Abstract
Hafnia alvei, a specific spoilage microorganism, has a strong capacity to destroy food protein and lead to spoilage. The aim of this study was to evaluate the phase-dependent regulation of lux-type genes on the spoilage characteristics of H. alvei H4. The auto-inducer synthase gene luxI and a regulatory gene luxR of the quorum sensing systems in H. alvei H4 were knocked out to construct the mutant phenotypes. On this basis, the research found that the luxI and luxR genes had a strong positive influence on not only flagella-dependent swimming ability and biofilm formation but also the production of putrescine and cadaverine. The luxR gene could downregulate putrescine production. The maximum accumulation of putrescine in wild type, ΔluxI, ΔluxR and ΔluxIR were detected at 24 h, reaching up to 695.23 mg/L, 683.02 mg/L, 776.30 mg/L and 724.12 mg/L, respectively. However, the luxI and luxR genes have a potential positive impact on the production of cadaverine. The maximum concentration of cadaverine produced by wild type, ΔluxI, ΔluxR and ΔluxIR were 252.7 mg/L, 194.5 mg/L, 175.1 mg/L and 154.2 mg/L at 72 h. Moreover, the self-organizing map analysis revealed the phase-dependent effects of two genes on spoilage properties. The luxI gene played a major role in the lag phase, while the luxR gene mainly acted in the exponential and stationary phases. Therefore, the paper provides valuable insights into the spoilage mechanisms of H. alvei H4.
1. Introduction
Food spoilage is mainly a consequence of the degrading enzymatic activity triggered by some spoilage microorganisms. For the spoilage microorganism, its excellent flagella-dependent swimming ability could help access an appropriate niche inside food, and strong biofilm formation could enhance the bacterium’s ability for colonizing a food surface, leading to resistance to antibacterial agents and food processing conditions [1]. Moreover, the spoilage microorganism has a potential ability to produce massive metabolic end products, such as saccharolytic, proteolytic, pectinolytic and lipolytic enzymes, leading to food spoilage [2]. Generally, decarboxylase-positive microorganisms are mainly involved in the decarboxylation of amines to produce biogenic amines (BAs), giving food an undesirable taste and “putrid odor” and affecting public health [3,4].
Hafnia alvei, a specific spoilage microorganism, has a strong capacity to destroy food protein, leading to spoilage. It is a Gram-negative bacterium with lux-type quorum sensing (QS) systems. Most genetic strains can grow over a wide range of temperatures, even at a minimum temperature of 0.2 to 3.7 °C [5]. With a psychrotrophic attribute, H. alvei has the opportunity to be dominantly found in spoiled food, including vacuum and air-conditioned packaged food and cryopreservation food, such as dairy [6], fish [7,8], meat [9] and other protein-rich food. Although many studies have shown that H. alvei is considered to be a typical contributor to spoilage with the production of BAs [10], the self-regulating mechanisms of spoilage characteristics in H. alvei are still in their infancy.
QS is a common regulatory mechanism of biological functions in the microorganism kingdom [11,12]. QS plays a key role in coordinating group behavior and addressing changes in external and internal environments [13,14]. That is to say, QS is a comprehensive regulator of various biological aspects of microbe metabolism processes [15]. The lux-type system is a typical kind of QS system with an auto-inducer (AI) signaling molecule for mediating intracellular signal recognition and is commonly found in H. alvei [16]. Two essential genes mainly accomplish the pathways of QS regulation: luxI, an AI synthase gene; and luxR, a regulatory gene [17]. The luxI gene can regulate the synthesis of AI and luxR can regulate reception with AI, which has an important influence on the expression of the target functional genes, including virulence modulation, exoenzyme enzyme synthesis and biofilm formation [18]. Therefore, we supposed that the luxI and luxR genes also have the potential ability to regulate spoilage behaviors.
The aim of this study was to determine the phase-dependent effect of luxI and luxR genes on spoilage characteristics in H. alvei H4. Through luxI and luxR gene knockouts, mutant phenotypes were constructed. Furthermore, growth ability, swimming motility, biofilm formation and BA formation capacity between wild and mutant strains were comparatively studied by self-organizing map (SOM) analysis.
2. Materials and Methods
2.1. Bacterial Strains and Reagents
Bacterial strains and plasmids used in this study are listed in Table 1. H. alvei H4 was cultured in Luria–Bertani (LB) (10 g tryptone, 5 g yeast extract power, 10 g NaCl, dissolved in 1 L deionized water) agar plates at 37 °C. The molecular biology reagents and precursor amino acids including L-histidine monohydrochloride monohydrate, L-tyrosine disodium salt hydrate, L-ornithine monohydrochloride, L-lysine monohydrochloride, L-tyrosine disodium salt hydrate, L-arginine monohydrochloride, L-phenylalanine hydrochloride and L-tryptophan hydrochloride were obtained from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Acetonitrile used for high-performance liquid chromatography (HPLC) was chromatographically pure, and other chemical reagents used in this study were of analytical grade; all of them were purchased from Bonuo biochemical reagent Co., Ltd. (Dalian, China). Tryptone, yeast extract power, NaCl, agarose, glucose, Tween-80, MgSO4, MnSO4, FeSO4, ammonium citrate, thiamine, K2PO4, CaCO3, pyridoxal-5-phosphate and bromocresol purple were also purchased from Bonuo biochemical reagent Co., Ltd. (Dalian, China).

