Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction Procedure
2.4. Cell Lines and Culture
2.5. Analytical Characterization of Blackberry Extract
2.5.1. HPLC-ESI-TOF-MS Analysis
2.5.2. Total Terpenoid Content
2.6. Antioxidant Capacity Assays
2.6.1. Folin–Ciocalteu Assay
2.6.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.6.4. DPPH Assay
2.7. Intracellular Antioxidant Activity Assay
2.8. Antimicrobial Analysis
2.9. In Vitro Antiproliferative Assays
2.10. In Vitro Anti-Inflammatory Assays
2.11. Statistical Analysis
3. Results and Discussion
3.1. Determination of Bioactive Compounds in Blackberry Extracts by HPLC-MS
3.2. Total Antioxidant Content (Folin–Ciocalteu) and Antioxidant Capacity (FRAP, TEAC and IC50) of the Extract
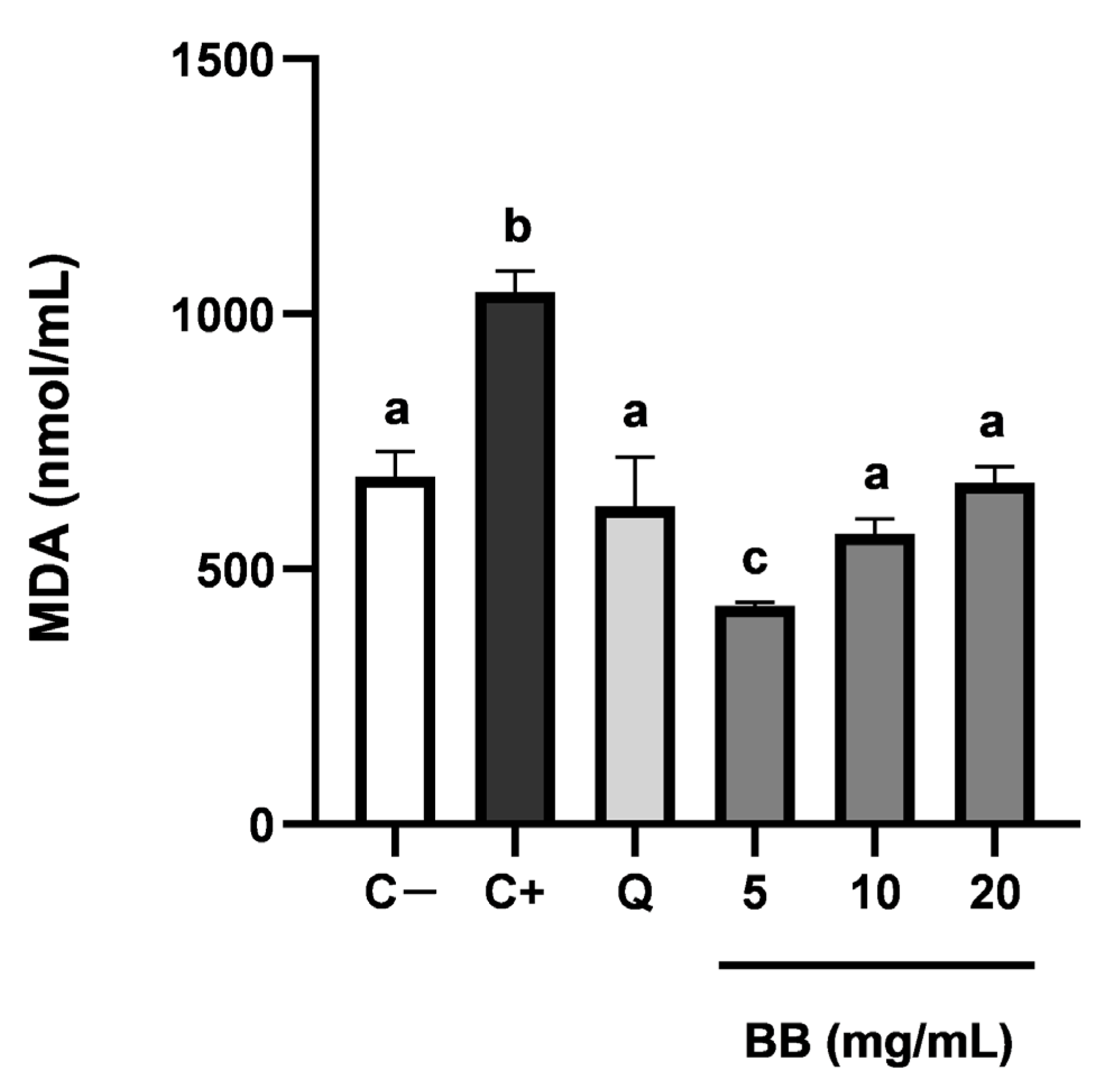
3.3. Inhibition of Intracellular Lipid Peroxidation
3.4. Antimicrobial Activity of Blackberry Extract
3.5. Antitumoral Activity of Blackberry Extract
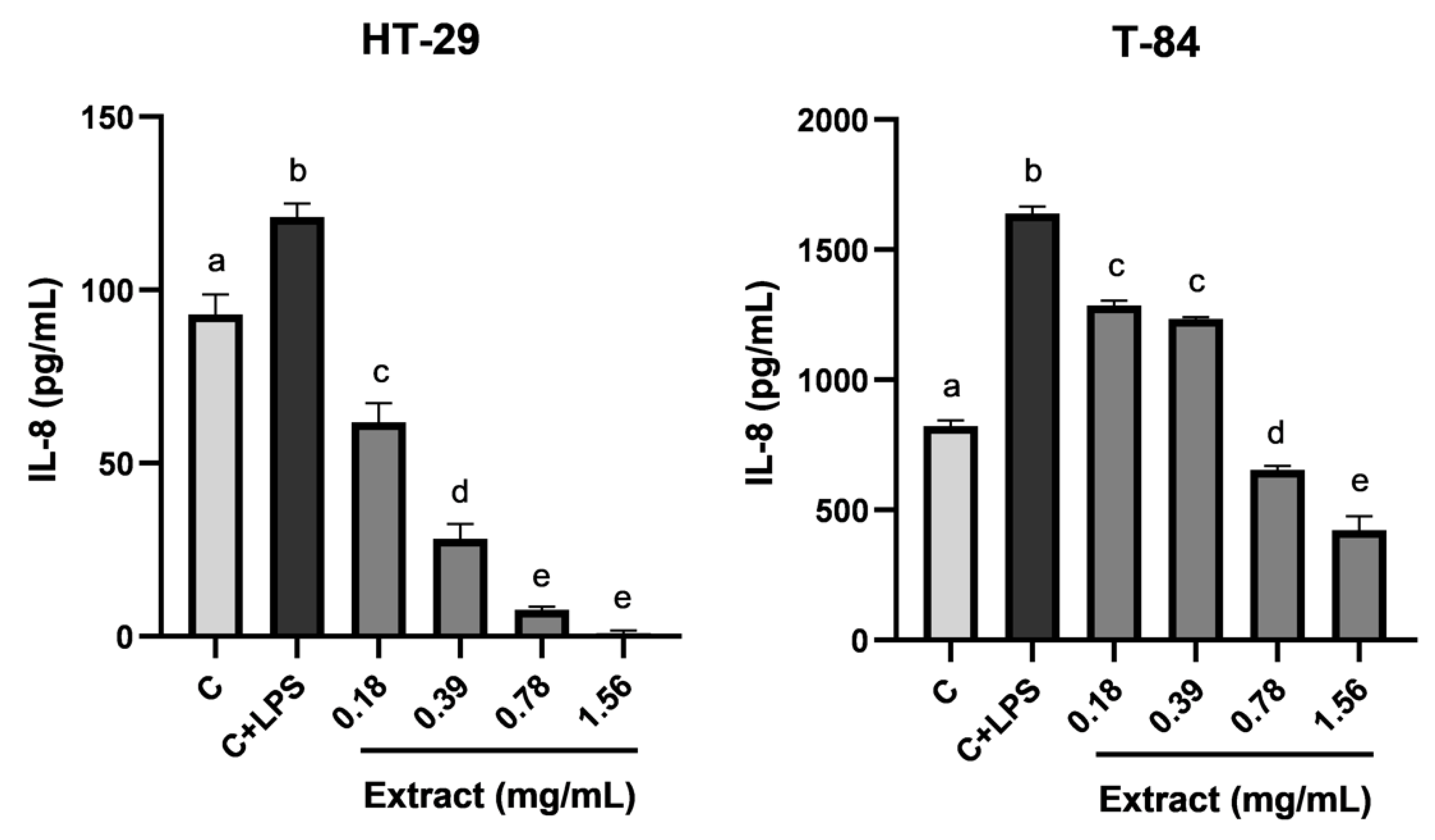
3.6. Anti-inflammatory Activity of Blackberry Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (Blackberry) Use as an Herbal Medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative Study of Red Berry Pomaces (Blueberry, Red Raspberry, Red Currant and Blackberry) as Source of Antioxidants and Pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; De Carvalho, I.S.; Končić, M.Z. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium Myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- D’Angelo, R.W.O.; Gonçalves, M.M.; Fachi, M.M.; Vilhena, R.d.O.; Pontarolo, R.; Maluf, D.F. UPLC–QToF-MS Characterization of Blackberry Extracts of Cultivars ‘Tupy’, ‘Guarani’, and ‘Xavante’: Development of Extract-Loaded Niosomes. Rev. Bras. Farmacogn. 