Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antibiotic Susceptibility
2.3. Probiotic Properties of L. coryniformis NA-3
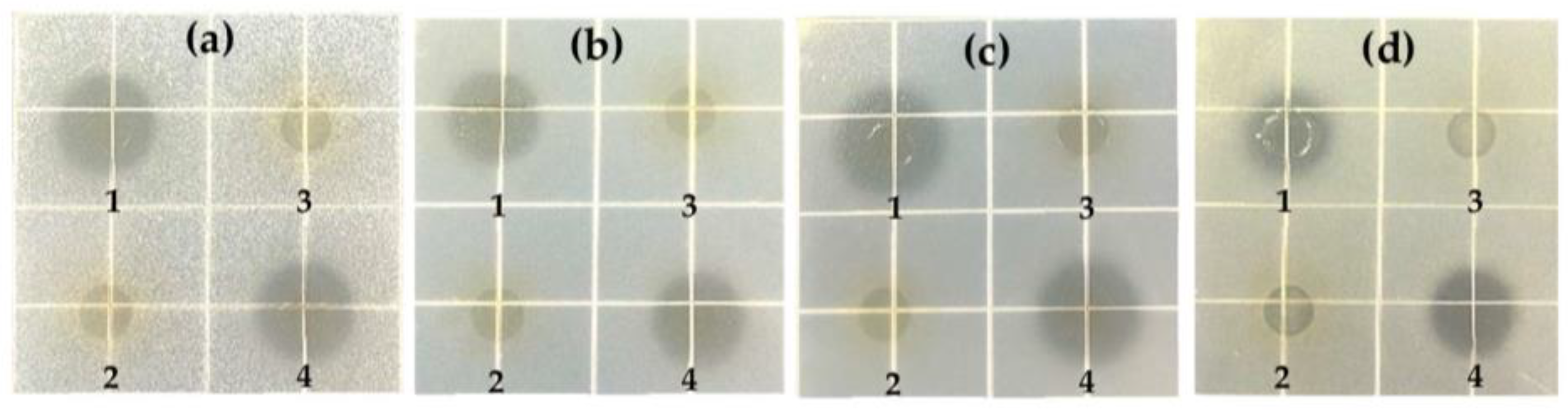
2.3.1. Antibacterial Activity
Cocultivation
Bacteriostatic Circle
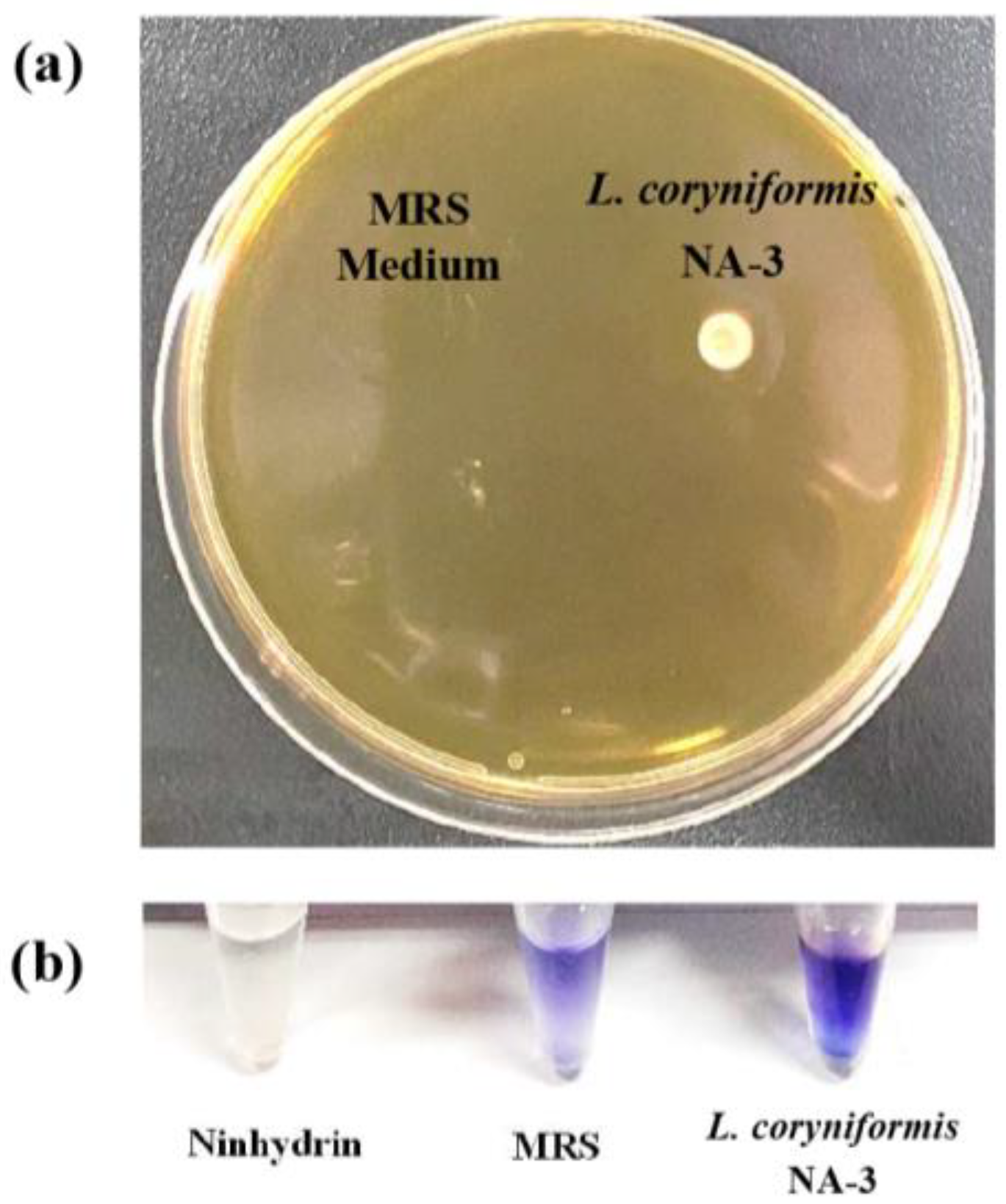
2.3.2. Cholesterol Removal and Bile Salt Hydrolase Production
2.4. Preparation of Live and Heat-Killed L. coryniformis NA-3 Cells
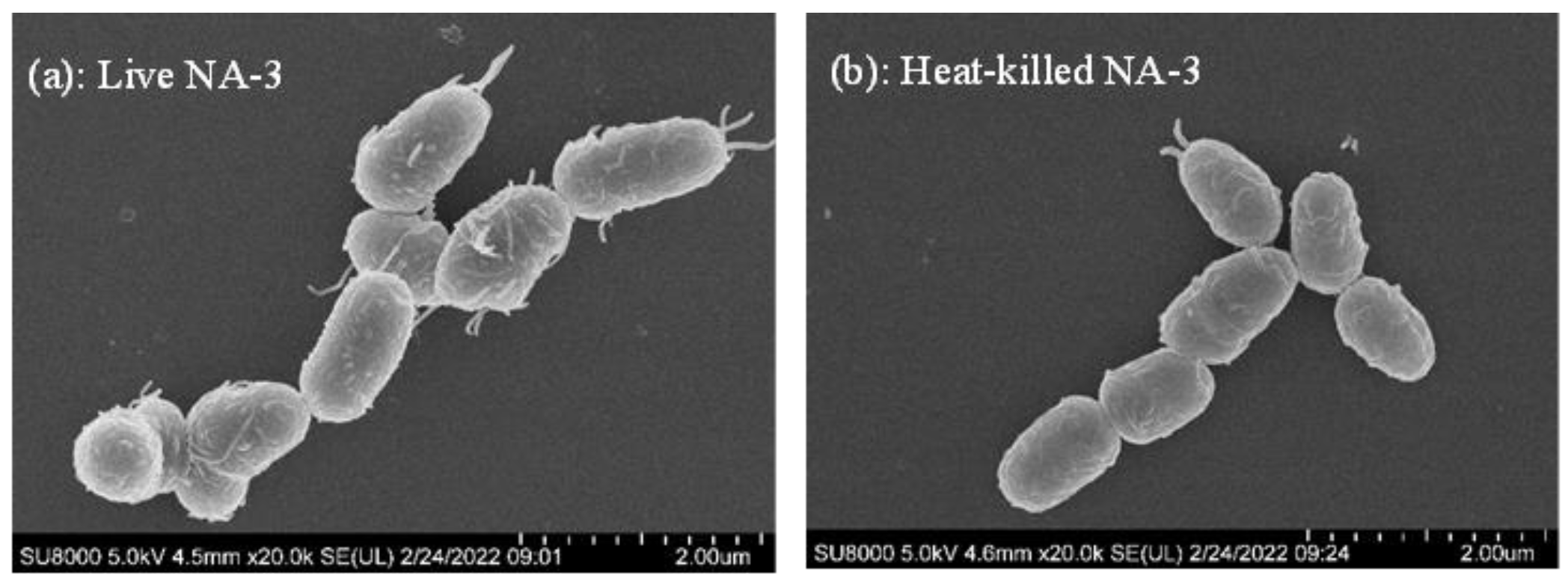
2.5. Observation of Live and Heat-Killed L. coryniformis NA-3 with Scanning Electron Microscope (SEM)
2.6. Antioxidant Activity of Live and Heat-Killed L. coryniformis NA-3
2.6.1. ABTS Scavenging
2.6.2. DPPH Scavenging
2.6.3. Nitric Oxide Scavenging
2.6.4. Hydroxyl Radical Scavenging
2.6.5. Superoxide Free Radical Scavenging
2.7. Anticancer Activity of Live and Heat-Killed L. coryniformis NA-3
2.8. The Immunoregulatory Activity of Live and Heat-Killed L. coryniformis NA-3
2.8.1. Determination of Nitric Oxide
2.8.2. Determination of IL-6 and TNF-α
2.8.3. Proliferation
2.8.4. Intracellular Reactive Oxygen Species Measurement
2.8.5. The Expression of the iNOS of Macrophages Treated with Live and Heat-Killed L. coryniformis NA-3
Western Blot
2.9. Statistical Analysis
3. Results and Discussion
3.1. Result of Antibiotic Susceptibility
3.2. Probiotic Properties of L. coryniformis NA-3
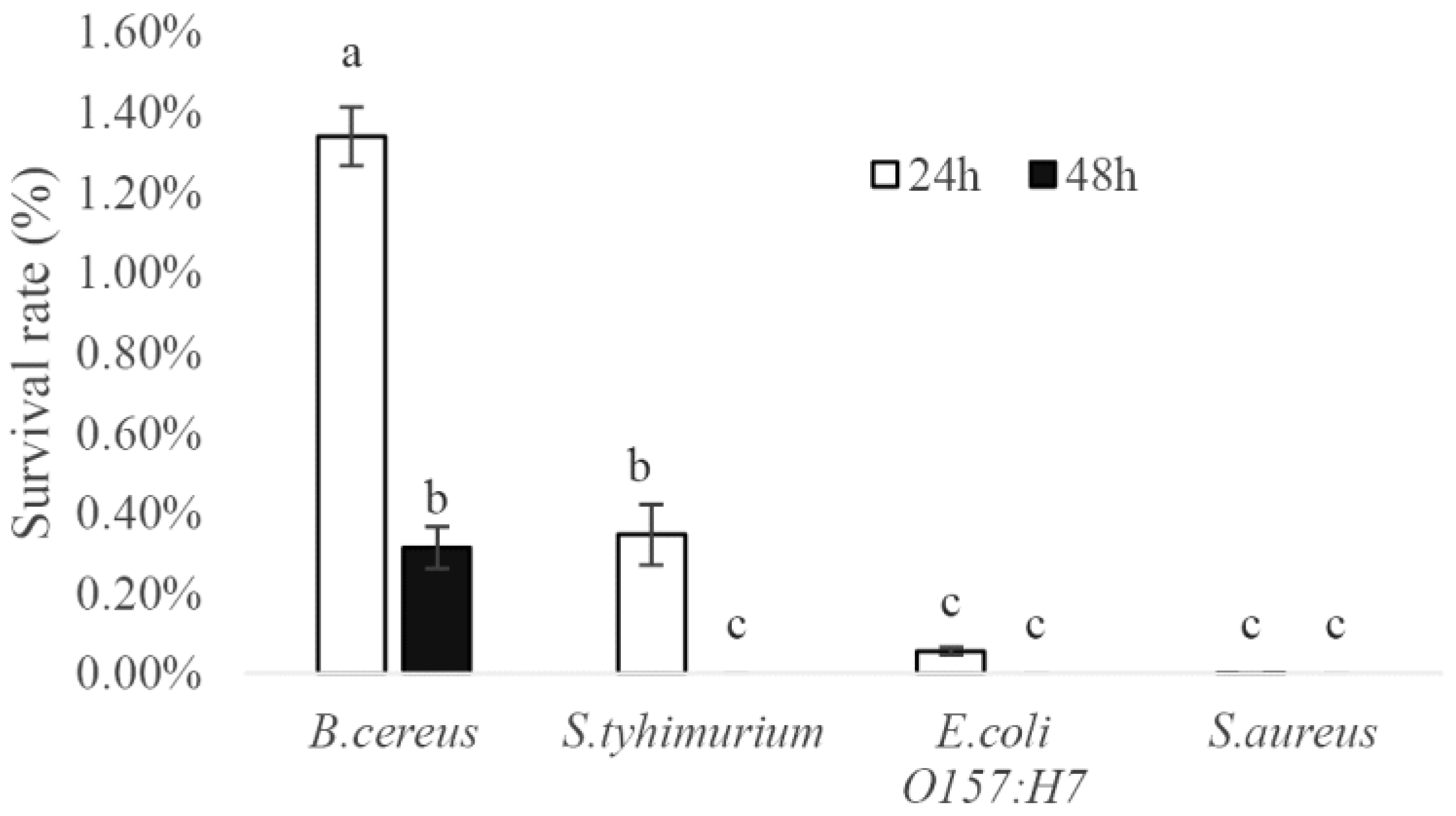
3.2.1. Antibacterial Result
3.2.2. Cholesterol Removal and BSH Production Ability
3.3. Observation of Live and Heat-Killed Strains
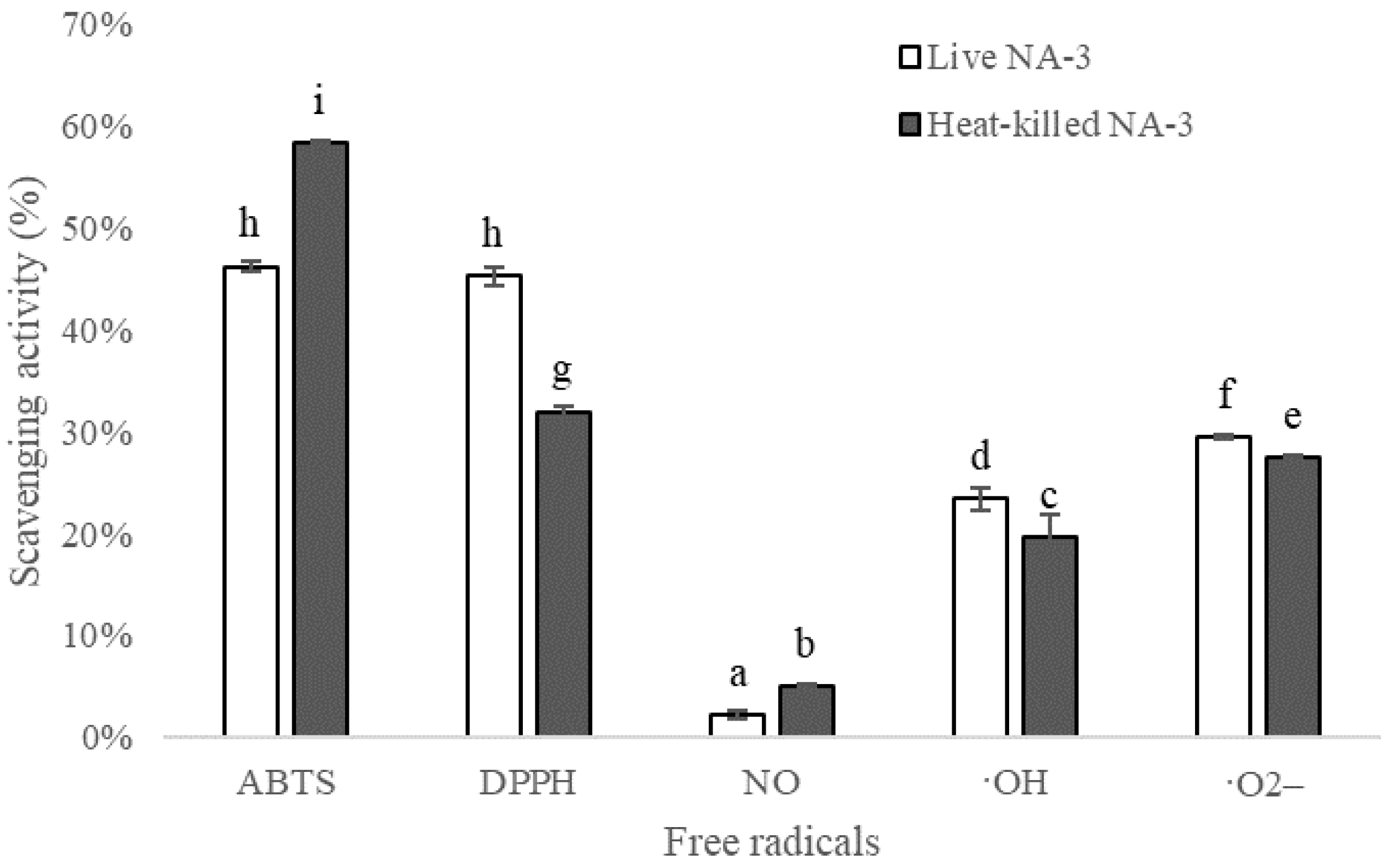
3.4. Antioxidant Activity
