |
| Crude protein estimation (mg/g DW) | 144.69 ± 11.37 a | 96.88 ± 0.00 b | 60.94 ± 2.71 c | 129.41 ± 0.10 ab |
| Phosphorous (mg/g DW) | 5.95 ± 0.08 a | 3.94 ± 0.02 b | 3.55 ± 0.05 c | 4.18 ± 0.08 b |
| Potassium (mg/g DW) | 31.54 ± 0.76 a | 30.91 ± 0.45 a | 26.11 ± 0.54 b | 26.49 ± 0.73 b |
| Sodium (mg/g DW) | 0.15 ± 0.01 c | 0.67 ± 0.03 b | 2.07 ± 0.02 a | 0.24 ± 0.00 c |
| Calcium (mg/g DW) | 47.51 ± 2.20 b | 46.63 ± 2.13 b | 90.54 ± 1.65 a | 29.70 ± 1.02 c |
| Magnesium (mg/g DW) | 4.98 ± 0.05 a | 2.48 ± 0.11 d | 3.75 ± 0.03 c | 4.04 ± 0.03 b |
| Copper (µg/g DW) | 11.86 ± 0.48 b | 11.23 ± 0.42 bc | 18.83 ± 0.91 a | 9.24 ± 0.26 c |
| Manganese (µg/g DW) | 48.15 ± 1.26 c | 54.91 ± 0.85 b | 40.51 ± 1.04 d | 61.59 ± 1.28 a |
| Iron (µg/g DW) | 66.84 ± 0.37 b | 73.33 ± 2.41 ab | 89.24 ± 4.60 a | 61.00 ± 4.82 b |
| Zinc (µg/g DW) | 67.42 ± 1.60 a | 51.60 ± 0.33 b | 72.72 ± 2.35 a | 76.92 ± 0.86 a |
| Species/Varieties | ||||
|---|---|---|---|---|
| C. moschata | D. chinensis | F. regia | V. cornuta | |
| Total polyphenols (mg GAEq/g FW) | 0.67 ± 0.04 d | 2.05 ± 0.19 c | 30.06 ± 0.78 a | 5.79 ± 0.25 b |
| Total flavonoids (mg CEq/g FW) | 0.30 ± 0.01 d | 1.06 ± 0.08 c | 4.19 ± 0.17 a | 2.89 ± 0.17 b |
| Total anthocyanins (mg MEq/g FW) | ND c | 0.65 ± 0.02 b | 1.36 ± 0.06 a | 1.20 ± 0.06 a |
| Total carotenoids (µg/g FW) | 13.85 ± 0.31 b | 7.45 ± 0.61 bc | 0.78 ± 0.05 c | 95.35 ± 5.57 a |
| Antiradical activity (DPPH) (IC50 mg/mL) | 11.16 ± 0.31 d | 4.24 ± 0.23 c | 0.80 ± 0.00 a | 1.52 ± 0.08 b |
| Species/Varieties | ||||
|---|---|---|---|---|
| C. moschata | D. chinensis | F. regia | V. cornuta | |
| Thiamine (B1) (mg/100 g DW) | 13.63 ± 0.34 a | 4.98 ± 0.10 b | 2.13 ± 0.00 c | 2.35 ± 0.01 c |
| Riboflavin (B2) (mg/100 g DW) | 0.03 ± 0.00 c | 0.16 ± 0.01 a | ND d | 0.11 ± 0.00 b |
| Nicotinamide (B3) (mg/100 g DW) | 218.90 ± 0.76 a | 169.69 ± 1.87 b | 107.70 ± 1.73 d | 128.89 ± 2.22 c |
| Folic acid (B9) (mg/100 g DW) | 13.13 ± 1.18 a | 3.16 ± 0.16 b | 0.43 ± 0.06 c | 0.40 ± 0.01 c |
| Total ascorbic acid (mg/100 g FW) | 0.85 ± 0.05 d | 24.70 ± 1.34 b | 8.90 ± 0.40 c | 42.55 ± 1.69 a |
| N° | Compounds | Class | LRIexp | LRIlit | SPME_Cm | SPME_Dch | SPME_Fr | SPME_Vc | |
|---|---|---|---|---|---|---|---|---|---|
| Relative abundance (%) | |||||||||
| 1 | Tricyclene | Mh | 928 | 924 | - | - | - | 1.4 ± 0.23 | |
| 2 | Benzaldehyde | ADH | Nt | 962 | 966 | 0.7 ± 0.04 | - | - | - |
| 3 | 6-methyl-5-hepten-2-one | KET | Nt | 987 | 988 | - | - | 4.1 ± 1.35 | - |
| 4 | Myrcene | Mh | 993 | 987 | - | - | - | 36.7 ± 1.58 | |
| 5 | cis-hexenyl acetate | EST | Nt | 1008 | 1006 | - | 31.2 ± 1.05 | - | - |
| 6 | Limonene | Mh | 1030 | 1028 | - | - | - | 2.5 ± 0.26 | |
| 7 | Eucalyptol | Om | 1032 | 1033 | - | - | - | 2.0 ± 0.03 | |