2020, 30, 519–527. [Google Scholar] [CrossRef]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of Blackberry Extract and Its Antiproliferative and Anti-Inflammatory Properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin Composition of Blackberry as Determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruits. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus fruticosus L.: Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I. Condensed Tannins in Extracts from European Medicinal Plants and Herbal Products. J. Pharm. Biomed. Anal. 2016, 121, 225–231. [Google Scholar] [CrossRef]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of Antioxidant Activity of Garden Blackberries (Rubus fruticosus L.) Extracts Obtained with Different Extraction Solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- D’Agostino, M.F.; Sicari, V.; Giuffrè, A.M.; Soria, A.C. Blackberries (Rubus Ulmifolius Schott) from Calabria (Italy): A Comprehensive Characterisation. Eur. Food Res. Technol. 2022, 248, 905–916. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible Berries: Bioactive Components and Their Effect on Human Health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Dragana, D.Č.; Ranitovi, A.S.; Cvetkovi, D.D.; Markov, S.L.; Vincic, M.N.; Djilas, S.M. Bioactivity of Blackberry (Rubus fruticosus L.) Pomace: Polyphenol Content, Radical Scavenging, Antimicrobial and Antitumor Activity. Acta Period. Technol. 2017, 323, 63–76. [Google Scholar]
- Halim, M.A.; Kanan, K.A.; Nahar, T.; Rahman, M.J.; Ahmed, K.S.; Hossain, H.; Mozumder, N.H.M.R.; Ahmed, M. Metabolic Profiling of Phenolics of the Extracts from the Various Parts of Blackberry Plant (Syzygium cumini L.) and Their Antioxidant Activities. LWT 2022, 167, 113813. [Google Scholar] [CrossRef]