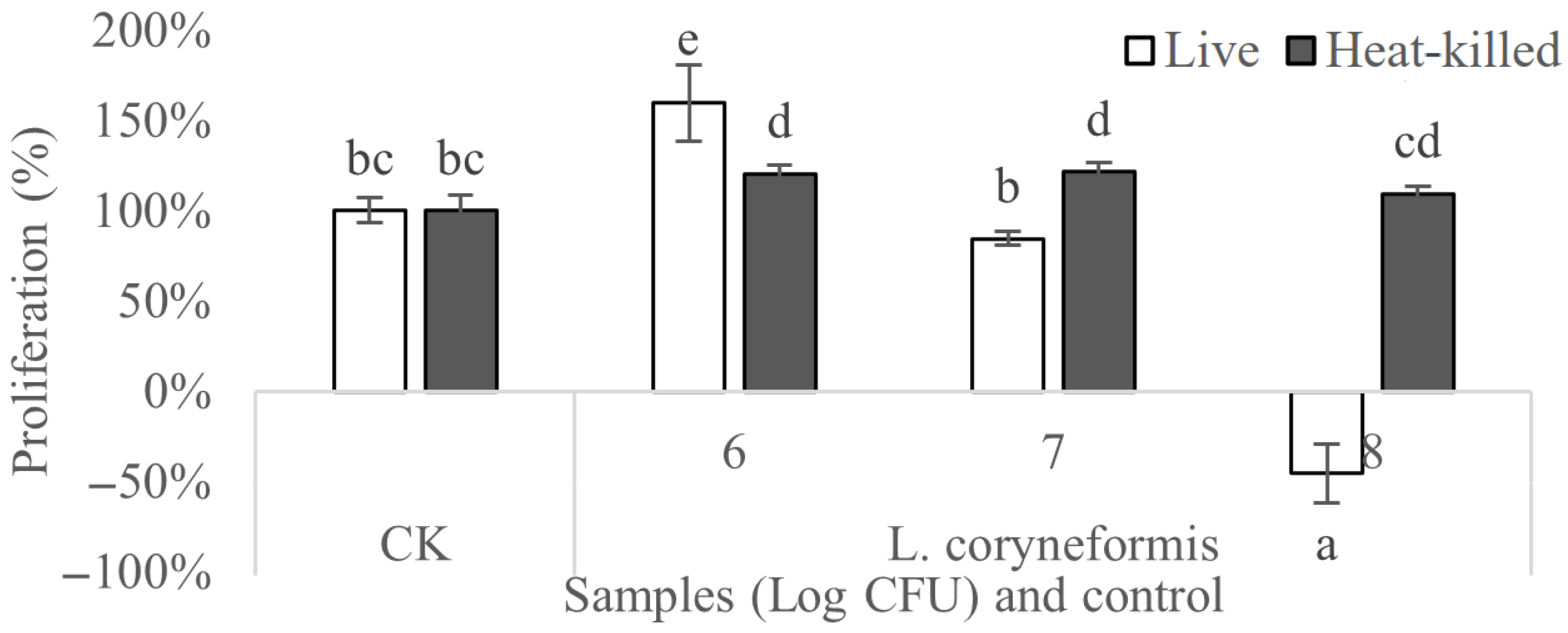
3.5. Anticancer Activity
3.6. Immunoregulation Activity
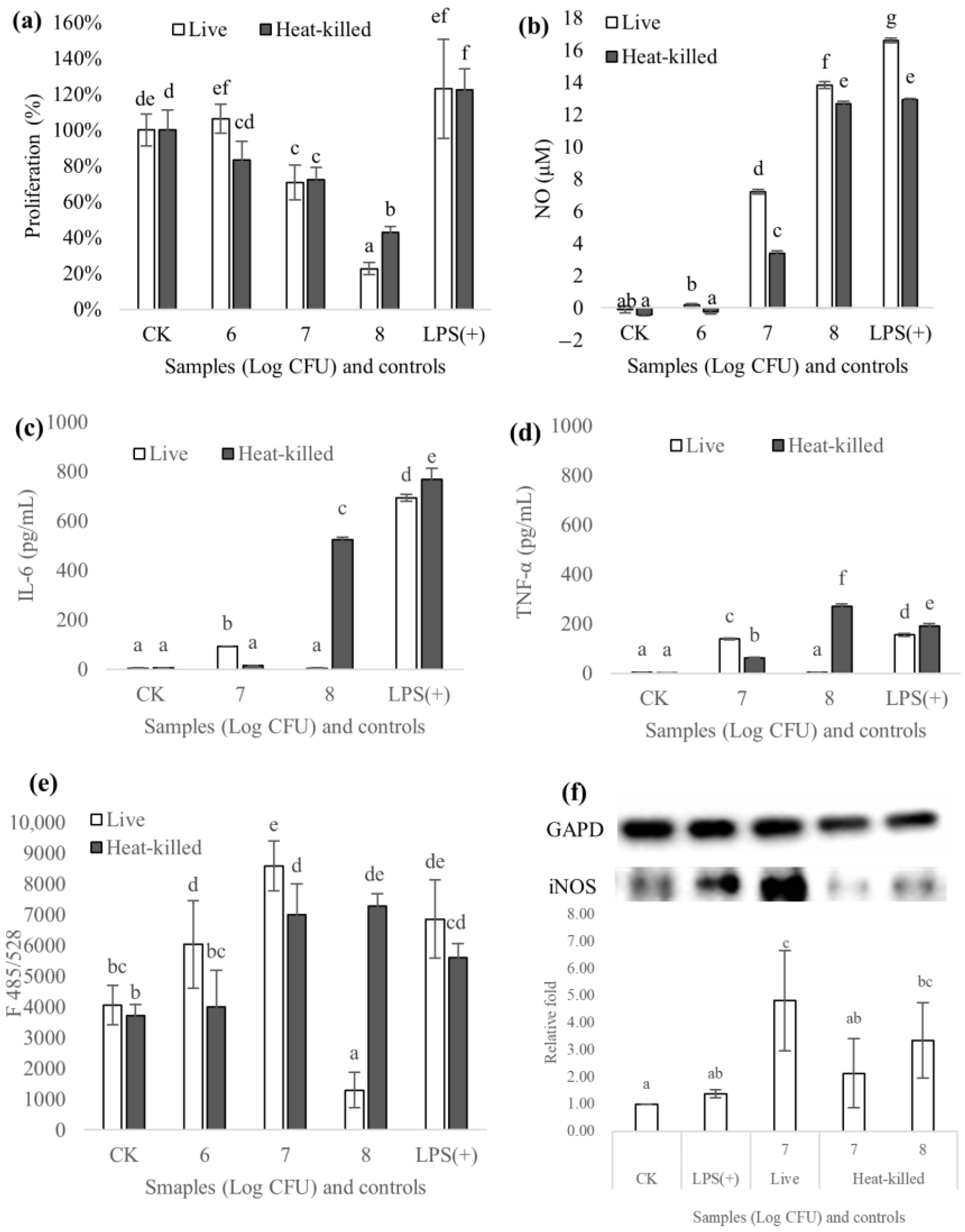
3.6.1. Proliferation
3.6.2. Production of NO and Cytokines (IL-6 and TNF-α)
3.6.3. Reactive Oxygen Species
3.6.4. Expression of iNOS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Application of lactic acid bacteria in green biorefineries. FEMS Microbiol. Lett. 2019, 366, fnz024. [Google Scholar] [CrossRef]
- Seon-Kyun, K.; Robin, B.G.; You-Tae, K.; Joongi, K.; Hyeri, K.; Hyoung, C.J.; Bum, K.H.; Ju-Hoon, L. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar]
- Fan, S.; Xue, T.; Bai, B.; Bo, T.; Zhang, J. Probiotic Properties Including the Antioxidant and Hypoglycemic Ability of Lactic Acid Bacteria from Fermented Grains of Chinese Baijiu. Foods 2022, 11, 3476. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, P.K.; Puniya, A.K.; Shukla, P. Catalytic interactions and molecular docking of bile salt hydrolase (BSH) from L. plantarum RYPR1 and its prebiotic utilization. Front. Microbiol. 2016, 7, 2116. [Google Scholar] [CrossRef]
- Yadav, M.; Dixit, M.; Shukla, P. Probiotics of diverse origin and their therapeutic applications: A review. J. Am. Coll. Nutr. 2020, 39, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lee, J.-E.; Lim, S.-M.; Kim, Y.-J.; Lee, N.-K.; Paik, H.-D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Chang, F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Song, M.W.; Lee, N.K.; Paik, H.D. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J. Food Sci. Technol. 2018, 55, 3174–3180. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.; Niu, Z.; Kang, H.; Wang, M.; Zhang, B.; Tian, H. Colonization and immunoregulation of Lactobacillus plantarum BF_15, a novel probiotic strain from the feces of breast-fed infants. Food Funct. 2020, 11, 3156–3166. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Pochart, P.; Flourié, B.; Pellier, P.; Santos, L.; Desjeux, J.F.; Rambaud, J.C. Effect of chronic ingestion of a fermented dairy product containing Lactobacillus acidophilus and Bifidobacterium bifidum on metabolic activities of the colonic flora in humans. Am. J. Clin. Nutr. 1990, 52, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Mukhtar, H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. 1995, 59, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Perdigon, G.; de Moreno, D.L.A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, I.; Choi, J.W.; Lee, C.W.; Cho, S.; Choi, T.G.; Sohn, M.; Park, Y.I. Micronized and heat-treated Lactobacillus plantarum LM1004 stimulates host immune responses via the TLR-2/MAPK/NF-κB signalling pathway in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 704–712. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Qiao, Y.; Shi, B. A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro. Food Nutr. Res. 2020, 64, 3744. [Google Scholar] [CrossRef] [PubMed]
- Tendencia, E.A. Disk diffusion method. In Laboratory Manual of Standardized Methods for Antimicrobial Sensitivity Tests for Bacteria Isolated from Aquatic Animals and Environment; Aquaculture Department, Southeast Asian Fisheries Development Center: Tigbauan, Philippines, 2004; pp. 13–29. [Google Scholar]