Table 1.
Bacterial strains and plasmids used in this study.
2.2. Construction of luxI and luxR Mutants and Complemented Strains
For characterizing the QS system in H. alvei H4, the luxI, luxR and luxIR gene knockout mutants and their complemented strains were constructed according to a report by Zhu et al. [21]. The luxI-F/R and luxR-F/R primers were designed as in Table 2 by Primer 3.0. The flanking regions of luxI and luxR were amplified by PCR with the primer pairs using pfu DNA polymerase. After confirming the sequence, the upstream and downstream regions of luxI with 628 bp and 654 bp, as well as luxR with 608 bp and 659 bp were digested by EcoRI/BamHI and BamHI/SphI, respectively, cloned into the plasmid pUC19 to create pUC19-ΔluxI, pUC19-ΔluxR and pUC19-ΔluxIR, and then introduced into E. coli TOP10 for identification by PCR. Subsequently, the plasmids were linked by a BamHI restriction site and the luxR, luxI and luxIR gene regions were replaced with a chloramphenicol resistance gene cassette (CmR) (1048 bp) previously amplified from the donor plasmid pKD3 using primers Cm-F/R, respectively. The plasmids including pUC19-ΔluxI::Cm, pUC19-ΔluxR::Cm and pUC19-ΔluxIR::Cm were created, then the target fragments, such as luxI::Cm, luxR::Cm and luxIR::Cm, were digested by SalI and inserted into the same sites of the suicide plasmid pCVD442 to acquire the recombinant plasmids pCVD442-ΔluxI::Cm, pCVD442-ΔluxR::Cm and pCVD442-ΔluxIR::Cm. The recombinant plasmids were introduced into E. coli β2155 (donor strain) by electroporator. E. coli β2155 harboring three different plasmids as pCVD442-ΔluxI::Cm, pCVD442-ΔluxR::Cm and pCVD442-ΔluxIR::Cm were conjugated with wild-type H. alvei H4, respectively. Recipient cells were plated on LB supplemented with 50 μg/mL Amp, 0.5 mM DAP and 10 μg/mL antibiotic chloramphenicol to select the successful clone recombinant plasmids that had integrated the vector by a single crossover of allelic exchange. Antibiotic-resistant colonies were selected and confirmed by PCR. The luxR, luxI and luxIR gene knockout mutant phenotypes were named as ΔluxI, ΔluxR and ΔluxIR, respectively.

Table 2.
Primers used for construction of mutant and complemented strains.
To build the corresponding complementary plasmids for the ΔluxI, ΔluxR and ΔluxIR mutants, the luxI and luxR genes were amplified by primers as before, and then the fragments were cloned into plasmid pET28a(+)/FaGH17A with kanamycin resistance to construct pET28a(+)/FaGH17A-ΔluxI, pET28a(+)/FaGH17A-ΔluxR and pET28a(+)/FaGH17A-ΔluxIR. The plasmids were primarily transformed into E.coli TOP10 and plasmid DNA was isolated and then transformed into the ΔluxI, ΔluxR and ΔluxIR mutants to produce the complementary strains comI, comR and comIR whose presence were confirmed by PCR analysis and sequencing.
2.3. Growth Curve
The growth of wild, mutant and complementation phenotypes was measured by optical density at OD600 nm every 6 h via an ultraviolet spectrophotometer. For comparative analysis of growth kinetics, the Gompertz model [22] was applied to fit the OD600 data. Per the parameters including maximum specific growth rate (Vmax), lag time (Lag) and maximal OD600 at stationary phase (Amax), the growth abilities of different strains were compared.
2.4. Swimming Motility Assay
As in a previous report [23], the swimming motility of wild, mutant and complementation phenotype strains were measured with some modifications. Briefly, 3 μL of the overnight cultured strain was placed in the center of a swimming agar plate including 1% tryptone, 0.5% NaCl and 0.3% agarose. After incubating at 30 °C for 48 h, the migration distances of different phenotypes were recorded by measuring the diameters of the colony zones.
2.5. Biofilm Formation Assay
According to the experimental method reported by Liu et al. [24], the biofilm formation abilities of H. alvei H4 strains were evaluated. Briefly, 200 μL of wild, mutant and complementation phenotypes were incubated at 30 °C for 48 h in a 96-well plate. Then the culture suspension was removed and the plate was rinsed thrice with PBS (pH 7.4, 0.01 M) and 200 μL methanol and 200μL of 0.1% crystal violet were added for immobilization and as dye. The plate was again rinsed thrice with deionized water and dried at 60 °C. The biofilm was extracted using 200 μL 33% acetic acid followed by a 20 min incubation at room temperature. The absorbance was recorded at 590 nm with an ultraviolet spectrophotometer.