| 8 | Phenylacetaldehyde | ADH | Nt | 1045 | 1049 | 0.4 ± 0.12 | - | - | 5.0 ± 0.28 |
| 10 | Nonanal | ADH | Nt | 1102 | 1102 | 0.7 ± 0.19 | 8.3 ± 0.49 | 19.0 ± 2.78 | 0.8 ± 0.28 |
| 11 | Phenylethyl alcohol | ACH | Nt | 1111 | 1112 | - | 2.4 ± 0.51 | - | - |
| 12 | Benzylnitrile | OTR | Nt | 1138 | 1143 | 6.2 ± 0.74 | - | - | 0.8 ± 0.03 |
| 13 | Camphor | Om | 1145 | 1146 | 2.4 ± 0.90 | - | - | ||
| 14 | p-dimethoxybenzene | ETR | Nt | 1168 | 1165 | 77.5 ± 3.88 | - | - | - |
| 15 | Octanoic acid | ACI | Nt | 1183 | 1191 | - | - | 6.2 ± 0.84 | - |
| 16 | Decanal | ADH | Nt | 1204 | 1200 | 0.8 ± 0.47 | 14.6 ± 1.75 | 29.8 ± 2.19 | 2.3 ± 0.18 |
| 17 | 2-pnenylethyl acetate | ADH | Nt | 1258 | 1256 | - | 2.8 ± 0.77 | - | - |
| 18 | 2-nitroethyl-benzene | OTR | Nt | 1304 | 1306 | 6.0 ± 0.15 | - | - | - |
| 19 | cis-α-bergamotene | Sh | 1416 | 1415 | - | 0.7 ± 0.06 | - | 0.7 ± 0.12 | |
| 20 | cis-geranylacetone | Ac | 1435 | 1434 | - | 2.1 ± 0.24 | 12.1 ± 0.25 | - | |
| 21 | trans-α-bergamotene | Sh | 1438 | 1441 | 0.1 ± 0.03 | - | - | - | |
| 22 | (E)-geranylacetone | Ac | 1456 | 1454 | 0.1 ± 0.02 | - | - | 1.0 ± 0.58 | |
| 23 | Germacrene D | Sh | 1482 | 1485 | 0.3 ± 0.06 | - | - | - | |
| 24 | α-selinene | Sh | 1495 | 1494 | - | 0.3 ± 0.07 | - | - | |
| 25 | Dihydro- β-agarofuran | Os | 1496 | 1501 | - | 29.7 ± 2.68 | - | - | |
| 26 | α-farnesene | Sh | 1508 | 1507 | - | - | - | 34.5 ± 4.52 | |
| 27 | δ-cadinene | Sh | 1524 | 1519 | - | 4.0 ± 0.05 | - | - | |
| 28 | α-calacorene | Sh | 1543 | 1542 | - | 0.2 ± 0.04 | - | - | |
| 29 | 3-hexen-1-ol benzoate | BZD | Nt | 1585 | 1585 | - | 1.3 ± 0.19 | - | 3.0 ± 0.45 |
| 30 | Precocene 2 | Chromene | Nt | 1658 | 1656 | 0.3 ± 0.07 | - | - | - |
| 31 | Ar-turmerone | Os | 1664 | 1664 | - | - | - | 1.0 ± 0.09 | |
| 32 | Benzyl benzoate | BZD | Nt | 1762 | 1676 | - | - | - | 2.1 ± 0.71 |
| 33 | Isopropyl myristate | Os | 1827 | 1759 | 0.4 ± 0.04 | - | - | - | |
| 34 | (E,E)-farnesyl acetate | Os | 1843 | 1831 | 0.7 ± 0.04 | - | 14.0 ± 0.55 | 3.1 ± 0.11 | |
| 35 | Hexahydrofarnesyl acetone | Os | 1844 | 1840 | - | - | - | 0.9 ± 0.07 | |
| 36 | Phenylethyl benzoate | EST | Nt | 1853 | 1847 | - | - | - | 1.9 ± 0.43 |
| 37 | Isopropyl palmitate | EST | Nt | 1981 | 1859 | 0.3 ± 0.05 | - | - | - |
| 38 | N-tetradecanol | ACH | Nt | 2056 | 1999 | 0.8 ± 0.08 | - | - | - |
| 39 | Dodecyl octanoate | EST | Nt | 2160 | 2160 | - | - | 4.5 ± 0.49 | - |
| 40 | trans-geranylgeraniol | Od | 2201 | 2201 | - | - | 10.3 ± 0.26 | - | |
| Class of compounds | SPME_Cm | SPME_Dch | SPME_Fr | SPME_Vc | |||||
| Monoterpene hydrocarbons (MHs) | - | - | - | 40.6 ± 2.61 | |||||
| Oxygenated monoterpenes (OMs) | - | 2.4 ± 0.90 | - | 2.0 ± 0.03 | |||||
