The Potential of Multi-Screening Methods and Omics Technologies to Detect Both Regulated and Emerging Mycotoxins in Different Matrices
Abstract
1. Introduction
2. Impact of Mycotoxins on Animal and Human Health
3. Challenges in the Analysis of Emerging and Hidden Mycotoxins
4. Transition from Targeted Analysis to Targeted/Untargeted Multiscreening-Based HRMS Approaches
4.1. Applications of Multiscreening Methods for Biofluids
4.2. Applications of Multiscreening Methods for Food Samples
4.3. Applications of Multiscreening Methods for Feed Samples
5. Omics Technologies as a Valuable and Emerging Tool to Assess Mycotoxicity
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gallo, A.; Ghilardelli, F.; Atzori, A.S.; Zara, S.; Novak, B.; Faas, J.; Fancello, F. Co-Occurrence of regulated and emerging mycotoxins in corn silage: Relationships with fermentation quality and bacterial communities. Toxins 2021, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors influencing production and control strategies. Aims Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Janić Hajnal, E.; Pleadin, J. Climate change—A global threat resulting in increasing mycotoxin occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food. Saf. 2024, 23, e13323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Tsiouris, V.; Tassis, P.; Raj, J.; Mantzios, T.; Kiskinis, K.; Vasiljević, M.; Delić, N.; Petridou, E.; Brellou, G.D.; Polizopoulou, Z.; et al. Investigation of a novel multicomponent mycotoxin detoxifying agent in amelioration of mycotoxicosis induced by aflatoxin-B1 and ochratoxin A in broiler chicks. Toxins 2021, 13, 367. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aflatoxins, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2012; Volume 100F.
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Dhakal, A.; Hashmi, M.F.; Sbar, E. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eissa, F.; Sebaei, A.S. A comparative study between the top 10 origin countries involved in the EU RASFF notifications on aflatoxins from 1997 to 2022. Microb. Risk Anal. 2023, 25, 100277. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin contamination: An overview on health issues, detection and management strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 toxins: Toxicity, occurrence and analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, D.; Cai, P.; Lin, H.; Ying, H.; Hu, Q.-N.; Wu, A. Elimination of Fusarium mycotoxin deoxynivalenol (DON) via microbial and enzymatic strategies: Current status and future perspectives. Trends Food Sci. Technol. 2022, 124, 96–107. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Patyal, A.; Thakur, R.; Sharma, H.; Shakya, S. Mitigating the health risks of mycotoxins: Concerns and solutions vis-à-vis food web. Int. J. Livest. Res. 2020, 10, 1–14. [Google Scholar]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, T.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F.; Gao, T. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020, 194, 110420. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Franchino, C.; Vita, V.; Iammarino, M.; De Pace, R. Monitoring of animal feed contamination by mycotoxins: Results of five years of official control by an accredited Italian laboratory. Microorganisms 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S.; et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-occurrence of mycotoxins in feed for cattle, pigs, poultry, and sheep in Navarra, a region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Oswald, I.P.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [PubMed]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.M. Balkan Endemic Nephropathy—Current Status and Future Perspectives. Clin. Kidney J. 2013, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of ochratoxin A on livestock production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef] [PubMed]
- Vörösházi, J.; Neogrády, Z.; Mátis, G.; Mackei, M. Pathological consequences, metabolism and toxic effects of trichothecene T-2 toxin in poultry. Poultry Sci. 2024, 103, 103471. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159, 7–24. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sdogati, S.; Pacini, T.; Bibi, R.; Caporali, A.; Verdini, E.; Orsini, S.; Ortenzi, R.; Pecorelli, I. Co-occurrence of aflatoxin B1, zearalenone and ochratoxin A in feed and feed materials in central Italy from 2018 to 2022. Foods 2024, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ toxicological mechanisms involving humans, livestock and their associated health concerns: A review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Bren, U.; Stone, M.P.; Guengerich, F.P. Inherent stereospecificity in the reaction of aflatoxin B(1) 8,9-epoxide with deoxyguanosine and efficiency of DNA catalysis. Chem. Res. Toxicol. 2009, 22, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of aflatoxins induces severe hepatotoxicity through multiple mechanisms. Front. Pharmacol. 2020, 11, 605823. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, F.; Gholami-Borujeni, F. Review, meta-analysis and carcinogenic risk assessment of aflatoxin M1 in different types of milks in Iran. Rev. Environ. Health 2022, 38, 511–518. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Ali, S.; Freire, L.G.D.; Rezende, V.T.; Noman, M.; Ullah, S.; Abdullah; Badshah, G.; Afridi, M.S.; Tonin, F.G.; de Oliveira, C.A.F. Occurrence of mycotoxins in foods: Unraveling the knowledge gaps on their persistence in food production systems. Foods 2023, 12, 4314. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Fut. Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A systematic review of global occurrence of emerging mycotoxins in crops and animal feeds, and their toxicity in livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- Okasha, H.; Song, B.; Song, Z. Hidden hazards revealed: Mycotoxins and their masked forms in poultry. Toxins 2024, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Russo, M.; La Rocca, C.; Pinto, O.; Mantovani, A. Modified mycotoxins, a still unresolved issue. Chemistry 2022, 4, 1498–1514. [Google Scholar] [CrossRef]
- Righetti, L.; Paglia, G.; Galaverna, G.; Dall’Asta, C. Recent advances and future challenges in modified mycotoxin analysis: Why HRMS has Become a key instrument in food contaminant research. Toxins 2016, 8, 361. [Google Scholar] [CrossRef]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and bioavailability assessment using Caco-2 cell monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; de Boevre, M.; Preußke, N.; de Saeger, S.; Birr, T.; Verreet, J.A.; Sönnichsen, F.D. Evaluation of high-resolution mass spectrometry for the quantitative analysis of mycotoxins in complex feed matrices. Toxins 2019, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Gaspari, A.; Grosso, M.; Rodríguez-Carrasco, Y.; Ritieni, A. Target analysis and retrospective screening of contaminants in ready-to-eat cooked ham samples through UHPLC-Q-Orbitrap HRMS. Food Chem. 2023, 408, 135244. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Vinas, P. High-resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Izzo, L.; Narváez, A.; Castaldo, L.; Gaspari, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Multiclass and multi-residue screening of mycotoxins, pharmacologically active substances, and pesticides in infant milk formulas through ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry analysis. J. Dairy Sci. 2022, 105, 2948–2962. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, X.; Liang, R.; Guo, T.; Xiao, Q.; Xia, B.; Wan, Y.; Zhou, Y. Determination of 63 mycotoxins in grain products by ultrahigh-performance liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. Food Cont. 2023, 150, 109772. [Google Scholar] [CrossRef]