2.6. Decarboxylase Detection
Based on Chang’s work [25], the decarboxylase production abilities of H. alvei H4 strains were estimated with some modifications. Briefly, one colony of wild-type, ΔluxI, ΔluxR and ΔluxIR strains were cultivated overnight in 5 mL of LB broth. Then 1 mL of culture was added to 9 mL of the decarboxylase media (LB supplemented with 0.05% glucose, 0.1% Tween-80, 0.02% MgSO4, 0.005% MnSO4, 0.004% FeSO4, 0.2% ammonium citrate, 0.001% thiamine, 0.2% K2PO4, 0.01% CaCO3, 0.005% pyridoxal-5-phosphate, 0.006% bromocresol purple, 2% aga) in a screw-cap test tube containing 0.1% precursor amino acid and cultivated for 24 h. Then the chromogenic reaction of the mixture was observed.
2.7. HPLC Analysis of BA Production
The determination of BAs was conducted based on the work of Wang et al. [26]. H. alvei H4 strains (wild type, ΔluxI, ΔluxR and ΔluxIR) were cultivated in LB supplemented with 0.005% pyridoxal-5-phosphate and 0.1% precursor amino acid for 24 h. Then 1 mL of culture was mixed with 9 mL 10% trichloroacetic acid in a centrifuge tube. After standing for 2 h at 4 °C, the mixture was homogenized for 10 min (3000× g). A 200 μL volume of supernatant was derivatized using 80 μL 2 mol/L NaOH and 800 μL 10 mg/mL dansyl chloride dissolved in acetone. After water-bath heating at 45 °C for 40 min, 50 μL ammonium hydroxide and 550 μL acetonitrile were added into the dansyl derivatives, homogenized for 5 min (3000× g) and filtrated through a 0.22 μm filter. Finally, 10 μL aliquots were injected for HPLC analysis.
The concentrations of BAs were determined by an HPLC system (ZORBAX, Agilent, Tokyo, Japan). An SB-C18 reversed-phase column (5 μm, 4.6 mm × 125 mm; Agilent, Tokyo, Japan) was used for chromatographic separation. The gradient elution program was operated with acetonitrile/water as the mobile phase.
2.8. Statistical Analysis
Each sample was subjected to three replicate trials, and all experiments were repeated three times. Results were presented as mean standard deviation (SD) and analyzed by t-test using SPSS 16.0 software, assuming statistical significance at p < 0.05. The Self-Organizing Map (SOM) was established by HMM toolbox (MATLAB7.8, The Math Works, R2009) to classify data patterns of the putrescine and cadaverine productivity of wild type, ΔluxI, ΔluxR and ΔluxIR. All of the graphs were made by origin version 8.0.
3. Results
3.1. Mutant and Complementation Strains Construction
H. alvei coordinates communal behavior as a function of population density by lux-type QS systems. This mechanism typically involves N-acyl homoserine lactones (AHLs), a kind of AI signaling molecule, described as a “language” of cell-to-cell communication, which is used by H. alvei to understand changes in its environment and consequently to apply specific strategies that allow adaptation to environmental stress in space and time [27]. The synthesis of AHLs is regulated by luxI, an AHL synthases gene. The sense of AHLs is regulated by luxR, a response transcriptional regulator gene. This could potentially lead to multiple target spoilage behaviors being regulated. For characterizing the QS system in H. alvei H4, the luxI, luxR and luxIR gene knockout mutants and their complemented strains were constructed. Through PCR analysis (Figure 1), the bands corresponding to the luxI, luxR and luxIR genes in the ΔluxI, ΔluxR and ΔluxIR mutant phenotypes, respectively, were not detected due to the target fragment being replaced with chloramphenicol. Furthermore, the corresponding lost bands recurred in the complementation strains, comI, comR and comIR, which is attributed to the recovery of the luxI, luxR and luxIR genes.
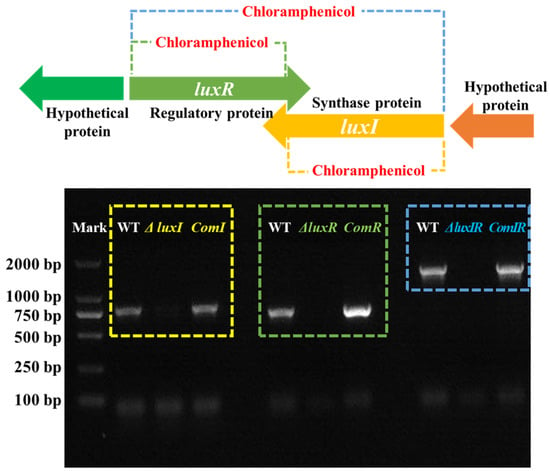
Figure 1.
PCR analysis of the mutant and complementation phenotypes.
