Effect of Damaged Starch and Wheat-Bran Arabinoxylans on Wheat Starch and Wheat Starch–Gluten Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Wheat Bran Arabinoxylans
2.3. Mechanical Damage of the Wheat Starch
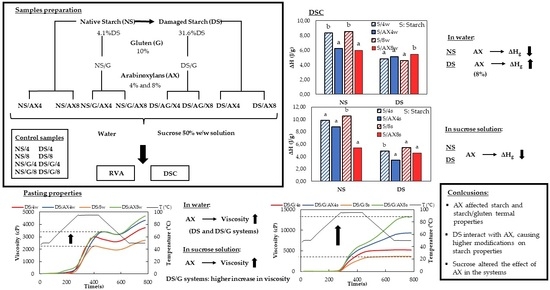
2.4. Samples Preparation
2.5. Pasting Profile
2.6. Differential Scanning Calorimetry (DSC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pasting Properties
- Water as a dispersion medium
- Sucrose solution as a dispersion medium
3.2. Differential Scanning Calorimetry Studies
- Water as a dispersion medium
- Sucrose solution as a dispersion medium
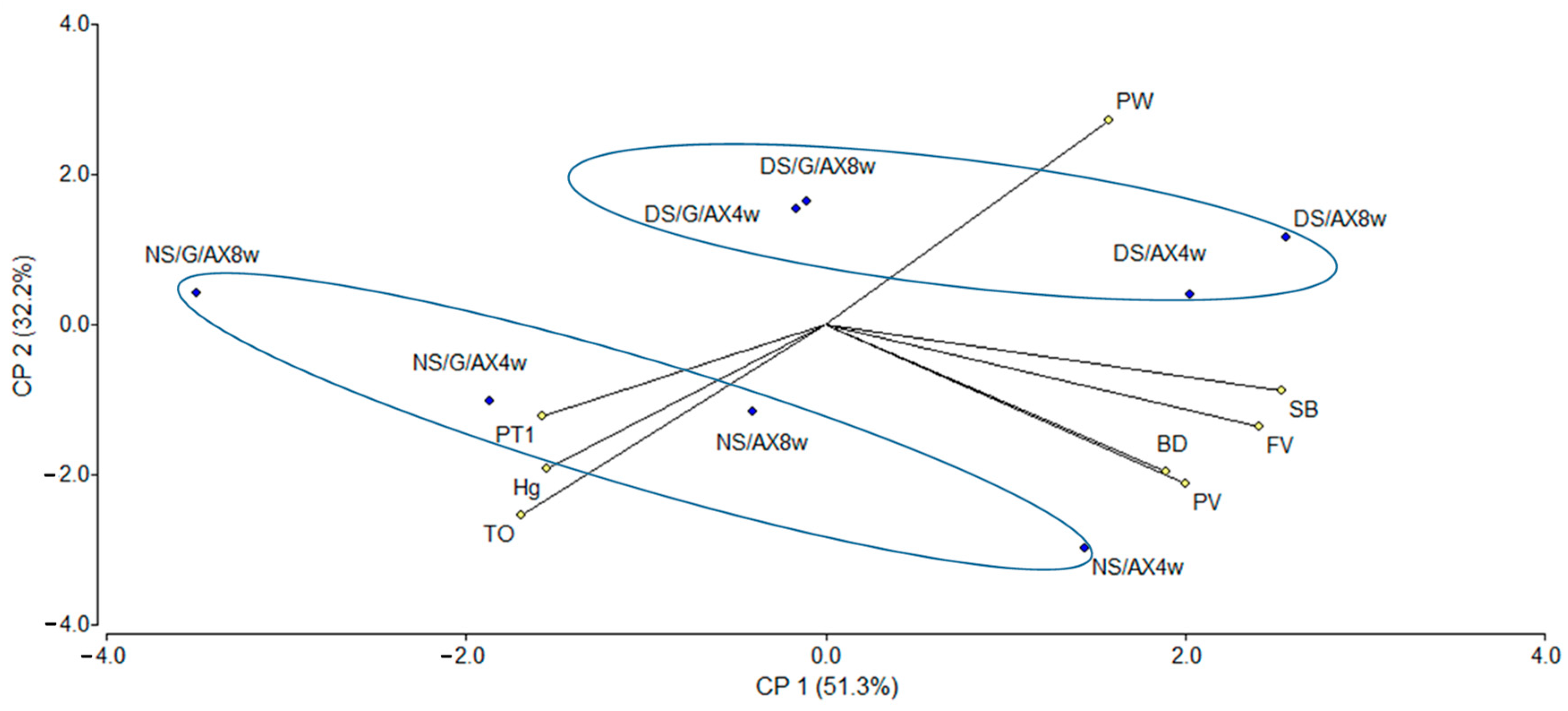
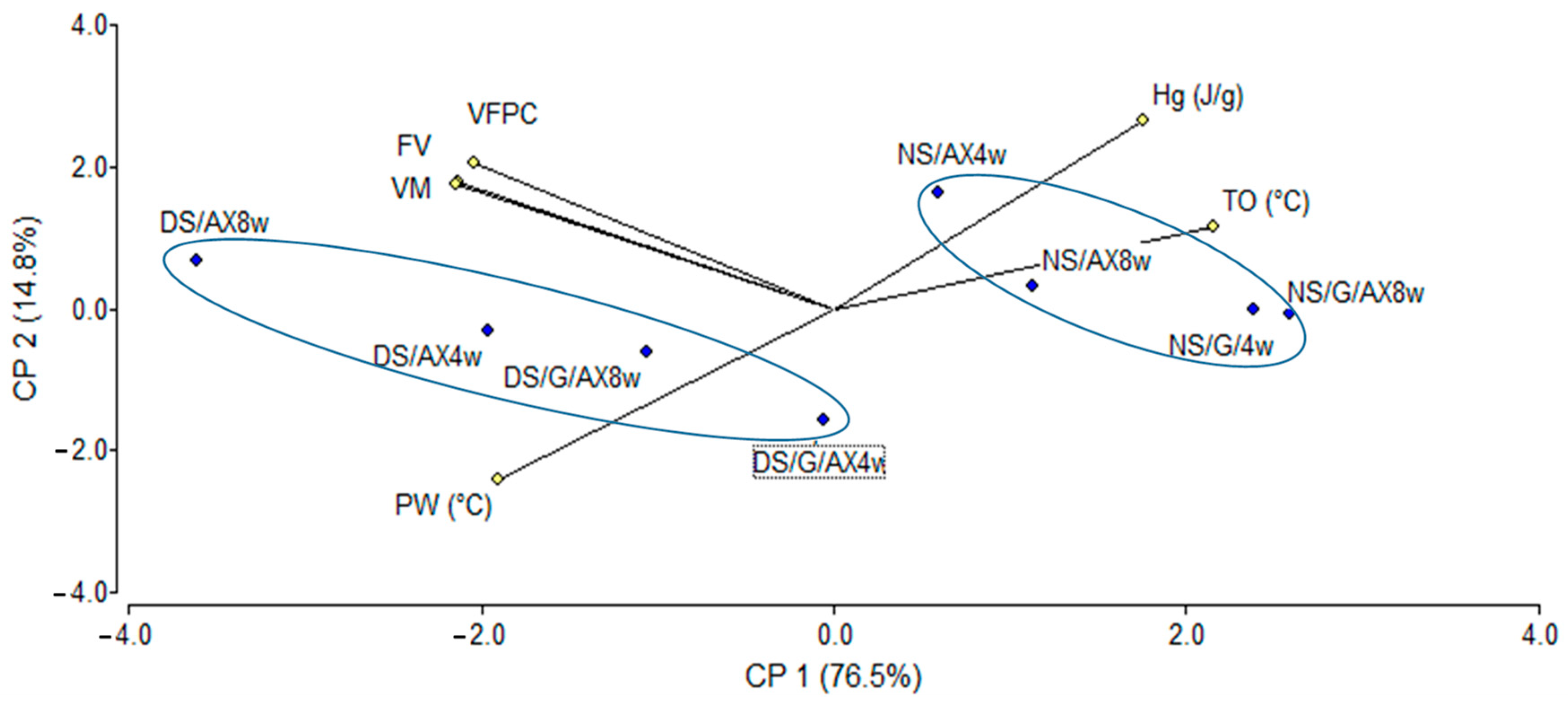
3.3. Principal Components Analysis (PCA) and Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C. Cereal Arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Yang, J.; Lu, Z.; Zhao, H.; Lu, F. Oxidative crosslinking of water-extractable wheat arabinoxylans by recombinant lipoxygenase and its effect on bread properties. Food Sci. Technol. 2019, 103, 1–7. [Google Scholar] [CrossRef]
- Hernández-Pinto, F.J.; Miranda-Medina, J.D.; Natera-Maldonado, A.; Vara-Aldama, O.; Ortueta-Cabranes, M.P.; Vázquez del Mercado-Pardiño, J.A.; El-Aidie, S.A.M.; Siddiqui, S.A.; Castro-Muñoz, R. Arabinoxylans: A review on protocols for their recovery, functionalities and roles in food formulations. Int. J. Biol. Macromol. 2024, 259, 12909–12923. [Google Scholar] [CrossRef] [PubMed]
- Moza, J.; Gujral, H.S. Influence of barley non-starchy polysaccharides on selected quality attributes of sponge cakes. Food Sci. Technol. 2017, 85, 252–261. [Google Scholar] [CrossRef]
- Teobaldi, A.G.; Barrera, G.N.; Severini, H.; Ribotta, P.D. Influence of damaged starch on thermal and rheological properties of wheat starch and wheat starch-gluten systems in water and sucrose. J. Sci. Food Agric. 2023, 103, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, X.; Guo, Y.; Wang, Q.; Peng, D.; Cao, L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr. Polym. 2010, 81, 784–789. [Google Scholar] [CrossRef]