| Sesquiterpene hydrocarbons (SHs) | 0.4 ± 0.04 | 5.2 ± 0.05 | - | - | |||||
| Oxygenated sesquiterpenes (OSs) | 1.1 ± 0.14 | 29.7 ± 2.68 | 14.0 ± 0.55 | 5.0 ± 0.09 | |||||
| Oxygenated diterpenes (ODs) | - | - | 10.3 ± 0.26 | - | |||||
| Apocarotenoids (ACs) | 0.1 ± 0.02 | 2.1 ± 0.24 | 12.1 ± 0.25 | 1.0 ± 0.58 | |||||
| Total non-terpene derivatives (NTs) | 93.7 ± 1.40 | 60.6 ± 0.80 | 63.6 ± 1.53 | 15.9 ± 1.91 | |||||
| Non-terpene derivative | Alcohol (ACH) | 0.8 ± 0.08 | 2.4 ± 0.51 | - | - | ||||
| Acids (ACI) | - | - | 6.2 ± 0.84 | - | |||||
| Aldehydes (ADH) | 2.6 ± 0.28 | 25.7 ± 2.57 | 48.8 ± 3.09 | 8.1 ± 0.58 | |||||
| Esters (EST) | 0.3 ± 0.05 | 32.5 ± 1.22 | 4.5 ± 0.49 | 7.0 ± 0.89 | |||||
| Eters (ETR) | 77.5 ± 3.88 | - | - | - | |||||
| Ketones (KET) | - | - | 4.1 ± 1.35 | ||||||
| Chromene | 0.3 ± 0.07 | - | - | - | |||||
| others | 12.2 ± 0.99 | - | - | 0.8 ± 0.03 | |||||
| Total identified | 95.3 ± 0.24 | 100 ± 0.00 | 100 ± 0.00 | 99.7 ± 0.43 | |||||
| Flower | Volatile Compound | Odor Description | References |
|---|---|---|---|
| C. moschata | p-dimethoxybenzene | Sweet, green, hay | [101] |
| Benzylnitrile | Bitter almonds, spicy | [102] | |
| 2-nitroethyl-benzene | Sweet, floral, spicy | [103] | |
| D. chinensis | cis-hexenyl acetate | Green | [104] |
| Dihydro- β-agarofuran | - | ||
| Decanal | Sweet, aldehydic, fatty, citrusy | [105,106] | |
| Nonanal | Candle-like, sweet orange-like, fatty and floral | [107] | |
| F. regia | Decanal | Sweet, aldehydic, fatty, citrusy | [105,106] |
| Nonanal | Candle-like, sweet orange-like, fatty, floral | [107] | |
| (E,E)-farnesyl acetate | Woody, floral | [108] | |
| Cis-geranylacetone | Magnolia, green, floral | [109] | |
| Trans-geranylgeraniol | - | ||
| Octanoic acid | Waxy, soapy | [110] | |
| V. cornuta | Myrcene | Resinous, herbaceous, balsamic, geranium-like | [111] |
| α-farnesene | Fruity, herbaceous | [112] | |
| Phenylacetaldehyde | Floral, lilac | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods 2024, 13, 939. https://doi.org/10.3390/foods13060939
Marchioni I, Gabriele M, Carmassi G, Ruffoni B, Pistelli L, Pistelli L, Najar B. Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods. 2024; 13(6):939. https://doi.org/10.3390/foods13060939
Chicago/Turabian StyleMarchioni, Ilaria, Morena Gabriele, Giulia Carmassi, Barbara Ruffoni, Luisa Pistelli, Laura Pistelli, and Basma Najar. 2024. "Phytochemical, Nutritional and Mineral Content of Four Edible Flowers" Foods 13, no. 6: 939. https://doi.org/10.3390/foods13060939
APA StyleMarchioni, I., Gabriele, M., Carmassi, G., Ruffoni, B., Pistelli, L., Pistelli, L., & Najar, B. (2024). Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods, 13(6), 939. https://doi.org/10.3390/foods13060939