3.2. Growth Ability, Swimming Motility and Biofilm Formation
The luxI, luxR and luxIR gene knockout mutant phenotypes of H. alvei H4 were successfully constructed and their growth ability, swimming motility and biofilm formation were investigated (Figure 2). As shown in Figure 2A, the growth curves of H. alvei H4 including wild, mutant and complementation phenotypes were obtained and fitted by Gompertz model. In wild-type H. alvei H4, the growth ability is extremely strong. After the lag time of 1.01 h, it quickly enters the exponential phase with a maximum specific growth rate at 0.26. Through the knockout of the luxI and luxR genes, it is found that the growth ability was not obviously affected in the lag phase. However, the luxI and luxR genes had an influence on growth rate in the exponential phase and maximal biomass in the stationary phase. The Vmax of mutants (around 0.21 h−1) was lower than wild and complementation types (0.26 h−1), especially the ΔluxI strain with a 23% descent rate. This result indicated that the QS system can only be triggered in the exponential phase when the microbial population density in the environment reaches the “quorum” threshold. H. alvei H4 also has a strong flagellar-dependent swimming ability and biofilm formation to ensure that it can move to seek a good nutritional matrix, excellently adhere to the food surface and resist antimicrobial substances. As shown in Figure 2B, H. alvei H4 flagella-dependent swimming is regulated by the luxI and luxR genes. The migration distance of the wild type increased rapidly with incubation time within 48 h and then remained constant by plate restriction, indicating that H. alvei H4 has a strong flagella-dependent swimming ability, while the strains without luxI or luxR genes were slightly inferior. Compared with the wild type, the migration distances of the ΔluxI, ΔluxR and ΔluxIR strains at 24 h were reduced by 50.0%, 64.3% and 54.3%, respectively. Furthermore, the biofilm formation ability of different phenotypes was evaluated by the crystal violet assay (Figure 2C). At 24 h, the biofilm yield of the H. alvei H4 mutant was significantly lower than that of the wild type. This phenomenon is similar to Pseudomonas aeruginosa [28] and Acinetobacter baumannii [29]. As the culture time prolonged, the differences between the wild type and the mutants in biofilm formation gradually increased. Thereby, the QS system is involved in the regulation of flagellar-dependent swimming ability and biofilm formation. These results also agree with Li’s work [30], where it is found that biofilm formation and swinging motility of H. alvei are regulated by the lux-type QS system.
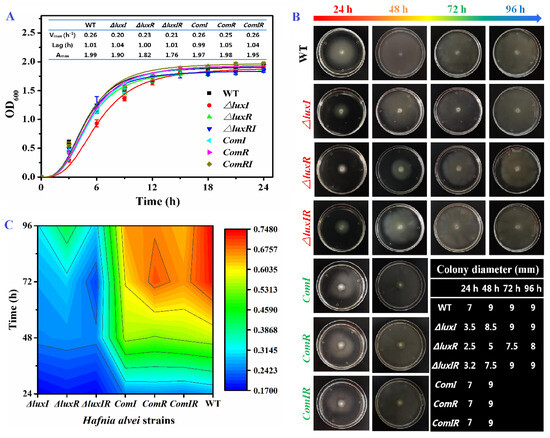
Figure 2.
Characterizations of wild, mutant and complementation phenotypes. (A) Growth curve fitted with the Gompertz model. (B) Swimming motility. (C) The contour of biofilm formation.
3.3. Decarboxylase Detection
Wild and mutant phenotypes of H. alvei H4 strains were cultivated with a variety of precursor amino acids. A chromogenic reaction can distinguish whether the strain produces decarboxylase [31]. As shown in Figure 3, both mediums with L-ornithine and L-lysine precursors changed color from orange to deep red, while the color of other mediums did not change significantly, indicating H. alvei H4 has an ability to produce putrescine and cadaverine. Unfortunately, through this experiment, the difference in the yield of decarboxylase between the mutant strains and the wild strain cannot be discriminated by the decarboxylase chromogenic reaction. Therefore, HPLC experiments were carried out for further study.
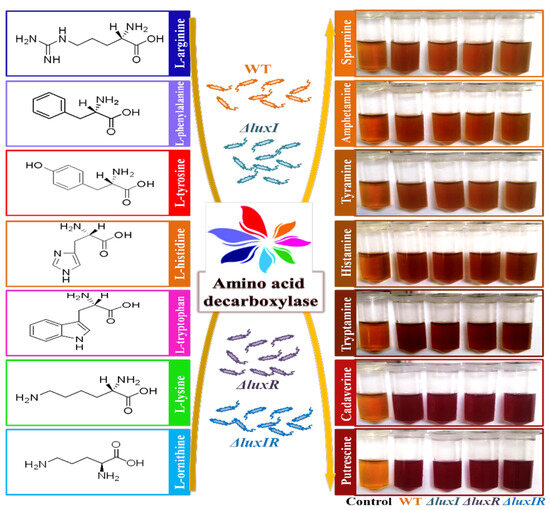
Figure 3.
Decarboxylase detection of wild and mutant phenotypes by mediums with different precursor amino acids.
3.4. Putrescine and Cadaverine Production
HPLC was applied to further quantitatively analyze the influence of luxI and luxR genes on putrescine and cadaverine production. As shown in Figure 4, two distinct high absorption peaks based on putrescine and cadaverine were found in the HPLC spectrum of biogenic amine production for each phenotypic strain of H. alvei H4. Through Figure 3 (Insert), it can be observed that the yield of the mutant strains of putrescine and cadaverine is significantly different from that of the wild type, especially cadaverine.
