Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Treatments
2.3.1. PEF Treatment
2.3.2. USN Treatment
2.4. DLLME
2.5. Mycotoxin Determination
2.6. Statistical Analysis
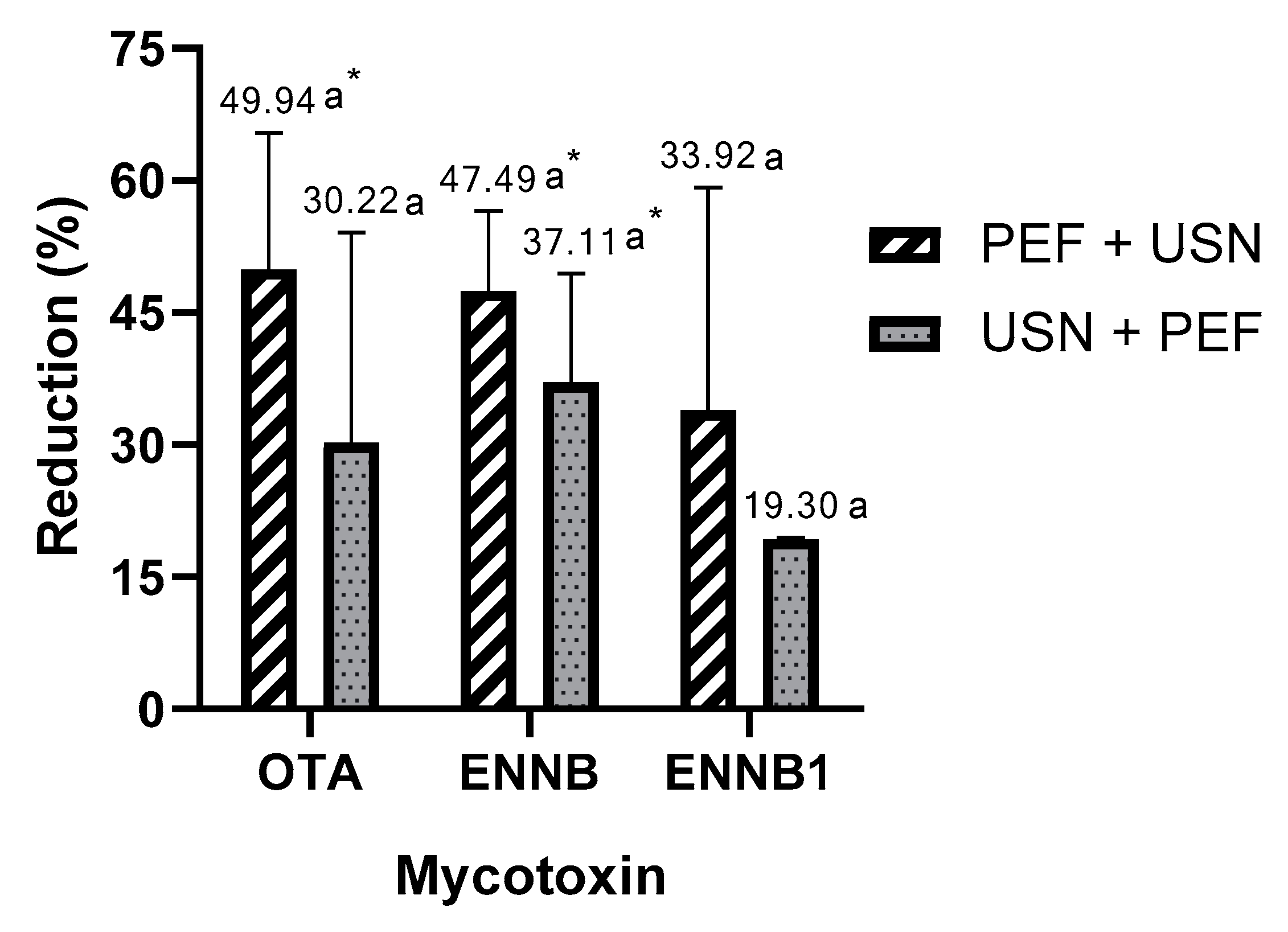
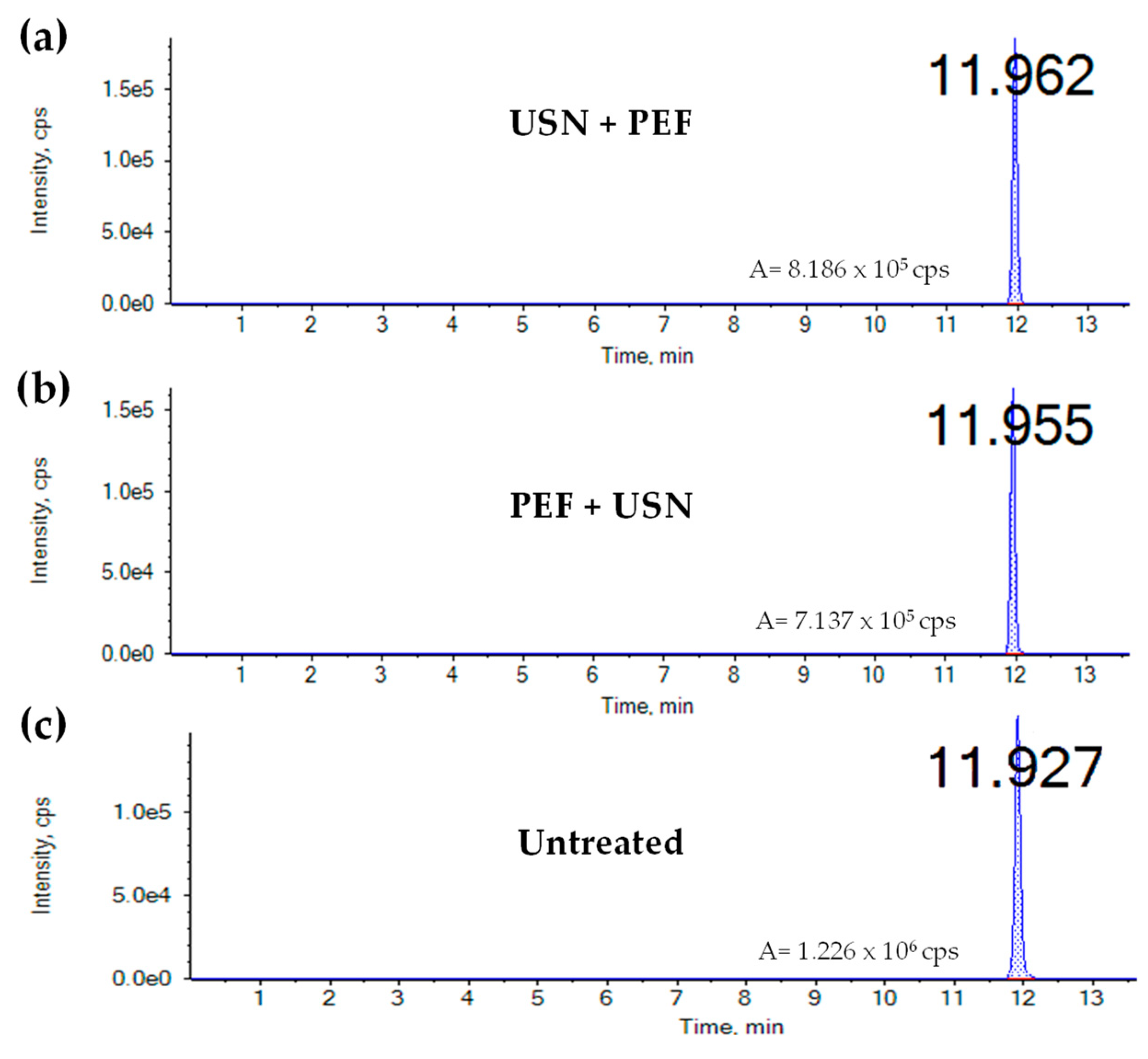
3. Results
4. Discussion
| Treatment | Type of Matrix | Mycotoxin | Treatment Conditions | % Reductions Achieved | Reference |
|---|---|---|---|---|---|
| PEF | Potato dextrose agar | AFs | Frequency 50 Hz, burst 10, energy 1 kJ, time 10 s (+ heat) | 79 to 96 | [33] |
| Model system | AFs | Frequency 50 Hz, burst 10, energy 1 kJ, time 10 s (+ pH 4 to 10) | 77 to 97 | [34] | |
| Juice and smoothie | ENNs and BEA | Voltage 30 kV, field strength 3 kV/cm, specific energy 500 kJ/kg | 43 to 70 | [12] | |
| Grape juice | AFs | Voltage 30 kV, field strength 3 kV/cm, specific energy 500 kJ/kg | 22 to 84 | [25] | |
| USN | Aqueous solution | AFB1, DON, ZEA, OTA | Frequency 20 kHz, power intensity 11 W/cm3, time 50 min | 61 to 97 | [13] |
| Aqueous solution | AFB1 | Frequency 20 kHz, power intensity 6.6 W/cm3, time 80 min | 85.1 | [27] | |
| Aqueous solution | AFs | Frequency 20 kHz, power output 1000 W, time 10 min | 41 | [26] | |
| HPP | Orange juice | AFB1 | Pressure 600 MPa, time 5 min | 24 29 | [40] |
| AOH | |||||
| Juice | ENNs | Pressure 600 MPa, time 5 min | 11 to 75 | [30] | |
| Grape juice | AFs | Pressure 500 MPa, time 5 min | 14 to 29 | [25] | |
| Vegetable juices | PAT | Pressure 600 MPa, time 5 min | 30 | [31] | |
| Apple Juice | PAT | Pressure 400 MPa, time 5 min, (+ heat) | 29 | [32] | |
| Combination of PEF and USN | Orange juice with milk | OTA, ENNB, ENNB1 | USN: Frequency 20 kHz, power 100 W, time 30 min PEF: Voltage 30 kV, field strength 3 kV/cm, Specific energy 500 kJ/kg | up to 50% for OTA (PEF + USN) up to 47% for ENNs (PEF + USN) | This study |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artés, F.; Allende, A. Minimal processing of fresh fruit, vegetables, and juices. In Emerging Technologies for Food Processing, 2nd ed.; Da-Wen, S., Ed.; Elsevier Academic Press: London, UK, 2014; pp. 583–597. [Google Scholar] [CrossRef]
- Informe el Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) Sobre Reco-Mendaciones Dietéticas Sostenibles y Recomendaciones de Actividad Física Para la Población Española. Available online: https://acortar.link/VmwOxU (accessed on 10 February 2023).
- Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO. Available online: https://apps.who.int/iris/handle/10665/42665 (accessed on 10 February 2023).
- Neoκleous, I.; Tarapata, J.; Papademas, P. Non-thermal processing technologies for dairy products: Their effect on safety and quality characteristics. Front. Sustain. Food Syst. 2022, 6, 184. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Gunes, G.; Turan, D. New Technologies and edible coatings for minimally processed and refrigerated (MPR) fruits and vegetables (fresh cuts and freshly squeezed juices). In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R., Eds.; Springer: Boston, MA, USA, 2017; pp. 587–617. [Google Scholar]
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2014, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.L.; Kubo, M.T.K.; Caetano-Silva, M.E.; Augusto, P.E.D. Ultrasound processing of fruits and vegetables, structural modification and impact on nutrient and bioactive compounds: A review. Int. J. Food Sci. Technol. 2021, 56, 4376–4395. [Google Scholar] [CrossRef]
- Arruda, T.R.; Vieira, P.; Silva, B.M.; Freitas, T.D.; do Amaral, A.J.B.; Vieira, E.N.R.; Leite Júnior, B.R.; de, C. What are the prospects for ultrasound technology in food processing? An update on the main effects on different food matrices, Drawbacks, and Applications. J. Food Process. Eng. 2021, 44, e13872. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Yang, R.; Zhao, W. Recent developments in the preservation of raw fresh food by pulsed electric field. Food Rev. Int. 2020, 38 (Suppl. 1), 247–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Liao, X.; Zhang, J.; Hou, Y.; Xiao, Z.; Chen, F.; Hu, X. Degradation of diazinon in apple juice by ultrasonic treatment. Ultrason. Sonochem. 2010, 17, 662–668. [Google Scholar] [CrossRef]
- Pallarés, N.; Barba, F.J.; Berrada, H.; Tolosa, J.; Ferrer, E. Pulsed electric fields (PEF) to mitigate emerging mycotoxins in juices and smoothies. Appl. Sci. 2020, 10, 6989. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Liu, Y.; Bai, F.; Bian, K. Effects of pulsed ultrasound at 20 KHz on the sonochemical degradation of mycotoxins. World Mycotoxin. J. 2019, 12, 357–366. [Google Scholar] [CrossRef]