- De Dominicis, E.; Commissati, I.; Gritti, E.; Catellani, D.; Suman, M. Quantitative targeted and retrospective data analysis of relevant pesticides, antibiotics and mycotoxins in bakery products by liquid chromatography-single-stage Orbitrap mass spectrometry. Food Addit. Contam Part A 2015, 32, 1617–1627. [Google Scholar] [CrossRef]
- Righetti, L.; Dreolin, N.; Celma, A.; McCullagh, M.; Barknowitz, G.; Sancho, J.V.; Dall’Asta, C. Travelling wave ion mobility-derived collision cross section for mycotoxins: Investigating interlaboratory and interplatform reproducibility. J. Agric. Food Chem. 2020, 68, 10937–10943. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Righetti, L.; Bergmann, A.; Galaverna, G.; Rolfsson, O.; Paglia, G.; Dall’Asta, C. Ion mobility-derived collision cross section database: Application to mycotoxin analysis. Anal. Chim. Acta 2018, 1014, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion mobility spectrometry in food analysis: Principles, current applications and future trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Jedziniak, P. Development of a multi-mycotoxin LC-MS/MS method for the determination of biomarkers in pig urine. Mycotoxin Res. 2021, 37, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, J.; Dai, Z.; Lin, Y.; Lu, G.; Yang, S.; You, Y.; Liu, H.; Wu, Y.; Jiang, G.; et al. Data-dependent acquisition based high-resolution mass spectrum for trace Alternaria mycotoxin analysis and sulfated metabolites identification. Food Chem. 2021, 364, 130450. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Rodríguez-Canás, I.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte method for the determination of regulated, emerging and modified mycotoxins in milk: QuEChERS extraction followed by UHPLC–MS/MS analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Tolosa, J.; Graziani, G.; Gaspari, A.; Ritieni, A. Target analysis and retrospective screening of mycotoxins and pharmacologically active substances in milk using an ultra-high-performance liquid chromatography/high-resolution mass spectrometry approach. J. Dairy Sci. 2020, 103, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Ghilardelli, F.; Masoero, F.; Gallo, A. Screening of regulated and emerging mycotoxins in bulk milk samples by high-resolution mass spectrometry. Foods 2021, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, M.O.; Braun, D.; Windisch, P.; Warth, B.; Ezekiel, C.N. Assessment of multiple mycotoxins in raw milk of three different animal species in Nigeria. Food Cont. 2022, 131, 108258. [Google Scholar] [CrossRef]