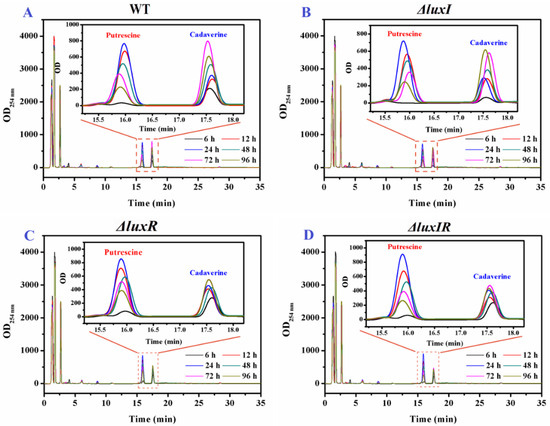
Figure 4.
The HPLC results of the BAs from H. alvei H4 strains including wild and mutant phenotypes, (A) wild type, (B) ΔluxI, (C) ΔluxR and (D) ΔluxIR.
The concentrations of putrescine were specifically analyzed in Figure 5A. All strains produced a large amount of putrescine in culture for 6–12 h. Compared with 6 h, the concentrations of putrescine in wild-type, ΔluxI, ΔluxR and ΔluxIR strains at 12 h increased by 19.7, 14.1, 8.44 and 10.8-fold, respectively. The maximum production and accumulation of putrescine in wild type, ΔluxI, ΔluxR and ΔluxIR were detected at 24 h, reaching up to 695.23 mg/L, 683.02 mg/L, 776.30 mg/L and 724.12 mg/L, respectively. For an in-depth analysis of the influence of luxI and luxR genes on putrescine productivity through data analysis, an identification model SOM including input layer and output layer was established. The input layer is a two-dimensional node matrix, where each node corresponds to a neuron representing the putrescine concentration of wild type, ΔluxI, ΔluxR and ΔluxIR throughout the culture (0–96 h). As shown in Figure 5B, the samples in the output layer were distinctly divided into four categories, and each category consisted of three samples belonging to wild type, ΔluxI, ΔluxR and ΔluxIR, respectively, without misclassified phenomena; hence, the accuracy rate of the prediction set was 100%. This demonstrated a significant difference in the production of putrescine between the various phenotypes. In Figure 5C, the topological function and distance function of the SOM are applied in 1000 iterations to describe the gap between the various phenotypic strains. The distance between wild type and ΔluxI and ΔluxR was bright yellow and dark yellow, respectively. The distance between ΔluxIR and ΔluxR is red, and the distance between ΔluxIR and ΔluxI is black, indicating that the ΔluxR phenotype is more distinct from the wild type in the putrescine accumulation process than the ΔluxI phenotype. In Figure 5D, the putrescine concentration profile is set to the modeled input, each neuron in the input layer is compared to each other neuron in the output layer by weight, and the dark to light color is applied depending on the magnitude of the weight [32]. Compared to the wild-type cluster, the ΔluxI cluster with the highest weight was black in the lag phase (6 h). After that, the weight of the ΔluxR cluster increased, replacing the ΔluxI cluster and turning into black from 12 h.
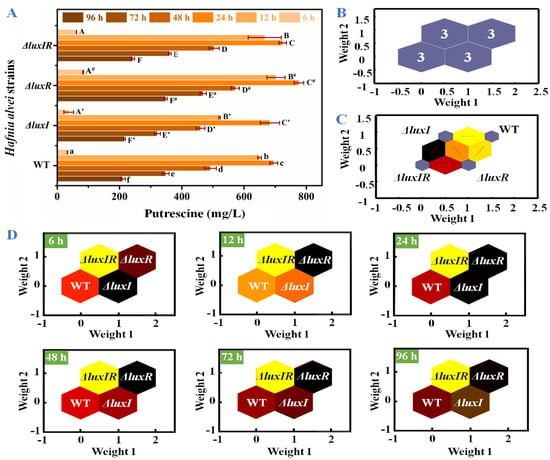
Figure 5.
(A) The concentrations of putrescine, (B) visualized clustering results of SOM, (C) weight distances between adjacent neurons, (D) weight analysis of the wild-type and mutant phenotypes at different times.
