Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Chemical Characterization of OPE and Pectin Fractions from OAW
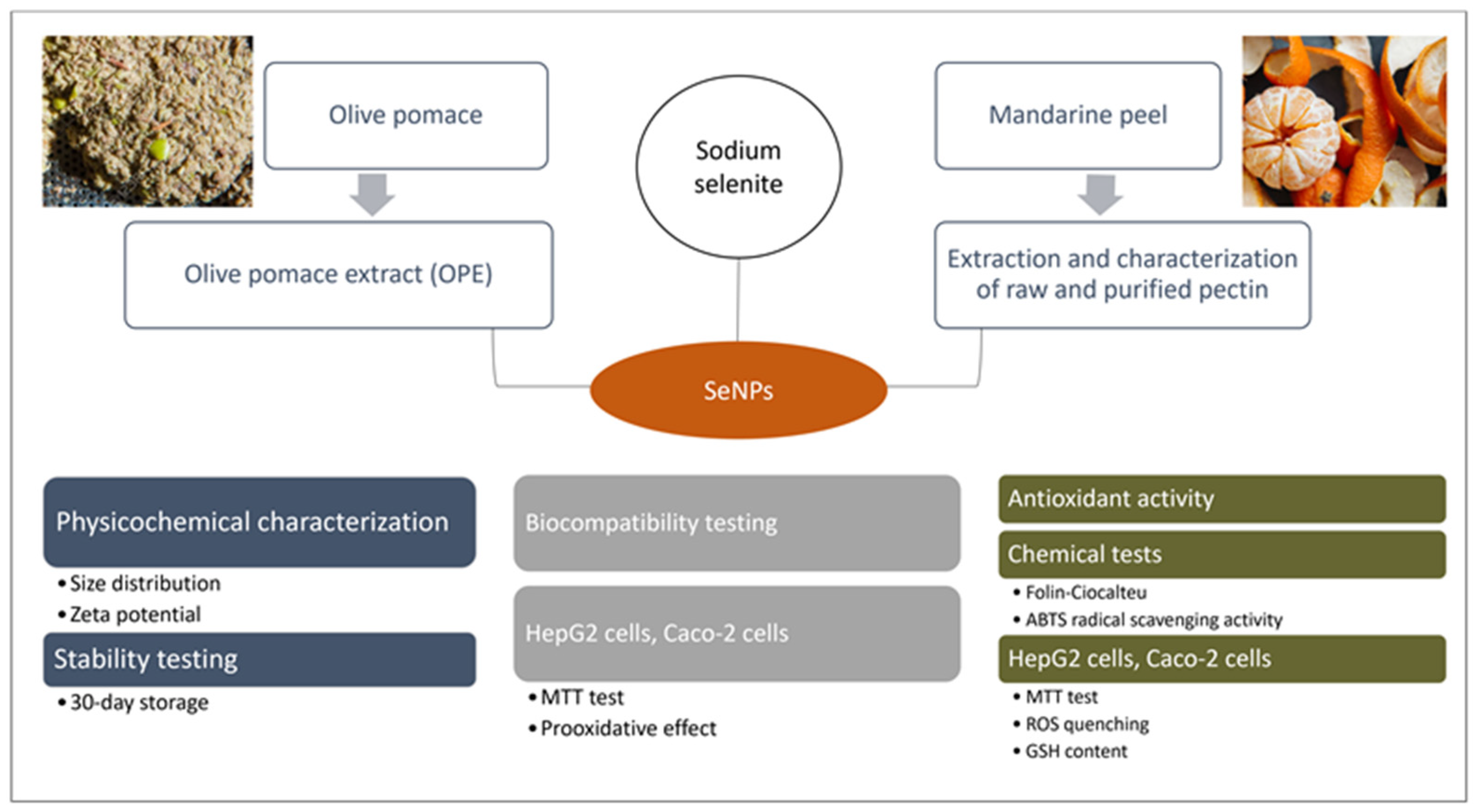
2.2. Synthesis of SeNPs
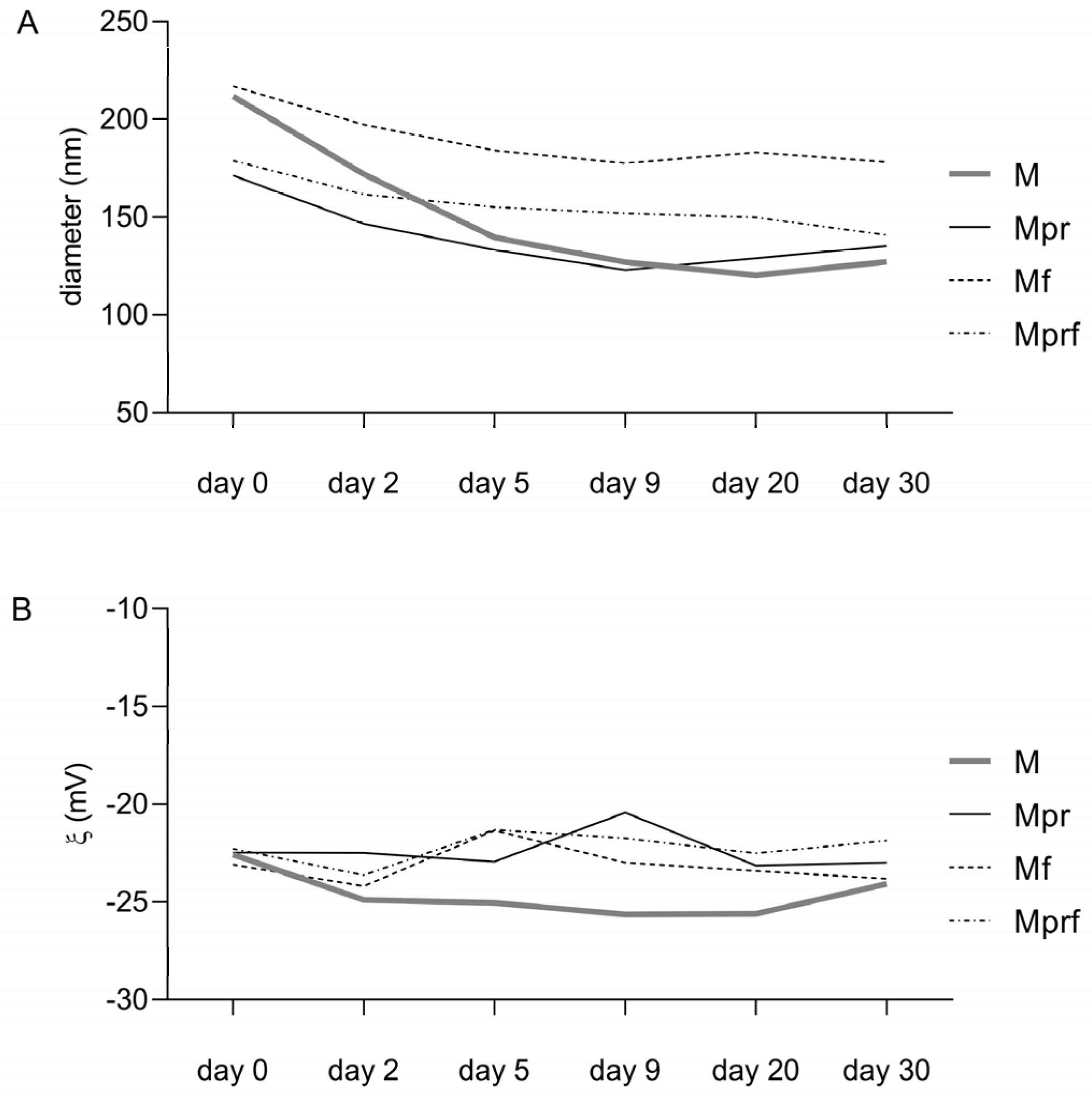
2.3. Physicochemical Characterization and Stability of SeNPs
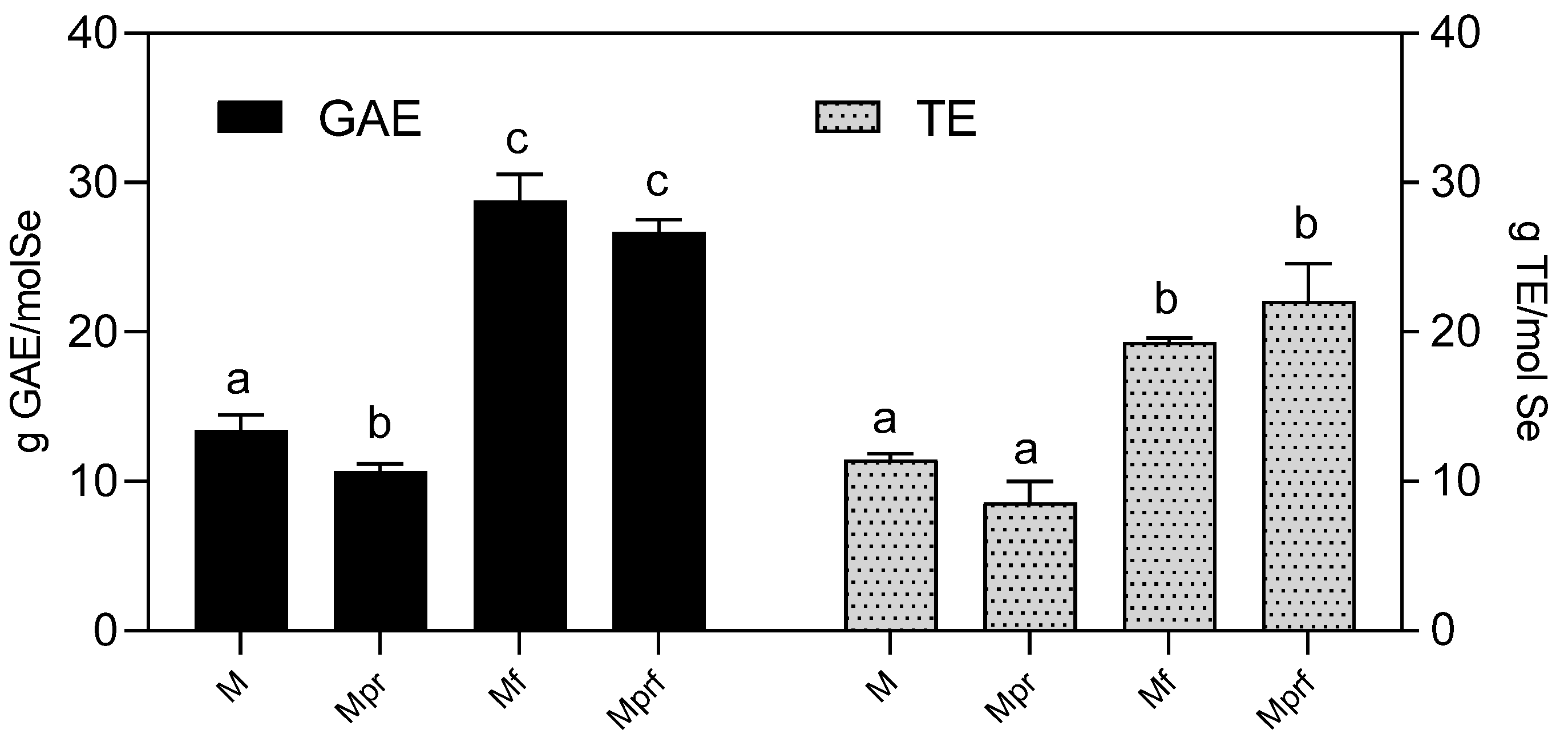
2.4. Measurement of SeNP Antioxidant Activity by Chemical Methods
2.5. Cell Cultures
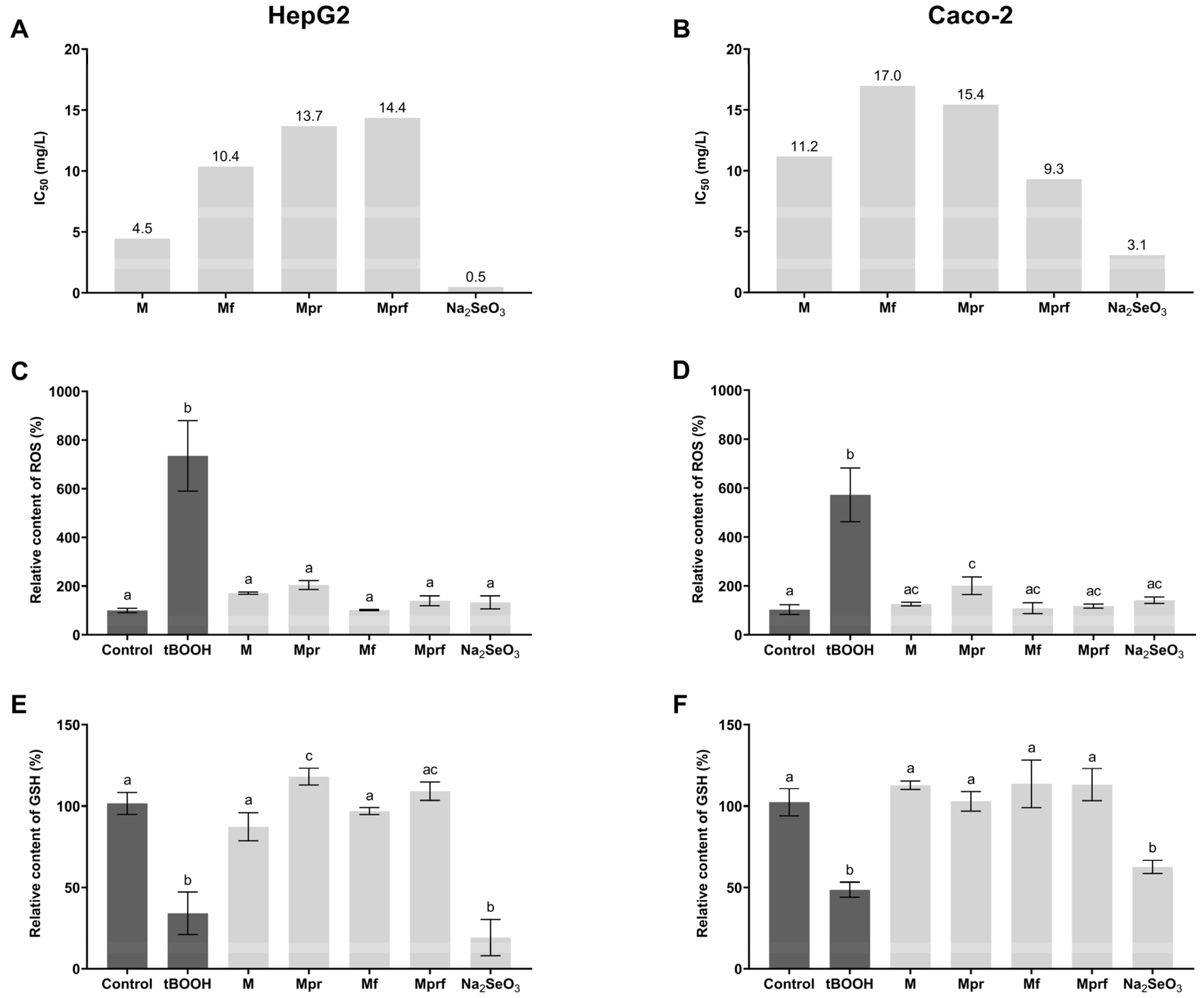
2.5.1. Measurement of SeNP Biocompatibility
2.5.2. Measurement of SeNP Antioxidant Activity in Cell Models
2.6. Measurement of Se Content
2.7. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Mandarin Peel-Derived Pectins
3.2. Physicochemical Characterization of SeNPs
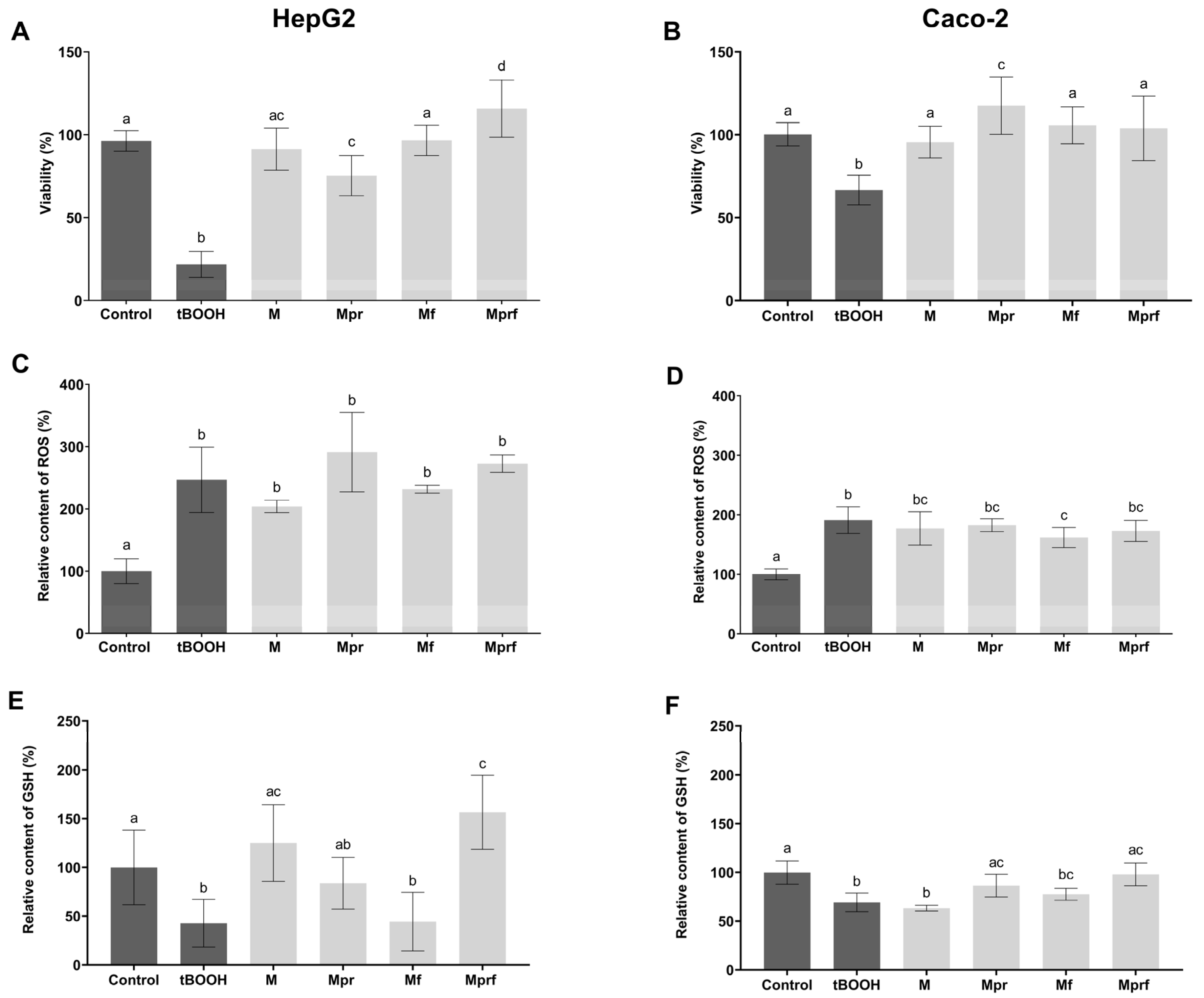
3.3. Biocompatibility of SeNPs