- González, O.A.; Escamilla, C.; Danaher, R.J.; Dai, J.; Ebersole, J.L.; Mumper, R.J.; Miller, C.S. Antibacterial Effects of Blackberry Extract Target Periodontopathogens. J. Periodontal Res. 2013, 48, 80–86. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MS n Identification and Quantification of Flavonol Glycosides in 28 Wild and Cultivated Berry Species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Martin-Smith, M.; Khatoon, T. Biological Activity of the Terpenoids and Their Derivatives. Fortschr. Arzneim. 1963, 42, 279–346. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer Effects of Bioactive Berry Compounds. Phytochem. Rev. 2014, 13, 295–322. [Google Scholar] [CrossRef]
- Martín-García, B.; Aznar-Ramos, M.J.; Verardo, V.; Gómez-Caravaca, A.M. Development of an Effective Sonotrode Based Extraction Technique for the Recovery of Phenolic Compounds with Antioxidant Activities in Cherimoya Leaves. Plants 2022, 11, 2034. [Google Scholar] [CrossRef]
- Łukowski, A.; Jagiełło, R.; Robakowski, P.; Adamczyk, D.; Karolewski, P. Adaptation of a Simple Method to Determine the Total Terpenoid Content in Needles of Coniferous Trees. Plant Sci. 2022, 314, 111090. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dayoub, J.C.; Ortiz, F.; Lõpez, L.C.; Venegas, C.; Del Pino-Zumaquero, A.; Roda, O.; Sánchez-Montesinos, I.; Acuña-Castroviejo, D.; Escames, G. Synergism between Melatonin and Atorvastatin against Endothelial Cell Damage Induced by Lipopolysaccharide. J. Pineal Res. 2011, 51, 324–330. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically. 11th Edition. 2018. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 1 August 2021).
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd Edition. 2017. Available online: https://clsi.org/standards/products/microbiology/documents/m38/ (accessed on 1 August 2021).