Likewise, the influence of the luxI and luxR genes on cadaverine production was specifically analyzed. As shown in Figure 6A, after 72 h of culture, the maximum concentration of cadaverine produced by wild strains was 252.7 mg/L, while the cadaverine concentrations of ΔluxI, ΔluxR and ΔluxIR were 194.5 mg/L, 175.1 mg/L and 154.2 mg/L, respectively, indicating that the luxI and luxR genes have a potential upregulation on the production of cadaverine. According to Wang’s work [26], similar results were obtained, where the amounts of putrescine and cadaverine of ΔluxI strains were always lower (p < 0.05) compared with wild-type H. alvei H4. The cadaverine concentrations of different phenotypic strains at 0–96 h were used as the basis neuron for SOM analysis in Figure 6B. According to the clustering property, it can be divided into four categories: wild type, ΔluxI, ΔluxR and ΔluxIR. The total identification accuracy is 100%. Through the self-organizing competition of the SOM network, the adjacent neurons were quantified. As shown in Figure 6C, the weight distances between neuron 1 of ΔluxIR and neuron 2 of ΔluxI was closest, suggesting that the cadaverine production process of the two is most similar. The weight distances between neuron 1 of ΔluxIR and neuron 4 of ΔluxR was most dissimilar. Moreover, the weight distance between wild type and ΔluxI was lighter than between wild type and ΔluxR, implying that luxR plays a more important role than luxI during the cadaverine production process. In further SOM analysis of different culture times as shown in Figure 6D, luxI plays a major role in the early stages of cadaverine production (6 h), and as the culture time prolongs (12–96 h), the role of the luxR gene gradually emerges.
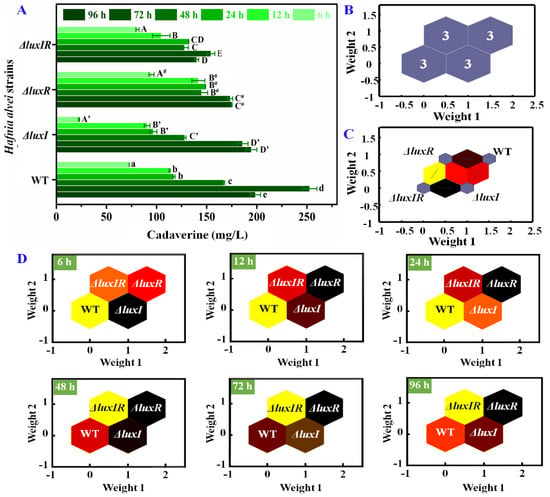
Figure 6.
(A) The concentrations of cadaverine, (B) visualized clustering results of SOM, (C) weight distances between adjacent neurons, (D) weight analysis of the wild-type and mutant phenotypes at different times.
In wild-type H. alvei H4, putrescine and cadaverine were mass-produced and accumulated during the exponential phase until the maximum was reached in the stationary phase. After that, putrescine and cadaverine were gradually consumed as available nitrogen sources, which may be due to a lack of nitrogen in the culture medium at the end of the culture period. Through comparative analysis of mutant and wild strains using SOM, it is not difficult to find that the knockout of any gene of luxI and luxR may significantly affect the production of putrescine and cadaverine, and the luxR gene has a greater impact. Similar to other corruption characteristics, the influence of the luxI gene on putrescine and cadaverine is mostly reflected in the lag phase, while the effect of the luxR gene is mainly concentrated in the exponential phase and stationary phase with a high density of bacteria. These results also agree with Yan’s work [33], where amino acid metabolism was associated with the luxI/R gene, which was also co-regulated in a growth phase-dependent manner. Choi et al. [34] also found that anthranilate metabolism was phase-dependently regulated by las/rhl quorum sensing in Pseudomonas aeruginosa. Anthranilate synthesis was especially activated by LasR in the log phase and repressed by RhlR; whereas, anthranilate degradation was repressed by LasR during the log phase and activated by RhlR in the late stationary phase. Bacterial quorum sensing (QS)-dependent gene expression is a dynamic response to cell density [35], thereby, it can be demonstrated in the effects of lux-type QS on spoilage characteristics in H. alvei H4. When H. alvei H4 grows in a lag phase with a slow growth rate and low cell density, the luxI gene is the main force of the QS system to regulate spoilage characteristics. According to Zhu’s work [36], the reason for this phenomenon may be that when the bacteria density is low, LuxI has to continuously strive to synthesize AHL signal molecules to achieve the threshold concentration and pair with the receptor protein LuxR to trigger the regulation of spoilage. With the growth of bacteria, the density of bacteria increases and the amount of AHL signal molecules is sufficient. The key point affecting QS regulation of spoilage characteristics, including mobility, biofilm formation and secretion of ornithine and lysine decarboxylase, is no longer the luxI gene but the luxR gene.
4. Conclusions
In this paper, the effects of the lux-type QS system on the growth characteristics and BA production of H. alvei H4 was explored. As the functions of the luxI and luxR genes are different, lux-type QS exhibited phase-differential regulation on growth, flagella-dependent swimming ability, biofilm formation and putrescine and cadaverine synthesis. In the lag phase, the population density is low, and luxI is the most critical factor affecting bacterial growth and BA production. Whereas, the luxR gene plays a major role in regulation of mobility, biofilm formation and production of putrescine and cadaverine in the exponential phase and stationary phase with a high density of bacteria. In short, the specific phase-dependent regulation mechanisms of the lux-type QS system on spoilage characteristics of H. alvei H4 and the transcriptional changes of luxI and luxR genes in the various growth phases (or different cell densities) and their functional characterizations will be subjects of our future research. The association of bacterial metabolism with the luxI and luxR genes still requires more research to determine the role of QS in H. alvei.