- Xu, H.; Tian, W.; Jia, L.; Cheng, B.; Ming, Z. Antibiotic susceptibility of potential probiotic lactobacilli isolated from the vagina of Chinese pregnant women. In Proceedings of the 2008 International Conference on BioMedical Engineering and Informatics (BMEI 2008), Sanya, China, 28–30 May 2008; Volume 2. [Google Scholar]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic susceptibility of potentially probiotic lactobacillus species. J. Food Prot. 1999, 61, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Dia, V.; Qiao, Y.; Shi, B. Antibacterial potential of a novel Lactobacillus casei strain isolated from Chinese northeast sauerkraut and the antibiofilm activity of its exopolysaccharides. Food Funct. 2020, 11, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ramírez, L.M.; Hernández-Ochoa, B.; Gómez-Manzo, S.; Marcial-Quino, J.; Cárdenas-Rodríguez, N.; Centeno-Leija, S.; García-Garibay, M. Evaluation of immunomodulatory activities of the heat-Killed probiotic strain Lactobacillus casei IMAU60214 on macrophages in vitro. Microorganisms 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Song, M.W.; Jang, H.J.; Kim, K.-T.; Paik, H.-D. Probiotic and antioxidant properties of novel Lactobacillus brevis KCCM 12203P isolated from kimchi and evaluation of immune-stimulating activities of its heat-killed cells in RAW 264.7 cells. J. Microbiol. Biotechnol. 2019, 29, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Tsai, T.-H.; Yu, C.-H.; Ho, S.-C. Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chem. 2007, 103, 181–187. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B.; Dia, V.P. Antioxidant and immunomodulatory properties of partially purified exopolysaccharide from Lactobacillus casei Isolated from Chinese Northeast sauerkraut. Immnological Investig. 2022, 51, 748–765. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Shi, B.; Dia, V.P. Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 2021, 27, 100178. [Google Scholar] [CrossRef]
- Teuber, M.; Meile, L.; Schwarz, F. Acquired antibiotic resistance in lactic acid bacteria from food. Antonie Van Leeuwenhoek 1999, 76, 115–137. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, R.; Sun, J.; Ran, J.; Ruan, X.; Zhu, Y. Partial purification and characterization of a broad-spectrum bacteriocin produced by a Lactobacillus plantarum zrx03 isolated from infant’s feces. Food Sci. Nutr. 2020, 8, 2214–2222. [Google Scholar] [CrossRef]
- Smet, I.D.; Hoorde, L.V.; Saeyer, N.D.; Woestyne, M.V.; Verstraete, W. In vitro study of bile salt hydrolase (BSH) activity of BSH isogenic Lactobacillus plantarum 80 strains and estimation of cholesterol lowering through enhanced BSH activity. Microb. Ecol. Health Dis. 1994, 7, 315–329. [Google Scholar]
- Liong, T.M.; Shah, P.N. Optimization of cholesterol removal by probiotics in the presence of prebiotics by using a response surface method. Appl. Environ. Microbiol. 2005, 71, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Sukhithasri, V.; Nisha, N.; Biswas, L.; Kumar, V.A.; Biswas, R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol. Res. 2013, 168, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.; et al. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Attur, M.; Abramson, S.B. Nitric oxide synthase and cyclooxygenases: Distribution, regulation, and intervention in arthritis. Curr. Opin. Rheumatol. 1999, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, A.; Tandrasasmita, O.; Tjandrawinata, R. Immunomodulatory and macrophage activating activity of Lactobacillus fermentum DLBSA204 in response to respiratory infection in a cellular model. Biosci. Biotechnol. Res. Asia 2016, 13, 1291–1302. [Google Scholar] [CrossRef]
- Jorjao, A.L.; de Oliveira, F.E.; Leao, M.V.; Carvalho, C.A.; Jorge, A.O.; de Oliveira, L.D. Live and heat-Killed Lactobacillus rhamnosus ATCC 7469 may induce modulatory cytokines profiles on macrophages RAW 264.7. Sci. World J. 2015, 2015, 716749. [Google Scholar] [CrossRef]
- Chen-Kai, C.; Shu-Chen, W.; Chih-Kwang, C.; Shih-Ying, C.; Zong-Tsi, C.; Pin-Der, D. Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac. J. Trop. Biomed. 2015, 5, 281–286. [Google Scholar]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Yu, F.; Li, Z.; Li, H.; Guo, C.; Fan, X. Ghrelin protects against palmitic acid or lipopolysaccharide-induced hepatocyte apoptosis through inhibition of MAPKs/iNOS and restoration of Akt/eNOS pathways. Biomed Pharm 2016, 84, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Slomiany, A. Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediat. Inflamm. 2010, 2010, 280464. [Google Scholar] [CrossRef] [PubMed]
| Type | Name | Contents/Pcs | Judgement Standard/mm | ||
|---|---|---|---|---|---|