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2016, 1, 1112–1116. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Gálvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to Its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, 1–12. [Google Scholar] [CrossRef]
- Connor, A.M.; Finn, C.E.; McGhie, T.K.; Alspach, P.A. Genetic and Environmental Variation in Anthocyanins and Their Relationship to Antioxidant Activity in Blackberry and Hybridberry Cultivars. J. Am. Soc. Hortic. Sci. 2005, 130, 680–687. [Google Scholar] [CrossRef]
- Mullen, W.; Larcombe, S.; Arnold, K.; Welchman, H.; Crozier, A. Use of Accurate Mass Full Scan Mass Spectrometry for the Analysis of Anthocyanins in Berries and Berry-Fed Tissues. J. Agric. Food Chem. 2010, 58, 3910–3915. [Google Scholar] [CrossRef]
- Santos, S.S.d.; Magalhães, F.d.S.; Paraíso, C.M.; Ogawa, C.Y.L.; Sato, F.; Santos Junior, O.d.O.; Visentainer, J.V.; Madrona, G.S.; Reis, M.H.M. Enhanced Conditions for Anthocyanin Extraction from Blackberry Pomace under Ultrasound Irradiation. J. Food Process Eng. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Zhao, D.K.; Shi, Y.N.; Petrova, V.; Yue, G.G.L.; Negrin, A.; Wu, S.B.; D’Armiento, J.M.; Lau, C.B.S.; Kennelly, E.J. Jaboticabin and Related Polyphenols from Jaboticaba (Myrciaria cauliflora) with Anti-Inflammatory Activity for Chronic Obstructive Pulmonary Disease. J. Agric. Food Chem. 2019, 67, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Čanadanović-Brunet, J.; Tumbas Šaponjac, V.; Stajčić, S.; Ćetković, G.; Čanadanović, V.; Ćebović, T.; Vulić, J. Polyphenolic Composition, Antiradical and Hepatoprotective Activities of Bilberry and Blackberry Pomace Extracts. J. Berry Res. 2019, 9, 349–362. [Google Scholar] [CrossRef]
- Moraes, D.P.; Machado, M.L.; Farias, C.A.A.; Barin, J.S.; Zabot, G.L.; Lozano-Sánchez, J.; Ferreira, D.F.; Vizzotto, M.; Leyva-Jimenez, F.J.; Da Silveira, T.L.; et al. Effect of Microwave Hydrodiffusion and Gravity on the Extraction of Phenolic Compounds and Antioxidant Properties of Blackberries (Rubus spp.): Scale-Up Extraction. Food Bioprocess Technol. 2020, 13, 2200–2216. [Google Scholar] [CrossRef]
- Jin Cho, M.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol Glycosides and Antioxidant Capacity of Various Blackberry and Blueberry Genotypes Determined by High-Performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Krstić, Đ.D.; Ristivojević, P.M.; Gašić, U.M.; Lazović, M.; Fotirić Akšić, M.M.; Milivojević, J.; Morlock, G.E.; Milojković-Opsenica, D.M.; Trifković, J. Authenticity Assessment of Cultivated Berries via Phenolic Profiles of Seeds. Food Chem. 2023, 402, 134184. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Liu, R.; Xu, Z.; Yang, M.; Wang, W.; Liu, P.; Sabia, G.; Wang, X.; Guo, D. Four New Lignans from the Stems of Kadsura Heteroclita. Planta Med. 2006, 72, 284–288. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Saeedi Saravi, S.S. The Chemistry, Pharmacology and Clinical Properties of Sambucus Ebulus: A Review. J. Med. Plants Res. 2010, 4, 095–103. [Google Scholar] [CrossRef]
- Shiraga, Y.; Okano, K.; Akira, T.; Fukaya, C.; Yokoyama, K.; Tanaka, S.; Fukui, H.; Tabata, M. Structures of Potent Antiulcerogenic Compounds from Cinnamomum Cassia. Tetrahedron 1988, 44, 4703–4711. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola Rosea Extract by Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Biochem. Res. Int. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Malakov, P.Y.; Papanov, G.Y. Neo-Clerodane Diterpenoids from Scutellaria Alpina. Phytochemistry 1998, 49, 2449–2452. [Google Scholar] [CrossRef]