3.4. Antioxidant Activity of SeNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results Chem. 2022, 4, 100581. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Production of high-value nanoparticles via biogenic processes using aquacultural and horticultural food waste. Materials 2017, 10, 852. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Green synthesis of metallic nanoparticles from natural resources and food waste and their environmental application. Macabresque Hum. Viol. Hate Genocide Mass Atrocity Enemy-Mak. 2018, 2018, 321–385. [Google Scholar] [CrossRef]
- Dmochowska, A.; Czajkowska, J.; Jędrzejewski, R.; Stawiński, W.; Migdał, P.; Fiedot-Toboła, M. Pectin based banana peel extract as a stabilizing agent in zinc oxide nanoparticles synthesis. Int. J. Biol. Macromol. 2020, 165, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hileuskaya, K.; Ladutska, A.; Kulikouskaya, V.; Kraskouski, A.; Novik, G.; Kozerozhets, I.; Kozlovskiy, A.; Agabekov, V. ‘Green’ approach for obtaining stable pectin-capped silver nanoparticles: Physico-chemical characterization and antibacterial activity. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 585, 124141. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Pectin modified metal nanoparticles and their application in property modification of biosensors. Carbohydr. Polym. Technol. Appl. 2021, 2, 100164. [Google Scholar] [CrossRef]
- Wang, D.; Geng, W.; Li, Q.; Li, G.; Zhang, D.; Zhang, H. Ultrasonic green synthesis of silver nanoparticles mediated by Pectin: Characterization and evaluation of the cytotoxicity, antioxidant, and colorectal carcinoma properties. Arab. J. Chem. 2022, 15, 103500. [Google Scholar] [CrossRef]