- Anderson, C.; Simsek, S. Mechanical profiles and pographical properties of films made from alkaline extracted arabinoxylans from wheat bran, maize, bran, or dried distillers grain. Food Hydrocoll. 2019, 86, 78–86. [Google Scholar] [CrossRef]
- Hashimoto, S.; Shogren, M.D.; Pomeranz, Y. Cereal pentosans: Their estimation and significance. I. Pentosans in wheat and milled wheat products. Cereal Chem. 1987, 64, 30–34. [Google Scholar]
- American Association of Cereal Chemists International (AACC). Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Teobaldi, A.G.; Barrera, G.N.; Sciarini, L.S.; Ribotta, P.D. Pasting, gelatinization, and rheological properties of wheat starch in the presence of sucrose and gluten. Eur. Food Res. Technol. 2021, 247, 1083–1093. [Google Scholar] [CrossRef]
- Kim, C.S.; Walker, E. Changes in starch pasting properties due to sugars and emulsifiers as determined by viscosity measurement. J. Food Sci. 1992, 57, 1009–1013. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, L.; Li, M.; He, X.; Hao, L.; Dai, Y. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols. Int. J. Biol. Macromol. 2019, 129, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Shazad, S.A.; Ababtain, I.A. Use of gum cordia (Cordia myxa) as a natural starch modifier; effect on pasting, thermal, textural, and rheological properties of corn starch. Foods 2020, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, M.; Zhang, M.; Yadav, M.P.; Jia, X.; Yin, L. Effect of wheat bran arabinoxylan on the gelatinization and long-term retrogradation behavior of wheat starch. Carbohydr. Polym. 2022, 291, 119581–119588. [Google Scholar] [CrossRef]
- Rojas, J.A.; Rossell, C.M.; Benedito de Barber, C. Pasting properties of different wheat flour-hydrocolloid systems. Food Hydrocoll. 1999, 13, 27–33. [Google Scholar] [CrossRef]
- Shi, X.; BeMiller, J.N. Effects of food gums on viscosities of starch suspensions during pasting. Carbohydr. Polym. 2002, 50, 7–18. [Google Scholar] [CrossRef]