- Gavahian, M.; Pallares, N.; al Khawli, F.; Ferrer, E.; Barba, F.J. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol. 2020, 106, 209–218. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. FCT 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Bulaić, M.; Nevistić, A.; Rot, T.; Babić, J.; Panjičko, M.; Kovač, T.; Šarkanj, B. Regulated mycotoxin occurrence and co-occurrence in croatian cereals. Toxins 2022, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Gacem, M.A.; Ould El Hadj-Khelil, A.; Boudjemaa, B.; Gacem, H. Mycotoxins occurrence, toxicity and detection methods. In Sustainable Agriculture Reviews 40; Lichtfouse, E., Ed.; Springer: Cham, Germany, 2020; Volume 40, pp. 1–42. [Google Scholar]
- Pallarés, N.; Carballo, D.; Ferrer, E.; Fernández-Franzón, M.; Berrada, H. Mycotoxin dietary exposure assessment through fruit juices consumption in children and adult population. Toxins 2019, 11, 684. [Google Scholar] [CrossRef]
- Mandappa, I.M.; Basavaraj, K.; Manonmani, H.K. Analysis of mycotoxins in fruit juices. In Fruit Juices; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: London, UK, 2018; pp. 763–777. [Google Scholar] [CrossRef]
- Colović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Ðuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Barkai-golan, R. Postharvest disease initiation. In Postharvest Diseases of Fruits and Vegetables; Barkai-golan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3–24. ISBN 978-0-444-50584-2. [Google Scholar]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Suman, M. Last decade studies on mycotoxins’ fate during food processing: An overview. Curr. Opin. Food Sci. 2021, 41, 70–80. [Google Scholar] [CrossRef]
- Pallarés, N.; Berrada, H.; Tolosa, J.; Ferrer, E. Effect of high hydrostatic pressure (HPP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT 2021, 142, 111000. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Sani, A.M.; Mohseni, S. Destruction of AFT by ultrasound treatment. J. Appl. Environ. Biol. Sci. 2015, 4, 198–202. [Google Scholar]
- Liu, Y.; Li, M.; Liu, Y.; Bian, K. Structures of reaction products and degradation pathways of aflatoxin B1 by ultrasound treatment. Toxins 2019, 11, 526. [Google Scholar] [CrossRef]