- Le, L.H.T.; Tran-Lam, T.-T.; Nguyen, H.Q.; Quan, T.C.; Nguyen, T.Q.; Nguyen, D.T.; Dao, Y.H. A study on multi-mycotoxin contamination of commercial cashew nuts in Vietnam. J. Food Compos. Anal. 2021, 102, 104066. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Li, X.; Zhou, S.; Ma, J.; Zhao, X.; Zhang, Q.; Yin, X. Determination of multi-mycotoxins in vegetable oil via liquid chromatography-high resolution mass spectrometry assisted by a complementary liquid–liquid extraction. Food Chem. X 2023, 20, 100887. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Suman, M.; Krska, R. Quantification of 730 Mycotoxins and Other Secondary Metabolites of Fungi and Plants in Grain Products. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Chen, D.; Liang, W.; Lu, X.; Zhao, C.; Xu, G. Suspect and nontarget screening of mycotoxins and their modified forms in wheat products based on ultrahigh-performance liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2023, 1708, 464370. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Ferreiroa, V.; Rodríguez-Canas, I.; Alfonso, A.; Sainz, M.J.; Aguín, O.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Occurrence of mycotoxins and mycotoxigenic fungi in silage from the north of Portugal at feed-out. Int. J. Food Microbiol. 2022, 365, 109556. [Google Scholar] [CrossRef] [PubMed]
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.; Zhang, Q.; Giorgi, M.; Li, P.; Logrieco, A.F.; Poapolathep, A. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using ultra high performance liquid chromatography-tandem mass spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Graziani, G.; Gaspari, A.; Izzo, L.; Tolosa, J.; Rodríguez-Carrasco, Y.; Ritieni, A. Target analysis and retrospective screening of multiple mycotoxins in pet food using UHPLC-Q-Orbitrap HRMS. Toxins 2019, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Lanaridi, O.; Warth, B.; Ansari, K.M. Metabolomics as an emerging approach for deciphering the biological impact and toxicity of food contaminants: The case of mycotoxins. Crit. Rev. Food Sci. Nutr. 2023. [CrossRef] [PubMed]
- Owolabi, I.O.; Siwarak, K.; Greer, B.; Rajkovic, A.; Dall’asta, C.; Karoonuthaisiri, N.; Uawisetwathana, U.; Elliott, C.T.; Petchkongkaew, A. Applications of mycotoxin biomarkers in human biomonitoring for exposome-health studies: Past, present, and future. Expo. Health 2023. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- García-Pérez, P.; Becchi, P.P.; Zhang, L.; Rocchetti, G.; Lucini, L. Metabolomics and chemometrics: The next-generation analytical toolkit for the evaluation of food quality and authenticity. Trends Food Sci. Technol. 