- Qiu, S.; Yadav, M.P.; Liu, Y.; Chen, H.; Tatsumi, E.; Yin, L. Effects of corn fiber gum with different molecular weights on the gelatinization behaviors of corn and wheat starch. Food Hydrocoll. 2016, 53, 180–186. [Google Scholar] [CrossRef]
- Tester, R.F. Influence of growth conditions on barley starch properties. Int. J. Biol. Macromol. 1997, 21, 37–45. [Google Scholar] [CrossRef]
- Xie, H.; Ying, R.; Huang, M. Effect of arabinoxylans with different molecular weights on the gelling properties of wheat starch. Int. J. Biol. Macromol. 2022, 209, 1676–1684. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Noda, S.; Hiroe, M.; Ishihara, S.; Asai, I.; Takahashi, R.; Nishinari, K. Functions of fenugreek gum with various molecular weights on the gelatinization and retrogradation behaviors of corn starch-1: Characterizations of fenugreek gum and investigations of corn starch/fenugreek gum composite system at a relatively high starch concentration; 15w/v%. Food Hydrocoll. 2008, 22, 763–776. [Google Scholar]
- Biliaderis, C.G.; Arvanitoyannis, I.; Izydorczyk, M.S.; Prokopowick, D.J. Effect of Hydrocolloids on gelatinization and structure formation in concentrated waxy maize and wheat starch gels. Starch/Stärke 1997, 49, 278–283. [Google Scholar] [CrossRef]
- Richardson, S.J.; Baianu, I.C.; Steinberg, M.P. Mobility of water in sucrose solutions determined by deuterium and oxygen-17 nuclear magnetic resonance measurements. J. Food Sci. 1987, 52, 806–809. [Google Scholar] [CrossRef]
- Si, X.; Li, T.; Zhang, Y.; Zhang, W.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Interactions between gluten and water-unextractable arabinoxylan during the thermal treatment. Food Chem. 2021, 345, 128785–128793. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zou, M.; Tian, M.; Gu, Z.; Yang, R. The impact of heating on the unfolding and polymerization process of frozen-stored gluten. Food Hydrocoll. 2018, 85, 195–203. [Google Scholar] [CrossRef]
- Rosell, C.M.; Foegeding, A. Interaction of hydroxypropylmethylcellulose with gluten proteins: Small deformation properties during thermal treatment. Food Hydrocoll. 2007, 21, 1092–1100. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Tester, R.F.; Gidley, M.J. Properties of damaged starch granules II. Crystallinity, molecular order and gelatinization of ball milled starches. J. Cereal Sci. 1994, 19, 209–217. [Google Scholar] [CrossRef]