- Gavahian, M.; Sheu, S.C.; Magnani, M.; Mousavi Khaneghah, A. Emerging technologies for mycotoxins removal from foods: Recent advances, roles in sustainable food consumption, and strategies for industrial applications. J. Food Process. Preserv. 2022, 46, e15922. [Google Scholar] [CrossRef]
- Barba, F.J.; Cortés, C.; Esteve, M.J.; Frígola, A. Study of Antioxidant Capacity and Quality Parameters in An Orange Juice-Milk Beverage After High-Pressure Processing Treatment. Food Bioproc. Tech. 2012, 5, 2222–2232. [Google Scholar] [CrossRef]
- Pallarés, N.; Sebastià, A.; Martínez-Lucas, V.; Queirós, R.; Barba, F.J.; Berrada, H.; Ferrer, E. High pressure processing impact on emerging mycotoxins (ENNA, ENNA1, ENNB, ENNB1) mitigation in different juice and juice-milk matrices. Foods 2022, 11, 190. [Google Scholar] [CrossRef]
- Hao, H.; Zhou, T.; Koutchma, T.; Wu, F.; Warriner, K. High hydrostatic pressure assisted degradation of patulin in fruit and vegetable juice blends. Food Control 2016, 62, 237–242. [Google Scholar] [CrossRef]
- Avsaroglu, M.D.; Bozoglu, F.; Alpas, H.; Largeteau, A.; Demazeau, G. Use of pulsed-high hydrostatic pressure treatment to decrease patulin in apple juice. High Press. Res. 2015, 35, 214–222. [Google Scholar] [CrossRef]
- Subramanian, V.; Shanmugam, N.; Ranganathan, K.; Kumar, S.; Reddy, R. Effect of combination processing on aflatoxin reduction: Process optimization by response surface methodology. J. Food Process. Preserv. 2017, 41, e13230. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Nadanasabhapathi, S.; Kumar, R.; Sunny Kumar, S. Effect of pH and pulsed electric field process parameters on the aflatoxin reduction in model system using response surface methodology: Effect of pH and PEF on aflatoxin reduction. J. Food Sci. Technol. 2018, 55, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruíz-Moyano, S.; Casquete, R.; Hernández, A.; Córdoba, M.G. Use of efficient drying methods to improve the safety and quality of dried Fig. J. Food Process. Preserv. 2019, 43, e13853. [Google Scholar] [CrossRef]
- de Nijs, M.; van den Top, H.; de Stoppelaar, J.; Lopez, P.; Mol, H. Fate of enniatins and deoxynivalenol during pasta cooking. Food Chem. 2016, 213, 763–767. [Google Scholar] [CrossRef]
- Galiassi, G.R.R.; Ramirez, M.V. Experimental study of thermal influence on vitamin c content in pasteurization of orange juice in two types of heating. Period. Tche. Quimica. 2022, 19, 69–75. [Google Scholar] [CrossRef]