Author Contributions
Conceptualization, J.B.; investigation, Q.Y.; writing—original draft preparation, G.Z.; writing—review and editing, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This article was supported by the national key research and development project (2022YFD2100500), the national natural science foundation of China (32102050), and Liaoning provincial education department basic scientific research projects (LJKMZ20220875).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We gratefully appreciate the editors’ efficient work in processing our manuscript and the anonymous reviewers’ careful work and thoughtful suggestions that have helped improve this paper substantially.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Davares, A.K.L.; Arsene, M.M.J.; Viktorovna, P.I.; Vyacheslavovna, Y.N.; Vladimirovna, Z.A.; Aleksandrovna, V.E.; Nikolayevich, S.A.; Nadezhda, S.; Anatolievna, G.O.; Nikolaevna, S.I.; et al. Quorum-Sensing Inhibitors from Probiotics as a Strategy to Combat Bacterial Cell-to-Cell Communication Involved in Food Spoilage and Food Safety. Fermentation 2022, 8, 711. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3538–3555. [Google Scholar] [CrossRef]
- Turna, N.S.; Chung, R.; McIntyre, L. A review of biogenic amines in fermented foods: Occurrence and health effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Tapia, O.; Elexpuru-Zabaleta, M.; Pifarre, K.T.; Armas Diaz, Y.; Battino, M.; Giampieri, F. The Molecular Weaponry Produced by the Bacterium Hafnia alvei in Foods. Molecules 2022, 27, 5585. [Google Scholar] [CrossRef]
- Hernandez-Galan, L.; Cattenoz, T.; Le Feunteun, S.; Canette, A.; Briandet, R.; Le-Guin, S.; Guedon, E.; Castellote, J.; Delettre, J.; Dugat Bony, E.; et al. Effect of dairy matrices on the survival of Streptococcus thermophilus, Brevibacterium aurantiacum and Hafnia alvei during digestion. Food Res. Int. (Ott. Ont.) 2017, 100 Pt 1, 477–488. [Google Scholar] [CrossRef]
- Orozova, P.; Sirakov, I.; Chikova, V.; Popova, R.; Al-Harbi, A.H.; Crumlish, M.; Austin, B. Recovery of Hafnia alvei from diseased brown trout, Salmo trutta L., and healthy noble crayfish, Astacus astacus (L.), in Bulgaria. J. Fish Dis. 2014, 37, 891–898. [Google Scholar] [CrossRef]
- Guo, Q.-Y.; Shan, K.; Yang, X.; Jiang, C.-J.; Zhu, L. Inhibitory effects of pH, salinity, and tea polyphenols concentration on the specific spoilage organisms isolated from lightly-salted large yellow croaker (Pseudosciaena crocea). Food Sci. Nutr. 2022, 10, 3062–3071. [Google Scholar] [CrossRef]
- Geeraerts, W.; De Vuyst, L.; Leroy, F.; Van Kerrebroeck, S. Monitoring of volatile production in cooked poultry products using selected ion flow tube-mass spectrometry. Food Res. Int. (Ott. Ont.) 2019, 119, 196–206. [Google Scholar] [CrossRef]
- Merchán, A.V.; Ruiz-Moyano, S.; Hernández, M.V.; Martín, A.; Lorenzo, M.J.; Benito, M.J. Characterization of autochthonal Hafnia spp. strains isolated from Spanish soft raw ewe’s milk PDO cheeses to be used as adjunct culture. Int. J. Food Microbiol. 2022, 373, 109703. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Ruan, L.-Y.; Chen, H.-J.; Luo, H.-Z.; Jiang, H.; Wang, J.-S.; Jia, A.-Q. Inhibition of Quorum Sensing and Virulence in Serratia marcescens by Hordenine. J. Agric. Food Chem. 2019, 67, 784–795. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Z.; Mao, X. Application of Quorum Sensing in Metabolic Engineering. J. Agric. Food Chem. 2023, 71, 5062–5074. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Ding, X.; Bin, P.; Yang, Y.; Zhu, G. Regulatory Mechanisms between Quorum Sensing and Virulence in Salmonella. Microorganisms 2022, 10, 2211. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef]
- Yan, C.; Li, X.; Zhang, G.; Bi, J.; Hao, H.; Hou, H. Quorum Sensing (QS)-regulated target predictions of Hafnia alvei H4 based on the joint application of genome and STRING database. Food Res. Int. (Ott. Ont.) 2022, 157, 111356. [Google Scholar] [CrossRef]
- Li, S.; Wu, S.; Ren, Y.; Meng, Q.; Yin, J.; Yu, Z. Characterization of differentiated autoregulation of LuxI/LuxR-type quorum sensing system in Pseudoalteromonas. Biochem. Biophys. Res. Commun. 2022, 590, 177–183. [Google Scholar] [CrossRef]
- Ridgway, W.; Ward, M.J.; Wetton, B.T. Quorum-sensing induced transitions between bistable steady-states for a cell-bulk ODE-PDE model with lux intracellular kinetics. J. Math. Biol. 2021, 84, 5. [Google Scholar] [CrossRef]