- Tester, R.F. Properties of damaged starch granules: Composition and swelling properties of maize, rice, pea and potato starch fractions in water at various temperatures. Food Hydrocoll. 1997, 11, 293–301. [Google Scholar] [CrossRef]
- Barrera, G.N.; León, A.E.; Ribotta, P.D. Effect of damaged starch on starch thermal behavior. Starch/Stärke 2012, 64, 786–793. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Eliasson, A.C.; Bengtsson, S.; Aman, P. The effects of water soluble arabinoxylan on gelatinization and retrogradation of starch. Starch/Stärke 1991, 43, 5–10. [Google Scholar] [CrossRef]
- Kim, W.W.; Yoo, B. Rheological and thermal effects of galactomannan addition to acorn starch paste. Food Sci. Technol. 2011, 44, 759–764. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Maurice, T.J.; Vose, J.R. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1674. [Google Scholar] [CrossRef]
- Krüger, A.; Ferrero, C.; Zaritzky, N.E. Modelling corn starch swelling in batch systems: Effect of sucrose and hydrocolloids. J. Food Eng. 2003, 58, 125–133. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. A new insight into the gelatinization process of native starches. Carbohydr. Polym. 2007, 67, 511–529. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Page, C.M.; Maurice, T.J.; Juliano, B.O. Thermal Characterization of rice starches: A polymeric approach to phase transitions of granular starch. J. Agric. Food Chem. 1986, 34, 6–14. [Google Scholar] [CrossRef]
- BeMiller, J.N. Pasting, paste, and gel properties of starch-hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Grossutti, M.; Dutcher, J.R. Correlation between chain architecture and hydration water structure in polysaccharides. Biomacromolecules 2016, 17, 1198–1204. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Ibraheem, M.A.; Abdo Qasem, A.A. Effect of hydrocolloid gums on the pasting, thermal, rheological and textural properties of chickpea starch. Foods 2019, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.S.; Navarro, A.S.; Yamul, D.K. Effect of hydrocolloids on the properties of wheat/potato starch mixtures. Starch Stärke 2016, 68, 753–761. [Google Scholar] [CrossRef]