- Corea, G.; Fattorusso, E.; Lanzotti, V.; Capasso, R.; Izzo, A.A. Antispasmodic Saponins from Bulbs of Red Onion, Allium cepa L. Var. Tropea. J. Agric. Food Chem. 2005, 53, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Gradillas, A.; Martínez-Alcázar, M.P.; Gutiérrez, E.; Ramos-Solano, B.; García, A. A Novel Strategy for Rapid Screening of the Complex Triterpene Saponin Mixture Present in the Methanolic Extract of Blackberry Leaves (Rubus Cv. Loch Ness) by UHPLC/QTOF-MS. J. Pharm. Biomed. Anal. 2019, 164, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.J.; Kim, K.B.; Han, H.S.; An, I.S.; Ahn, K.J. 23-Hydroxytormentic Acid Protects Human Dermal Fibroblasts by Attenuating UVA-Induced Oxidative Stress. Photodermatol. Photoimmunol. Photomed. 2017, 33, 92–100. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, P. 23-Hydroxytormentic Acid Reduces Cerebral Ischemia/Reperfusion Damage in Rats through Anti-Apoptotic, Antioxidant, and Anti-Inflammatory Mechanisms. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1045–1054. [Google Scholar] [CrossRef]
- Olech, M.; Ziemichód, W.; Nowacka-jechalke, N. The Occurrence and Biological Activity of Tormentic Acid—A Review. Molecules 2021, 26, 3797. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Jazić, M.R.; Vulić, J.J.; Kukrić, Z.Z.; Topalić-Trivunović, L.N.; Savić, A.V. Chemical Composition, Biological Potentials and Antimicrobial Activity of Wild and Cultivated Blackberries. Acta Period. Technol. 2018, 49, 65–79. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A Comparative Study on Composition and Antioxidant Activities of Supercritical Carbon Dioxide, Hexane and Ethanol Extracts from Blackberry (Rubus fruticosus) Growing in Poland. J. Sci. Food Agric. 2017, 97, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Small Berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Graßmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Porte, S.; Joshi, V.; Shah, K.; Chauhan, N.S. Plants’ Steroidal Saponins—A Review on Its Pharmacology Properties and Analytical Techniques. World J. Tradit. Chinese Med. 2022, 8, 350–385. [Google Scholar] [CrossRef]
- Higashi-Okai, K.; Ishikawa, A.; Yasumoto, S.; Okai, Y. Potent Antioxidant and Radical-Scavenging Activity of Chenpi—Compensatory and Cooperative Actions of Ascorbic Acid and Citric Acid. J. UOEH 2009, 31, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A First Evidence in the Synergism and Bioactivities. Food Rev. Int. 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The Synergistic Effect of Triterpenoids and Flavonoids—New Approaches for Treating Bacterial Infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Pasteurization of Blackberry Juice Preserves Polyphenol-Dependent Inhibition for Lipid Peroxidation and Intracellular Radicals. J. Food Compos. Anal. 2015, 42, 56–62. [Google Scholar] [CrossRef]
- Hassan, H.A.; Abdel-Aziz, A.F. Evaluation of Free Radical-Scavenging and Anti-Oxidant Properties of Black Berry against Fluoride Toxicity in Rats. Food Chem. Toxicol. 2010, 48, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; Jin, C.H.; Choi, D.S.; Kang, S.Y.; Kim, D.S.; Byun, M.W.; Jeong, I.Y. Blackberry Extract Attenuates Oxidative Stress through Up-Regulation of Nrf2-Dependent Antioxidant Enzymes in Carbon Tetrachloride-Treated Rats. J. Agric. Food Chem. 2011, 59, 11442–11448. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.C.; Andelković, A.S.M.; Radovanović, A.B.; Andelković, M.Z. Antioxidant and Antimicrobial Activity of Polyphenol Extracts from Wild Berry Fruits Grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Antimicrobial Activity of Pentacyclic Triterpenes Isolated from African Combretaceae. Phytochemistry 2003, 63, 81–88. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, H.T.; Wu, Z.Y.; Jhan, Y.L.; Shyu, C.L.; Chou, C.H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia Scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Baysal, G.; Olcay, H.S.; Keresteci, B.; Özpinar, H. The Antioxidant and Antibacterial Properties of Chitosan Encapsulated with the Bee Pollen and the Apple Cider Vinegar. J. Biomater. Sci. Polym. Ed. 2022, 33, 995–1011. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry Extracts Inhibit Growth and Stimulate Apoptosis of Human Cancer Cells in Vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic Composition, Antioxidant and Antiproliferative Effects of Wild and Cultivated Blackberries (Rubus fruticosus L.) Pomace. Int. J. Food Sci. Technol. 2019, 54, 194–201. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Nicácio, A.E.; Boeing, J.S.; Garcia, F.P.; Nakamura, C.V.; Visentainer, J.V.; Maldaner, L. Rapid Extraction Method Followed by a D-SPE Clean-up Step for Determination of Phenolic Composition and Antioxidant and Antiproliferative Activities from Berry Fruits. Food Chem. 2020, 309, 125694. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Cueva, C.; Tamargo, A.; Quintela, J.C.; de la Fuente, E.; Walker, A.W.; Moreno-Arribas, M.V.; Bartolomé, B. Application of the Dynamic Gastrointestinal Simulator (Simgi®) to Assess the Impact of Probiotic Supplementation in the Metabolism of Grape Polyphenols. Food Res. Int. 2020, 129, 108790. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-Inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Lail, H.L.; Feresin, R.G.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a Treatment for Obesity-Induced Inflammation: Evidence from Preclinical Models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Rizki, D.R.; Purnama, A.; Duta, T.F. Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Sci. Pharm. 2023, 91, 15. [Google Scholar] [CrossRef]
| Peak No. | Retention Time (Min) | m/z Exp. | m/z Calc. | Error (ppm) | Molecular Formula | Score | Proposed Compound | Quantification (μg/g d.w.) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids and derivatives | ||||||||
| 3 | 0.433 | 333.0574 | 333.0610 | −4.8 | C16H14O8 | 93.93 | Jaboticabin | 27.68 ± 0.26 |
| 7 | 4.979 | 223.0598 | 223.0606 | −3.6 | C11H12O5 | 97.37 | Sinapic acid | 17.83 ± 0.12 |
| 9 | 5.476 | 385.1107 | 385.1135 | −7.3 | C17H22O10 | 92.67 | Sinapic acid hexoside | 13.35 ± 0.03 |
| 16 | 6.639 | 183.0249 | 183.0293 | −2.4 | C8H8O5 | 99.18 | Methylgallic acid | 19.18 ± 0.07 |
| 20 | 7.707 | 433.0406 | 433.0407 | −0.2 | C19H14O12 | 84.53 | Ellagic acid-pentoside | 27.98 ± 0.02 |
| 21 | 7.888 | 433.0412 | 433.0407 | 2.7 | C19H14O12 | 92.90 | Ellagic acid-pentoside isomer | 25.62 ± 0.16 |
| 24 | 8.726 | 300.9994 | 300.9984 | 3.3 | C14H6O8 | 100 | Ellagic acid | 19.46 ± 0.13 |
| 29 | 9.276 | 447.0560 | 447.0564 | −0.9 | C20H16O12 | 93.98 | Ellagic acid 2-rhamnoside | 20.89 ± 0.10 |
| 32 | 9.917 | 315.0119 | 315.0141 | −7.0 | C15H8O8 | 85.96 | 3-O-Methylellagic acid | 10.41 ± 0.05 |
| Flavonoids and derivatives | ||||||||
| 6 | 4.367 | 463.0850 | 463.0877 | −5.8 | C21H20O12 | 95.62 | Quercetin-O-hexoside | 3.37 ± 0.09 |
| 10 | 5.487 | 577.1396 | 577.1405 | −1.6 | C30H26O12 | 85.36 | B-type procyanidin dimer | 7.57 ± 0.12 |
| 11 | 5.716 | 315.1226 | 315.1232 | −1.9 | C18H20O5 | 85.82 | 4-hydroxy-5,7,4′-trimethoxyflavan | 5.89 ± 0.01 |
| 14 | 6.335 | 289.0714 | 289.0712 | 0.7 | C15H14O6 | 87.91 | Epicatechin | 7.90 ± 0.03 |
| 15 | 6.517 | 577.1364 | 577.1346 | 3.1 | C30H26O12 | 86.47 | B-type procyanidin dimer isomer | 7.94 ± 0.05 |
| 25 | 8.801 | 609.1475 | 609.1456 | 3.1 | C27H30O16 | 99.80 | Rutin | 8.46 ± 0.15 |
| 26 | 9.046 | 463.0886 | 463.0877 | 1.9 | C21H20O12 | 99.59 | Quercetin 3-galactoside | 5.63 ± 0.09 |
| 27 | 9.051 | 609.1460 | 609.1456 | 0.7 | C27H30O16 | 99.55 | Rutin isomer | 5.50 ± 0.18 |