| Resistant (R) | Moderately Susceptible (MS) | Susceptible (S) | |||
| Group 1 Inhibitors of cell wall synthesis | |||||
| β-lactam | Oxacillin | 1 μg | |||
| Penicillin | 10 U | ≤19 | 20–27 | ≥28 | |
| Ampicillin | 10 μg | ≤12 | 13–15 | ≥16 | |
| Piperacillin | 100 μg | ≤18 | 19–20 | ≥21 | |
| Imipenem | 10 μg | No standard | |||
| Cephalosporins | Cephalexin | 30 μg | ≤14 | 15–16 | ≥17 |
| Cefamezin | 30 μg | ≤15 | 16–17 | ≥18 | |
| Cefuroxime | 30 μg | ≤15 | 16–17 | ≥18 | |
| Ceftazidime | 30 μg | ≤15 | 16–17 | ≥18 | |
| Ceftriaxone | 30 μg | ≤13 | 14–20 | ≥21 | |
| Cefoperazone | 75 μg | ≤15 | 16–18 | ≥19 | |
| Glycopeptides | Vancomycin | 30 μg | ≤14 | 15–16 | ≥17 |
| Group 2 Inhibitors of protein synthesis | |||||
| Aminoglycosides | Amikacin | 30 μg | ≤15 | 16–17 | ≥18 |
| Gentamicin | 10 μg | ≤12 | - | ≥13 | |
| Kanamycin | 30 μg | ≤13 | 14–17 | ≥18 | |
| Streptomycin | 10 μg | ≤11 | 12–14 | ≥15 | |
| Tetracyclines | Doxycycline | 30 μg | |||
| Tetracycline | 30 μg | ≤14 | 15–18 | ≥19 | |
| Minocycline | 30 μg | No standard | |||
| Single antibiotics | Chloramphenicol | 30 μg | ≤13 | 14–17 | ≥18 |
| Macrolides | Erythromycin | 15 μg | ≤13 | 14–17 | ≥18 |
| Azithromycin | 15 μg | No standard | |||
| Lincosamides | Clindamycin | 2 μg | ≤8 | 9–11 | ≥12 |
| Lincomycin | 2 μg | No standard | |||
| Amides | Florfenicol | 30 μg | No standard | ||
| Group 3 Inhibitors of nucleic acid synthesis | |||||
| Sulfonamides | Compound Sulfamethoxa | 25 μg | ≤10 | 11–15 | ≥16 |
| Quinolones | Norfloxacin | 10 μg | ≤13 | 14–18 | ≥19 |
| Ciprofloxacin | 5 μg | ≤13 | 14–18 | ≥19 | |