2024, 147, 104481. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Richard-Forget, F.; Atanasova, V.; Chéreau, S. Using metabolomics to guide strategies to tackle the issue of the contamination of food and feed with mycotoxins: A review of the literature with specific focus on Fusarium mycotoxins. Food Cont. 2021, 121, 107610. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ghilardelli, F.; Bonini, P.; Lucini, L.; Masoero, F.; Gallo, A. Changes of milk metabolomic profiles resulting from a mycotoxins-contaminated corn silage intake by dairy cows. Metabolites 2021, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef] [PubMed]
- Flasch, M.; Bueschl, C.; Woelflingseder, L.; Schwartz-Zimmermann, H.E.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Stable isotope-assisted metabolomics for deciphering xenobiotic metabolism in mammalian cell culture. ACS Chem. Biol. 2020, 15, 970–981. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zheng, N.; Guo, L.; Song, X.; Zhao, S.; Wang, J. Biological system responses of dairy cows to aflatoxin B1 exposure revealed with metabolomic changes in multiple biofluids. Toxins 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.; Jiang, Y.; Adeyemi, J.; Oliveira, A.; Vyas, D.; Adesogan, A. Biomarker of aflatoxin ingestion: 1H NMR-based plasma metabolomics of dairy cows fed aflatoxin B1 with or without sequestering agents. Toxins 2018, 10, 545. [Google Scholar] [CrossRef]
- Gerdemann, A.; Behrens, M.; Esselen, M.; Humpf, H.U. Metabolic profiling as a powerful tool for the analysis of cellular alterations caused by 20 mycotoxins in HepG2 cells. Arch. Toxicol. 2022, 96, 2983–2998. [Google Scholar] [CrossRef]
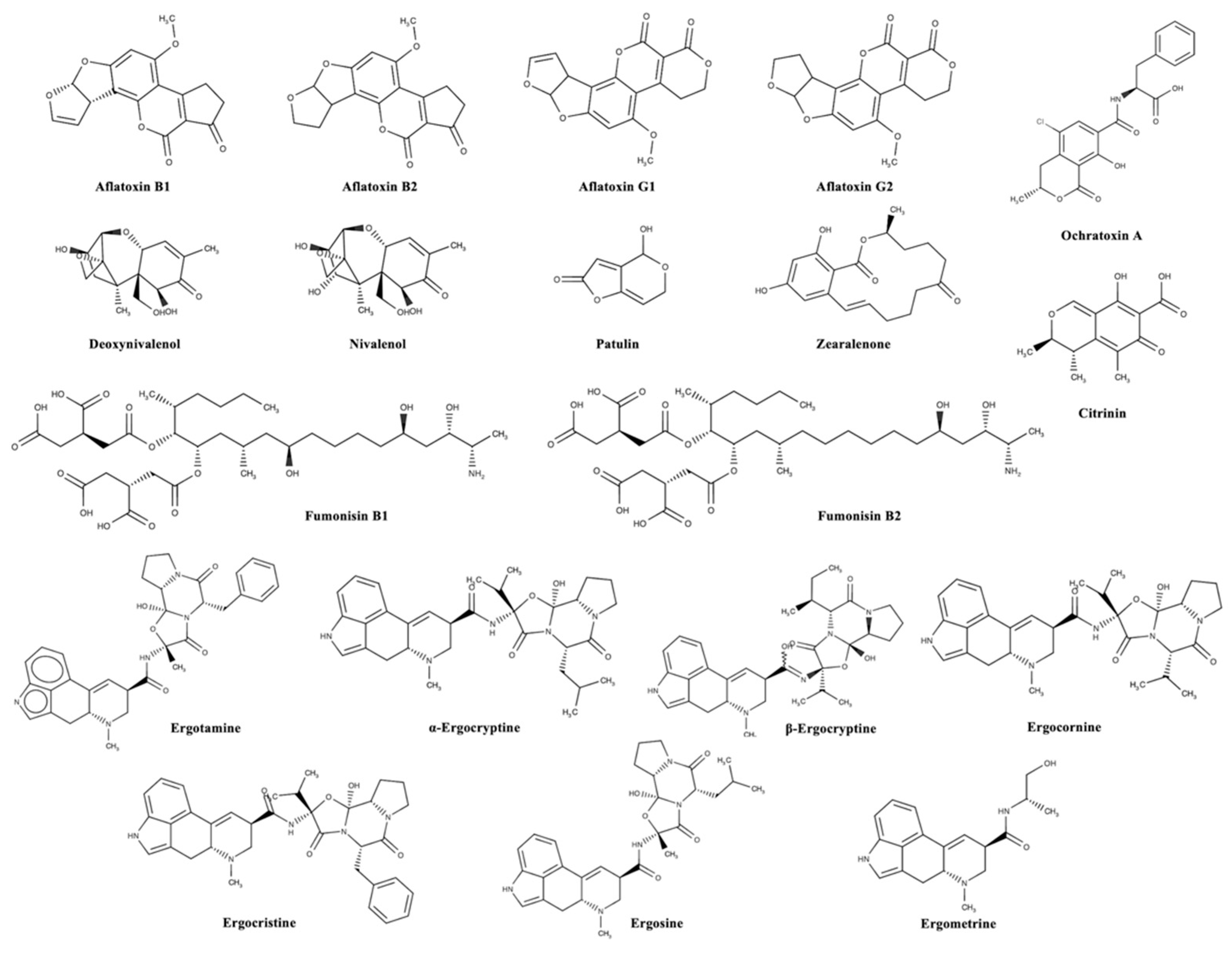
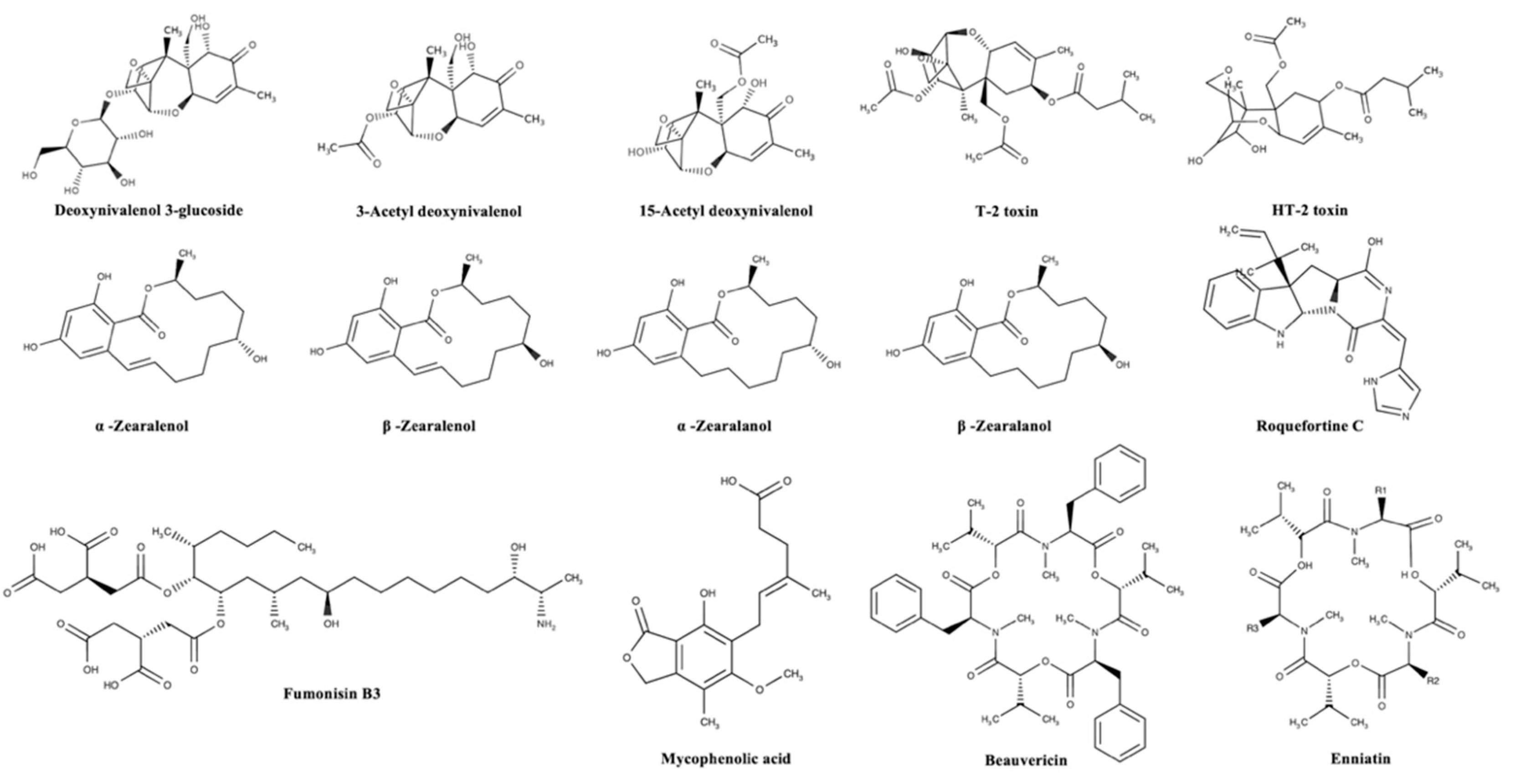
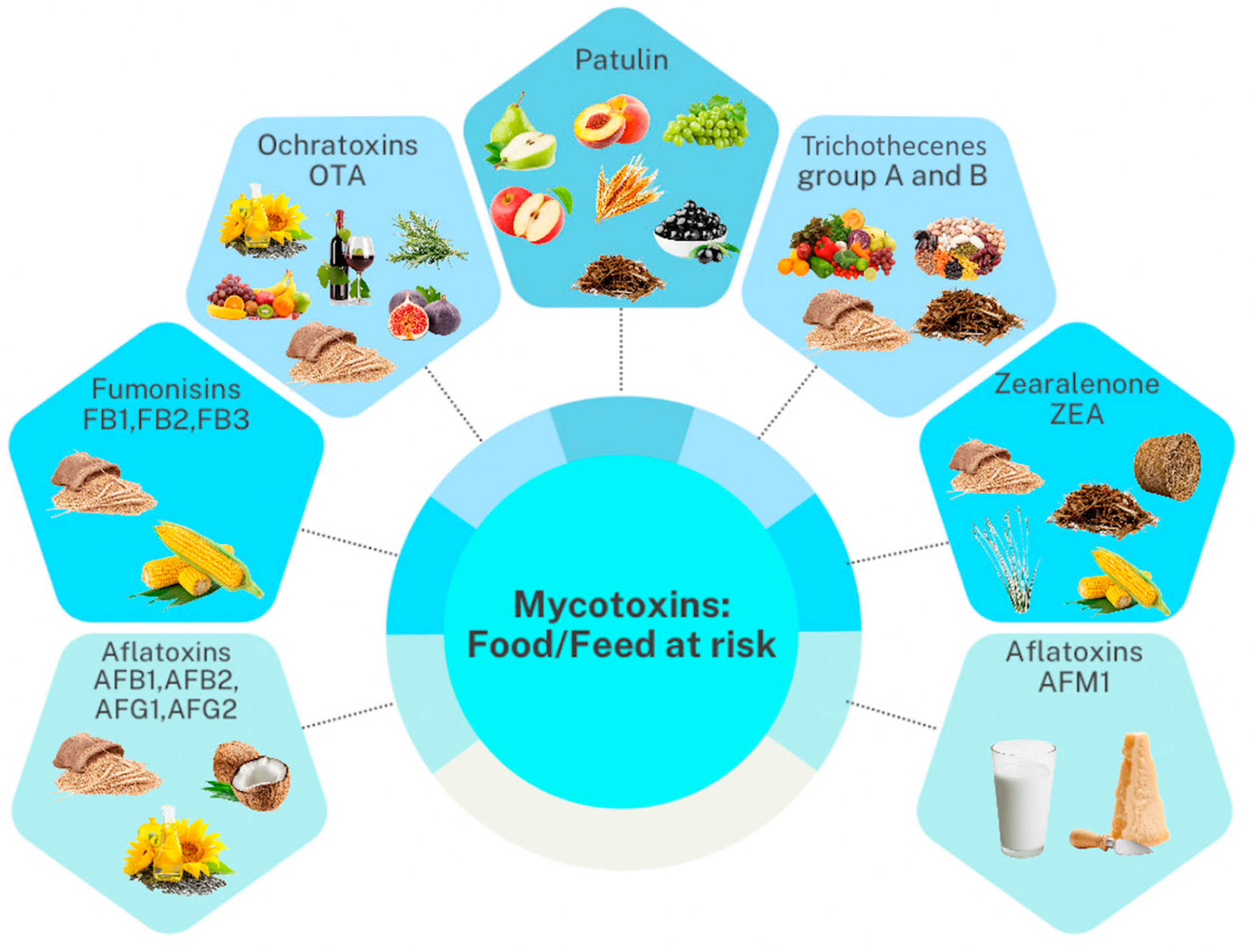

| Mycotoxin Group | Animal Species | Legal Limit (mg kg−1) |
|---|---|---|
| Aflatoxins (AFB1, AFB2, AFG1, AFG2) | Cows | 0.005 |
| Other species | 0.010 | |
| Cow, goat, sheep, poultry | 0.020 | |
| Type A-Trichothecenes (T-2 and HT-2 toxins) | All species | 0.25 |
| Type B-Trichothecenes (Deoxynivalenol, DON) | Swine | 0.9 |
| Calves, lambs, and young goats | 2.0 | |
| Other species | 5.0 | |
| Fumonisins (FB1, FB2, FB3) | Swine, equine, rabbits, and pet animals | 5.0 |
| Poultry, calves, lambs, and young goats | 20 | |
| Adult ruminants and minks | 50 | |
| Zearalenone (ZEN) | Piglets | 0.1 |
| Fattening pigs | 0.25 | |
| Calves, cows, sheep, and goats | 0.5 | |
| Ochratoxin A (OTA) | Swine | 0.05 |