- Dahal, S.; Lee, H.J.; Gu, K.; Ryu, D. Heat stability of ochratoxin a in an aqueous buffered model system. J. Food Prot. 2016, 79, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. Stability of ochratoxin a in oats during roasting with reducing sugars. Food Control 2020, 118, 107382. [Google Scholar] [CrossRef]
- Pallarés, N.; Sebastià, A.; Martínez-Lucas, V.; González-Angulo, M.; Barba, F.J.; Berrada, H.; Ferrer, E. High pressure processing impact on alternariol and aflatoxins of grape juice and fruit juice-milk based beverages. Molecules 2021, 26, 3769. [Google Scholar] [CrossRef] [PubMed]

| Orange Juice | Milk | Water | Pectin | Sugar | Citric Acid |
|---|---|---|---|---|---|
| 50 mL | 20 mL | 30 mL | 0.3 g | 7.5 g | 0.1 g |
| Mycotoxin | Precursor Ion (m/z) | Quantifier Product Ion (m/z) | Qualifier Product Ion (m/z) | tR (min) | DP | EP | CE | CXP |
|---|---|---|---|---|---|---|---|---|
| OTA | 404 | 239 | 102 | 101 | 91 | 10 | 37 | 16 |
| ENNB | 6575 | 1963 | 2141 | 12 | 81 | 10 | 45 | 18 |
| ENNB1 | 6714 | 196 | 210 | 12,2 | 111 | 10 | 43 | 12 |
| OTA | ENNB | ENNB1 | |
|---|---|---|---|
| PEF + USN | 50.06 ± 15.45 µg/L | 52.51 ± 9.04 µg/L | 66.08 ± 25.29 µg/L |
| USN + PEF | 69.78 ± 23.90 µg/L | 62.89 ± 12.30 µg/L | 80.70 ± 0.20 µg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastià, A.; Calleja-Gómez, M.; Pallarés, N.; Barba, F.J.; Berrada, H.; Ferrer, E. Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage. Foods 2023, 12, 1582. https://doi.org/10.3390/foods12081582
Sebastià A, Calleja-Gómez M, Pallarés N, Barba FJ, Berrada H, Ferrer E. Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage. Foods. 2023; 12(8):1582. https://doi.org/10.3390/foods12081582
Chicago/Turabian StyleSebastià, Albert, Mara Calleja-Gómez, Noelia Pallarés, Francisco J. Barba, Houda Berrada, and Emilia Ferrer. 2023. "Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage" Foods 12, no. 8: 1582. https://doi.org/10.3390/foods12081582
APA StyleSebastià, A., Calleja-Gómez, M., Pallarés, N., Barba, F. J., Berrada, H., & Ferrer, E. (2023). Impact of Combined Processes Involving Ultrasound and Pulsed Electric Fields on ENNs, and OTA Mitigation of an Orange Juice-Milk Based Beverage. Foods, 12(8), 1582. https://doi.org/10.3390/foods12081582