| 28 | 9.213 | 463.0854 | 463.0877 | −5.0 | C21H20O12 | 97.48 | Quercetin-O-glucoside | 3.96 ± 0.02 |
| 30 | 9.577 | 477.0656 | 477.0669 | −2.7 | C21H18O13 | 98.21 | Quercetin 3-glucuronide | 4.01 ± 0.01 |
| 34 | 10.199 | 505.1000 | 505.0982 | 3.6 | C23H22O13 | 99.46 | Quercetin-O-acetylhexoside | 3.24 ± 0.11 |
| Ellagitannins | ||||||||
| 19 | 7.519 | 935.0790 | 935.0791 | −0.1 | C41H28O26 | 99.97 | Casuarictin | 21.02 ± 0.23 |
| 22 | 8.290 | 934.0745 | 934.0712 | 2.0 | C41H28O26 (X2) | 83.61 | Sanguiin H6 | 25.99 ± 0.18 |
| 23 | 8.381 | 934.0761 | 934.0712 | 1.5 | C41H28O26 (X2) | 89.47 | Sanguiin H6 isomer | 35.09 ± 0.27 |
| Lignans | ||||||||
| 33 | 9.984 | 571.2175 | 571.2179 | −0.7 | C30H36O11 | 89.59 | Kadsurarin | 28.17 ± 0.14 |
| 35 | 10.509 | 571.2134 | 571.2179 | −7.9 | C30H36O11 | 85.74 | Kadsurarin isomer | 12.37 ± 0.06 |
| 36 | 10.740 | 341.1370 | 341.1389 | −5.6 | C20H22O5 | 99.89 | Kadsurenin B | 8.85 ± 0.10 |
| Anthocyanins (MS+) | ||||||||
| 12 | 5.859 | 449.1074 | 449.1084 | −2.2 | C21H21O11 | 99.91 | Cyanidin-3-O-glucoside | 1635.15 ± 13.24 |
| 17 | 6.691 | 595.1646 | 595.1663 | −2.9 | C27H31O15 | 92.30 | Cyanidin-3-O-rutinoside | 505.34 ± 5.37 |
| 18 | 7.268 | 419.0981 | 419.0919 | 0.7 | C20H19O10 | 98.62 | Cyanidin-3-O-arabinoside | 145.05 ± 1.63 |
| 38 | 11.499 | 465.1038 | 465.1033 | 1.1 | C21H21O12 | 96.29 | Delphinidin-3-O-galactoside | 32.46 ± 0.97 |
| 39 | 11.501 | 611.1598 | 611.1612 | −2.3 | C27H31O16 | 92.89 | Cyanidin-3,5-O-diglucoside | 132.11 ± 2.08 |
| 40 | 11.672 | 465.1042 | 465.1033 | 1.9 | C21H21O12 | 99.40 | Delphinidin-3-O-glucoside | 63.56 ± 1.05 |
| 41 | 11.830 | 611.1587 | 611.1612 | 2.1 | C27H31O16 | 91.56 | Cyanidin-3-O-sophoroside | 94.96 ± 1.58 |
| 43 | 13.572 | 465.1058 | 465.1033 | 5.4 | C21H21O12 | 82.42 | Delphinidin-3-O-galactoside | 11.38 ± 0.46 |
| Peak No. | Retention Time (Min) | m/z Exp. | m/z Calc. | Error (ppm) | Molecular Formula | Score | Proposed Compound | % |
|---|---|---|---|---|---|---|---|---|
| Terpenoids | ||||||||
| 5 | 4.023 | 443.1918 | 443.1917 | 0.2 | C21H32O10 | 95.27 | Ebuloside (iridoide) | 0.54 |
| 8 | 5.189 | 517.2258 | 517.2285 | −5.2 | C24H38O12 | 84.32 | Cinnamoside | 0.63 |
| 12 | 6.117 | 347.1699 | 347.1706 | −2 | C16H28O8 | 99.96 | Rhodioloside A | 0.37 |
| 28 | 9.811 | 507.2219 | 507.2230 | −2.2 | C26H36O10 | 96.51 | Scutalpin F | 0.70 |
| 34 | 11.338 | 725.4130 | 725.4112 | 2.5 | C38H62O13 | 99.64 | Tropeoside B1 | 1.69 |
| 39 | 12.509 | 501.3188 | 501.3216 | −5.6 | C30H46O6 | 89.55 | Dihydroxyurs-12-ene-23,28-dioic acid | 0.64 |
| 41 | 13.760 | 487.3404 | 487.3423 | −3.9 | C30H48O5 | 89.45 | Tormentic acid | 0.25 |
| 42 | 13.760 | 709.4182 | 709.4163 | 2.7 | C38H62O12 | 83.89 | Elephanoside A | 0.12 |
| 43 | 14.042 | 663.3893 | 663.3897 | −0.6 | C40H56O8 | 97.18 | 2a,3a-dihydroxy-24[(3-methoxy-4-hydroxy-trans-cinnamoyl)oxy]urs-12-en-28oic acid | 2.02 |
| 44 | 14.119 | 519.3326 | 519.3322 | 0.8 | C30H48O7 | 91.53 | Dihydroxytormentic acid | 0.35 |
| 45 | 14.203 | 533.3121 | 533.3114 | 1.3 | C30H46O8 | 99.98 | Tetrahydroxyurs-12-ene-23,28-dioic acid | 1.02 |
| 46 | 14.324 | 533.3116 | 533.3114 | 0.4 | C30H46O8 | 94.27 | Tetrahydroxyurs-12-ene-23,28-dioic acid isomer a | 1.83 |
| 47 | 14.401 | 533.3124 | 533.3114 | 1.9 | C30H46O8 | 98.53 | Tetrahydroxyurs-12-ene-23,28-dioic acid isomer b | 0.30 |
| 48 | 14.673 | 519.3313 | 519.3322 | −1.7 | C30H48O7 | 90.55 | Dihydroxytormentic acid isomer | 0.63 |
| 49 | 14.736 | 517.3172 | 517.3165 | 1.4 | C30H46O7 | 96.27 | Corosin | 0.92 |
| 50 | 15.157 | 517.3168 | 517.3165 | 0.6 | C30H46O7 | 99.91 | Corosin isomer a | 12.06 |
| 51 | 15.454 | 503.3370 | 503.3373 | −0.6 | C30H48O6 | 98.98 | Hydroxytormentic acid | 15.05 |
| 52 | 15.553 | 503.3375 | 503.3373 | 0.4 | C30H48O6 | 95.72 | Hydroxytormentic acid isomer a | 7.21 |