| Poultry | 0.1 |
| Matrix | Multiscreening Method | Most Relevant Finding | Reference |
|---|---|---|---|
| Commercial grain products | UHPLC–ESI-Q/Orbitrap HRMS | Establishment of a customized accurate-mass database and mass spectral library for 63 mycotoxins. The method was highly sensitive, accurate, and high-throughput. | [69] |
| Biological matrices (plasma, urine, feces) | Targeted and untargeted LC-ESI-HRMS (Xevo® TQ-S mass spectrometer) | Targeted quantification of regulated mycotoxins (AFs, OTA, and Fusarium mycotoxins) and emerging mycotoxins (Alternaria mycotoxins and ENNs) | [75] |
| Biological sample (urine) | HPLC-ESI-MSMS (QTRAP® 6500 mass spectrometer) | Sensitive, selective, and simultaneous determination of 35 mycotoxins and metabolites like nivalenol, citrinin, dihydrocitrinone, fusarenon-X, altertoxin I, tentoxin, and hydrolyzed fumonisin B1. | [76] |
| Tomato samples | UHPLC-ESI-Q-Orbitrap | Detection of 24 sulfated metabolites, with two metabolites (AME-sulfated and AOH-sulfated) identified in Alternaria fungi-inoculated samples. | [77] |
| Raw milk | UHPLC-ESI-MSMS (Agilent 6460 triple quadrupole mass spectrometer) | Quantification of 40 mycotoxins in milk through QuEChERS extraction and UHPLC-MS/MS analysis. High occurrence (in low amounts) of beauvericin and enniatins in milk. | [78] |
| Commercial UHT milk (whole, semi-skim, and skim types) | UHPLC-ESI-Q-Exactive Orbitrap HRMS | Simultaneous analysis of 30 regulated and emerging mycotoxins through a sensitive, efficient, and quick method (chromatography run time of 8 min). No analyzed sample was contaminated with mycotoxins. | [79] |
| Bulk milk (from different dairy farms) | UHPLC-ESI-Q-Exactive Focus™ Orbitrap HRMS | Fusarium mycotoxins, together with tetrapeptide tentoxin, α-zearalenol, mycophenolic acid, and apicidin, were some of the most discriminant metabolites of the feeding system. | [80] |
| Raw milk (from different animal species) | Ultra-sensitive LC-MS/MS (Sciex QTrap® 6500+ mass spectrometer) | Samples collected in Niger showed that at least one mycotoxin was detected in 97% of all samples, with BEA (87%) being the most frequent. AFM1 was absent in camel milk samples. | [81] |
| Commercial cashew nuts | UPLC-MS/MS system (TSQ Quantis triple quadrupole) | Optimization of an analytical method for the measurement of 18 mycotoxins in commercial samples from Vietnam. FB1 showed the highest variation according to the seasoning techniques. | [82] |