- Hou, H.M.; Zhu, Y.L.; Wang, J.Y.; Jiang, F.; Qu, W.Y.; Zhang, G.L.; Hao, H.S. Characteristics of N-Acylhomoserine Lactones Produced by Hafnia alvei H4 Isolated from Spoiled Instant Sea Cucumber. Sensors 2017, 17, 772. [Google Scholar] [CrossRef]
- Dehio, C.; Meyer, M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 1997, 179, 538–540. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Hou, H.M.; Zhang, G.L.; Wang, Y.F.; Hao, H.S. AHLs Regulate Biofilm Formation and Swimming Motility of Hafnia alvei H4. Front. Microbiol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Xia, F.; Zhang, C.; Jiang, Q.; Wu, Z.; Cao, S.; Wu, P.; Gao, Y.; Cheng, X. Microbiome analysis and growth behaviors prediction of potential spoilage bacteria inhabiting harvested edible mushrooms. J. Plant Dis. Prot. 2024, 131, 77–90. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Chen, S.; Huang, J.; Li, T.; Duan, C.; Zhang, L.H.; Xu, Z. CzcR Is Essential for Swimming Motility in Pseudomonas aeruginosa during Zinc Stress. Microbiol. Spectr. 2022, 10, e0284622. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Dong, P.; Ni, L.; Luo, X.; Zhang, Y.; Zhu, L. Inhibitory effects of clove and oregano essential oils on biofilm formation of Salmonella Derby isolated from beef processing plant. LWT 2022, 162, 113486. [Google Scholar] [CrossRef]
- Chang, M.; Chang, H.C. Development of a screening method for biogenic amine producing Bacillus spp. Int. J. Food Microbiol. 2012, 153, 269–274. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, G.; Bi, J.; Hou, H. Transcriptome Reveals Regulation of Quorum Sensing of Hafnia alvei H4 on the Coculture System of Hafnia alvei H4 and Pseudomonas fluorescens ATCC13525. Foods 2024, 13, 336. [Google Scholar] [CrossRef]
- Hongman, H.; Yifang, W.; Gongliang, Z.; Yaolei, Z.; Longquan, X.; Hongshun, H.; Yue, W.; Meishan, L.J. Effects of Sulfide Flavors on AHL-Mediated Quorum Sensing and Biofilm Formation of Hafnia alvei. J. Food Sci. 2018, 83, 2550–2559. [Google Scholar] [CrossRef]
- Yan, H.; Liu, C.; Yu, W.; Zhu, X.; Chen, B. The aggregate distribution of Pseudomonas aeruginosa on biochar facilitates quorum sensing and biofilm formation. Sci. Total Environ. 2023, 856, 159034. [Google Scholar] [CrossRef]
- Law, S.K.K.; Tan, H.S. The role of quorum sensing, biofilm formation, and iron acquisition as key virulence mechanisms in Acinetobacter baumannii and the corresponding anti-virulence strategies. Microbiol. Res. 2022, 260, 127032. [Google Scholar] [CrossRef]
- Li, T.; Mei, Y.; He, B.; Sun, X.; Li, J. Reducing Quorum Sensing-Mediated Virulence Factor Expression and Biofilm Formation in Hafnia alvei by Using the Potential Quorum Sensing Inhibitor L-Carvone. Front. Microbiol. 2018, 9, 3324. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.W.; Cheng, W. Development of simplified models for nondestructive hyperspectral imaging monitoring of TVB-N contents in cured meat during drying process. J. Food Eng. 2017, 192, 53–60. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, M.; Pan, H.; Li, S.; Ren, B.; Ren, Z.; Xing, N.; Qi, L.; Ren, Q.; Xu, S.; et al. Does time difference of the acetylcholinesterase (AChE) inhibition in different tissues exist? A case study of zebra fish (Danio rerio) exposed to cadmium chloride and deltamethrin. Chemosphere 2017, 168, 908–916. [Google Scholar] [CrossRef]
- Yan, C.; Li, X.; Zhang, G.; Zhu, Y.; Bi, J.; Hao, H.; Hou, H. Quorum Sensing-Mediated and Growth Phase-Dependent Regulation of Metabolic Pathways in Hafnia alvei H4. Front. Microbiol. 2021, 12, 567942. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.-Y.; Park, S.J.; Park, S.-J.; Kim, S.-K.; Ha, C.; Im, S.-J.; Lee, J.-H. Growth phase-differential quorum sensing regulation of anthranilate metabolism in Pseudomonas aeruginosa. Mol. Cells 2011, 32, 57–65. [Google Scholar] [CrossRef]
- Boo, A.; Ledesma Amaro, R.; Stan, G.-B. Quorum sensing in synthetic biology: A review. Curr. Opin. Syst. Biol. 2021, 28, 100378. [Google Scholar] [CrossRef]
- Zhu, Y.; Sang, X.; Li, X.; Zhang, Y.; Hao, H.; Bi, J.; Zhang, G.; Hou, H. Effect of quorum sensing and quorum sensing inhibitors on the expression of serine protease gene in Hafnia alvei H4. Appl. Microbiol. Biotechnol. 2020, 104, 7457–7465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).