- Lee, M.H.; Baek, M.A.; Cha, D.S.; Park, H.J.; Lim, S.T. Freeze-thaw stabilization of sweet potato starch gel by polysaccharide gums. Food Hydrocoll. 2002, 16, 345–352. [Google Scholar] [CrossRef]
| System | Medium | |||
|---|---|---|---|---|
| Water | Sucrose | |||
| Samples | ||||
| Control | AX | Control | AX | |
| Starch | NS/4w | NS/AX4w | NS/4s | NS/AX4s |
| NS/8w | NS/AX8w | NS/8s | NS/AX8s | |
| DS/4w | DS/AX4w | DS/4s | DS/AX4s | |
| DS/8w | DS/AX8w | DS/8s | DS/AX8s | |
| Starch–Gluten | NS/G/4w | NS/G/AX4w | NS/G/4s | NS/G/AX4s |
| NS/G/8w | NS/G/AX8w | NS/G/8s | NS/G/AX8s | |
| DS/G/4w | DS/G/AX4w | DS/G/4s | DS/G/AX4s | |
| DS/G/8w | DS/G/AX8w | DS/G/8s | DS/G/AX8s | |
| Sample | PV (cP) | BD (cP) | FV (cP) | SB (cP) | Pt (°C) |
|---|---|---|---|---|---|
| NS/4w | 3887 ± 148 a | 705 ± 7 a | 4771 ± 147 a | 1589 ± 6 a | 85.9 ± 0.5 a |
| NS/AX4w | 3646 ± 54 a | 1017 ± 18 b | 4754 ± 23 a | 2125 ± 58 b | 87.6 ± 0.6 a |
| NS/8w | 2938 ± 141 a | 587 ± 63 a | 3477 ± 182 a | 1127 ± 104 a | 88.8 ± 0.0 b |
| NS/AX8w | 2945 ± 78 a | 795 ± 102 a | 3935 ± 57 a | 1785 ± 123 b | 82.7.3 ± 1.8 a |
| NS/G/4w | 3082 ± 68 a | 630 ± 8 a | 3731 ± 67 a | 1278 ± 7 a | 88.8 ± 0.0 a |
| NS/G/AX4w | 2994 ± 55 a | 585 ± 61 a | 3757 ± 91 a | 1348 ± 97 a | 89.2 ± 0.5 a |
| NS/G/8w | 2360 ± 20 a | 460 ± 2 a | 2882 ± 4 a | 981 ± 14 a | 90.5 ± 0.0 a |
| NS/G/AX8w | 2342 ± 153 a | 374 ± 151 a | 3133 ± 168 a | 1164 ± 165 a | 90.0 ± 0.6 a |
| DS/4w | 2995 ± 31 a | 453 ± 77 a | 3726 ± 20 a | 1184 ± 26 a | 84.8 ± 0.0 a |
| DS/AX4w | 3077 ± 164 a | 906 ± 11 b | 4315 ± 224 a | 2143 ± 71 b | 86.8 ± 0.6 b |
| DS/8w | 2266 ± 33 a | 518 ± 62 a | 2727 ± 94 a | 851 ± 0 a | 88.0 ± 1.0 b |
| DS/AX8w | 3243 ± 6 b | 621 ± 34 b | 4679 ± 156 b | 2057 ± 117 b | 67.7 ± 2.3 a |