| Commercial vegetable oils | UHPLC-ESI-Q-Exactive Plus Orbitrap-MS | Aflatoxins (B1 and B2) and zearalenone were observed in 50% of the real samples analyzed, with a total of 10 real samples. The developed method was simple and low-cost, with great potential to screen mycotoxins in complex oil matrices. | [83] |
| Commercial grain products | UHPLC-QTrap 5500 MS/MS system | Simultaneous quantification of 730 mycotoxins and other secondary fungal metabolites and plant toxins. The enniatins and deoxynivalenol discriminated were found in the majority of the samples. | [84] |
| Commercial wheat flours | UHPLC-HRMS (Q-Exactive HF Orbitrap) | A screening method for detecting both parent and modified mycotoxins was developed. In particular, an in-house MS/MS database containing 82 mycotoxins divided into eight categories was constructed. | [85] |
| Maize and grass silage (from different dairy farms) | UHPLC–ESI-MS–IT–TOF combined with UHPLC-ESI-MS/MS | A high co-occurrence of Fusarium mycotoxins was found, with low contamination levels. DON and BEA were the most frequent compounds in silage (82%). | [86] |
| Swine, poultry, and dairy feeds | UHPLC-ESI- MS/MS (ExionLC™ AD system coupled with QTRAP 5500 tandem mass spectrometer) | FBs, ZEN, AFB1, and deoxynivalenol were the most prevalent mycotoxins in the samples analyzed. | [87] |
| Dry pet food | UHPLC-ESI-Q-Orbitrap HRMS | Comprehensive method combining quantification of 28 mycotoxins and post-target screening for another 245 fungal and bacterial metabolites. Emerging Fusarium mycotoxins were the most commonly detected mycotoxins. | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapris, M.; Errico, M.; Rocchetti, G.; Gallo, A. The Potential of Multi-Screening Methods and Omics Technologies to Detect Both Regulated and Emerging Mycotoxins in Different Matrices. Foods 2024, 13, 1746. https://doi.org/10.3390/foods13111746
Lapris M, Errico M, Rocchetti G, Gallo A. The Potential of Multi-Screening Methods and Omics Technologies to Detect Both Regulated and Emerging Mycotoxins in Different Matrices. Foods. 2024; 13(11):1746. https://doi.org/10.3390/foods13111746
Chicago/Turabian StyleLapris, Marco, Michela Errico, Gabriele Rocchetti, and Antonio Gallo. 2024. "The Potential of Multi-Screening Methods and Omics Technologies to Detect Both Regulated and Emerging Mycotoxins in Different Matrices" Foods 13, no. 11: 1746. https://doi.org/10.3390/foods13111746
APA StyleLapris, M., Errico, M., Rocchetti, G., & Gallo, A. (2024). The Potential of Multi-Screening Methods and Omics Technologies to Detect Both Regulated and Emerging Mycotoxins in Different Matrices. Foods, 13(11), 1746. https://doi.org/10.3390/foods13111746