| Levofloxacin | 5 μg | ≤13 | 14–18 | ≥19 | |
| Group 4 Inhibitors of cytoplasmic membrane synthesis | |||||
| Polymyxins | Polymyxin B | 300 IU | ≤8 | 9–11 | ≥12 |
| Oxacillin | Penicillin | Ampicillin | Piperacillin | Imipenem |
| -(R) | 38 (S) | 37 (S) | 36 (S) | -(R) * |
| Cephalexin | Cefamezin | Cefuroxime | Ceftazidime | Ceftriaxone |
| 22 (S) | 25 (S) | 25 (S) | 27 (S) | 15 (MS) |
| Cefoperazone | Vancomycin | Amikacin | Gentamicin | Kanamycin |
| 29 (S) | -(R) | 18 (S) | 18 (S) | 16 (MS) |
| Streptomycin | Doxycycline | Tetracycline | Minocycline | Chloramphenicol |
| 15 (S) | 38 (S) | 35 (S) | 44 * | 41 (S) |
| Erythromycin | Azithromycin | Clindamycin | Lincomycin | Florfenicol |
| 40 (S) | 35 * | 40 (S) | 12 * | 42 * |
| Compound Sulfamethoxa | Norfloxacin | Ciprofloxacin | Levofloxacin | Polymyxin B |
| 25 (S) | -(R) | 14 (MS) | 21 (S) | 23 (S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Qiao, Y.; Peng, Q.; Shi, B. Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities. Foods 2023, 12, 1118. https://doi.org/10.3390/foods12051118
Xu X, Qiao Y, Peng Q, Shi B. Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities. Foods. 2023; 12(5):1118. https://doi.org/10.3390/foods12051118
Chicago/Turabian StyleXu, Xiaoqing, Yu Qiao, Qing Peng, and Bo Shi. 2023. "Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities" Foods 12, no. 5: 1118. https://doi.org/10.3390/foods12051118
APA StyleXu, X., Qiao, Y., Peng, Q., & Shi, B. (2023). Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities. Foods, 12(5), 1118. https://doi.org/10.3390/foods12051118