| DS/G/4w | 2426 ± 26 a | 464 ± 21 a | 2839 ± 30 a | 878 ± 18 a | 88.4 ± 0.6 a |
| DS/G/AX4w | 2785 ± 6 b | 643 ± 23 b | 3618 ± 21 b | 1476 ± 37 b | 88.0 ± 0.0 a |
| DS/G/8w | 1776 ± 1 a | 380 ± 170 a | 2134 ± 8 a | 738 ± 1 a | 90.5 ± 0.1 a |
| DS/G/AX8w | 2629 ± 83 b | 611 ± 182 a | 3666 ± 148 b | 1648 ± 247 b | 79.8 ± 13.8 a |
| Sample | FV (cP) | MV (cP) | FPCV (cP) |
|---|---|---|---|
| NS/4s | 11,539 ± 164 a | 11,692 ± 190 a | 9959 ± 86 b |
| NS/AX4s | 12,293 ± 90 b | 12,315 ± 113 a | 8095 ± 124 a |
| NS/8s | 7477 ± 91 a | 7708 ± 104 a | 7210 ± 6 b |
| NS/AX8s | 11,470 ± 481 b | 11,470 ± 481 b | 5478 ± 139 a |
| NS/G/4s | 6177 ± 120 a | 6371 ± 120 a | 5809 ± 100 b |
| NS/G/AX4s | 7556 ± 194 b | 7561 ± 196 b | 5090 ± 190 a |
| NS/G/8s | 4085 ± 192 a | 4230 ± 173 a | 3771 ± 179 a |
| NS/G/AX8s | 8210 ± 109 b | 8210 ± 109 b | 4040 ± 114 a |
| DS/4s | 9549 ± 0 a | 9716 ± 27 a | 8560 ± 29 a |
| DS/AX4s | 13,661 ± 176 b | 13,772 ± 319 b | 8806 ± 281 a |
| DS/8s | 6594 ± 51 a | 6660 ± 45 a | 5868 ± 88 a |
| DS/AX8s | 18,781 ± 1049 b | 18,794 ± 1049 b | 9768 ± 346 b |
| DS/G/4s | 5248 ± 238 a | 5301 ± 250 a | 4578 ± 281 a |
| DS/G/AX4s | 9387 ± 246 b | 9390 ± 246 b | 5755 ± 37 b |
| DS/G/8s | 3611 ± 9 a | 3639 ± 26 a | 3070 ± 25 a |
| DS/G/AX8s | 13,276 ± 147 b | 13,377 ± 289 b | 6515 ± 100 b |
| Sample | ΔHg (J/g) | TO (°C) | PW (°C) |
|---|---|---|---|
| NS/4w | 8.34 ± 0.23 b | 55.2 ± 0.1 a | 7.1 ± 0.0 a |
| NS/AX4w | 6.22 ± 0.02 a | 56.4 ± 0.3 b | 6.6 ± 0.5 a |
| NS/8w | 8.52 ± 0.14 b | 55.2 ± 0.0 a | 6.9 ± 0.1 a |
| NS/AX8w | 5.99 ± 0.55 a | 57.1 ± 0.2 b | 6.8 ± 0.3 a |
| NS/G/4w | 8.08 ± 0.34 b | 55.5 ± 0.0 a | 7.0 ± 0.1 a |
| NS/G/AX4w | 6.67 ± 0.36 a | 56.3 ± 0.4 a | 6.7 ± 0.5 a |
| NS/G/8w | 8.27 ± 0.08 b | 55.4 ± 0.1 a | 7.3 ± 0.0 b |
| NS/G/AX8w | 6.46 ± 0.35 a | 57.0 ± 0.0 b | 7.1 ± 0.1 a |
| DS/4w | 4.81 ± 0.08 a | 51.0 ± 0.4 a | 9.5 ± 0.2 a |
| DS/AX4w | 5.10 ± 0.44 a | 50.1 ± 0.5 a | 10.5 ± 0.2 b |
| DS/8w | 4.60 ± 0.09 a | 51.1 ± 0.0 b | 8.9 ± 0.8 a |
| DS/AX8w | 5.43 ± 0.04 b | 50.0 ± 0.3 a | 11.0 ± 0.4 a |
| DS/G/4w | 4.10 ± 0.38 a | 51.5 ± 0.2 a | 9.3 ± 0.3 a |