| 53 | 15.998 | 503.3371 | 503.3373 | −0.4 | C30H48O6 | 93.47 | Hydroxytormentic acid isomer b | 6.39 |
| 54 | 16.090 | 503.3366 | 503.3373 | −1.4 | C30H48O6 | 96.65 | Hydroxytormentic acid isomer c | 2.20 |
| 55 | 16.275 | 501.3214 | 501.3216 | −0.4 | C30H46O6 | 92.55 | Dihydroxyurs-12-ene-23,28-dioic acid isomer a | 4.41 |
| 56 | 16.367 | 501.3207 | 501.3216 | −1.8 | C30H46O6 | 95.06 | Dihydroxyurs-12-ene-23,28-dioic acid isomer b | 1.13 |
| 57 | 16.497 | 503.3361 | 503.3373 | −1.2 | C30H48O6 | 90.25 | Hydroxytormentic acid isomer d | 0.40 |
| 58 | 16.760 | 501.3202 | 501.3216 | −2.8 | C30H46O6 | 98.15 | Dihydroxyurs-12-ene-23,28-dioic acid isomer c | 2.85 |
| 59 | 16.875 | 487.3424 | 487.3423 | 0.2 | C30H48O5 | 99.57 | Tormentic acid isomer a | 1.75 |
| 60 | 17.167 | 487.3422 | 487.3423 | −0.2 | C30H48O5 | 99.76 | Tormentic acid isomer b | 10.62 |
| 61 | 17.391 | 485.3272 | 485.3267 | 1 | C30H46O5 | 99.12 | Hydroxyurs-12-ene-23,28 dioic acid | 6.26 |
| 62 | 17.497 | 517.3167 | 517.3165 | 0.4 | C30H46O7 | 100 | Corosin isomer b | 6.85 |
| 63 | 17.668 | 471.3481 | 471.3474 | 1.5 | C30H48O4 | 91.18 | Rubitic acid | 4.40 |
| 64 | 17.774 | 471.3472 | 471.3474 | −0.4 | C30H48O4 | 100 | Rubitic acid isomer | 3.84 |
| 65 | 12.979 | 721.3815 | 721.3799 | 3.4 | C38H58O13 | 83.24 | Suavissimoside F1 | 2.56 |
| Other compounds | ||||||||
| 1 | 0.414 | 353.0708 | 353.0720 | −3.4 | C12H18O12 | 99.21 | 6-O-(beta-D-glucopyranosyloxy)-L-ascorbic acid | |
| 2 | 0.433 | 173.0069 | 173.0086 | −9.8 | C6H6O6 | 96.29 | Dehydroascorbic acid | |
| 4 | 1.240 | 219.0481 | 219.0505 | −11 | C8H12O7 | 96.56 | Dimethyl citrate | |
| Total terpenoid content (mg/g d.w.) | 63.80 | |||||||
| Blackberry Extract | Quercetin | Ascorbic Acid | |
|---|---|---|---|
| Folin–Ciocalteu (mg GAE/g d.w.) | 31.1 ± 4.9 | 1019.7 ± 5.4 | 1260.2 ± 10.3 |
| FRAP (μmol Fe2+/g d.w.) | 637.8 ± 3.2 | 5927.2 ± 7.6 | 8639.7 ± 15.1 |
| DPPH (IC50 μg d.w./mL) | 97.1 ± 2.4 | 15.3 ± 0.1 | 9.6 ± 0.2 |
| TEAC (μmol TE/g d.w.) | 576.6 ± 8.3 | 1096.3 ± 8.7 | 1723.1 ± 10.4 |
| Strain Type | MBC/MFC, mg/mL | |
|---|---|---|
| Gram-positive | L. monocytogenes | 25 |
| L. innocua | 25 | |
| S. aureus | 25 | |
| E. faecalis | 12.5 | |
| B. cereus | 12.5 | |
| Gram-negative | S. enterica | 100 |
| P. aeruginosa | 100 | |
| S. sonnei | 50 | |
| E. coli | 12.5 | |
| Fungi | C. sake | 25 |
| Z. bailii | 50 | |
| P. expansum | 100 | |
| A. niger | 100 |
| Cellular Line | IC50, mg/mL | Selectivity Index |
|---|---|---|
| HT-29 (Human grade II colorectal adenocarcinoma) | 4.9 ± 0.2 | 22.1 |
| T-84 (Human colorectal carcinoma) | 5.9 ± 0.3 | 18.4 |
| SW-837 (Human grade IV rectum adenocarcinoma) | 5.9 ± 0.2 | 18.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. https://doi.org/10.3390/foods12071505
Gil-Martínez L, Mut-Salud N, Ruiz-García JA, Falcón-Piñeiro A, Maijó-Ferré M, Baños A, De la Torre-Ramírez JM, Guillamón E, Verardo V, Gómez-Caravaca AM. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods. 2023; 12(7):1505. https://doi.org/10.3390/foods12071505
Chicago/Turabian StyleGil-Martínez, Lidia, Nuria Mut-Salud, José Antonio Ruiz-García, Ana Falcón-Piñeiro, Mònica Maijó-Ferré, Alberto Baños, José Manuel De la Torre-Ramírez, Enrique Guillamón, Vito Verardo, and Ana María Gómez-Caravaca. 2023. "Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits" Foods 12, no. 7: 1505. https://doi.org/10.3390/foods12071505
APA StyleGil-Martínez, L., Mut-Salud, N., Ruiz-García, J. A., Falcón-Piñeiro, A., Maijó-Ferré, M., Baños, A., De la Torre-Ramírez, J. M., Guillamón, E., Verardo, V., & Gómez-Caravaca, A. M. (2023). Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods, 12(7), 1505. https://doi.org/10.3390/foods12071505