| DS/G/AX4w | 4.64 ± 0.02 a | 51.0 ± 0.4 a | 9.8 ± 0.3 a |
| DS/G/8w | 4.68 ± 0.13 a | 51.3 ± 0.1 b | 9.5 ± 0.2 a |
| DS/G/AX8w | 5.99 ± 0.28 b | 50.6 ± 0.2 a | 10.4 ± 0.2 b |
| Sample | ΔHg (J/g) | TO (°C) | PW (°C) |
|---|---|---|---|
| NS/4w | 8.34 ± 0.23 b | 55.2 ± 0.1 a | 7.1 ± 0.0 a |
| NS/AX4w | 6.22 ± 0.02 a | 56.4 ± 0.3 b | 6.6 ± 0.5 a |
| NS/8w | 8.52 ± 0.14 b | 55.2 ± 0.0 a | 6.9 ± 0.1 a |
| NS/AX8w | 5.99 ± 0.55 a | 57.1 ± 0.2 b | 6.8 ± 0.3 a |
| NS/G/4w | 8.08 ± 0.34 b | 55.5 ± 0.0 a | 7.0 ± 0.1 a |
| NS/G/AX4w | 6.67 ± 0.36 a | 56.3 ± 0.4 a | 6.7 ± 0.5 a |
| NS/G/8w | 8.27 ± 0.08 b | 55.4 ± 0.1 a | 7.3 ± 0.0 b |
| NS/G/AX8w | 6.46 ± 0.35 a | 57.0 ± 0.0 b | 7.1 ± 0.1 a |
| DS/4w | 4.81 ± 0.08 a | 51.0 ± 0.4 a | 9.5 ± 0.2 a |
| DS/AX4w | 5.10 ± 0.44 a | 50.1 ± 0.5 a | 10.5 ± 0.2 b |
| DS/8w | 4.60 ± 0.09 a | 51.1 ± 0.0 b | 8.9 ± 0.8 a |
| DS/AX8w | 5.43 ± 0.04 b | 50.0 ± 0.3 a | 11.0 ± 0.4 a |
| DS/G/4w | 4.10 ± 0.38 a | 51.5 ± 0.2 a | 9.3 ± 0.3 a |
| DS/G/AX4w | 4.64 ± 0.02 a | 51.0 ± 0.4 a | 9.8 ± 0.3 a |
| DS/G/8w | 4.68 ± 0.13 a | 51.3 ± 0.1 b | 9.5 ± 0.2 a |
| DS/G/AX8w | 5.99 ± 0.28 b | 50.6 ± 0.2 a | 10.4 ± 0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teobaldi, A.G.; Barrera, G.N.; Ribotta, P.D. Effect of Damaged Starch and Wheat-Bran Arabinoxylans on Wheat Starch and Wheat Starch–Gluten Systems. Foods 2024, 13, 689. https://doi.org/10.3390/foods13050689
Teobaldi AG, Barrera GN, Ribotta PD. Effect of Damaged Starch and Wheat-Bran Arabinoxylans on Wheat Starch and Wheat Starch–Gluten Systems. Foods. 2024; 13(5):689. https://doi.org/10.3390/foods13050689
Chicago/Turabian StyleTeobaldi, Andrés Gustavo, Gabriela Noel Barrera, and Pablo Daniel Ribotta. 2024. "Effect of Damaged Starch and Wheat-Bran Arabinoxylans on Wheat Starch and Wheat Starch–Gluten Systems" Foods 13, no. 5: 689. https://doi.org/10.3390/foods13050689
APA StyleTeobaldi, A. G., Barrera, G. N., & Ribotta, P. D. (2024). Effect of Damaged Starch and Wheat-Bran Arabinoxylans on Wheat Starch and Wheat Starch–Gluten Systems. Foods, 13(5), 689. https://doi.org/10.3390/foods13050689