- Qiu, W.-Y.; Wang, Y.-Y.; Wang, M.; Yan, J.-K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surfaces B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Galić, E.; Radić, K.; Golub, N.; Čepo, D.V.; Kalčec, N.; Vrček, E.; Vinković, T. Utilization of olive pomace in green synthesis of selenium nanoparticles: Physico-chemical characterization, bioaccessibility and biocompatibility. Int. J. Mol. Sci. 2022, 23, 9128. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Albahari, P.; Zovko Končić, M.; Radić, K.; Jurmanović, S.; Jug, M. Solvent extraction and chromatographic determination of polyphenols in olive pomace. Food Health Dis. 2017, 6, 7–14. Available online: https://hrcak.srce.hr/182916 (accessed on 12 January 2023).
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; González, L.-M. Process development and simulation of pectin extraction from orange peels. Food Bioprod. Process. 2015, 96, 86–98. [Google Scholar] [CrossRef]
- Ranganna, S. Hand Book of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata-McGraw-Hill: New Delhi, India, 1995; ISBN 9780074518519. [Google Scholar]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.; Wokosin, D.; Girkin, J.; Grant, M. Measurement of the intracellular distribution of reduced glutathione in cultured rat hepatocytes using monochlorobimane and confocal laser scanning microscopy. Toxicol. Vitr. 2002, 16, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Vitali, D.; Radić, M.; Cetina-Čižmek, B.; Dragojević, I.V. Caco-2 cell uptake of Ca, Mg and Fe from biscuits as affected by enrichment with pseudocereal/inulin mixtures. Acta Aliment. 2011, 40, 480–489. [Google Scholar] [CrossRef]
- Radić, K.; Dukovski, B.J.; Čepo, D.V. Influence of pomace matrix and cyclodextrin encapsulation on olive pomace polyphenols’ bioaccessibility and intestinal permeability. Nutrients 2020, 12, 669. [Google Scholar] [CrossRef]
- Jurmanović, S.; Jug, M.; Safner, T.; Radić, K.; Domijan, A.; Pedisić, S.; Šimić, S.; Jablan, J.; Vitali čepo, D. Utilization of olive pomace as a source of polyphenols: Optimization of microwave-assisted extraction and characterization of spray-dried ex-tract. J. Food Nutr. Res. 2019, 58, 51–62. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Mohamed, H. Extraction and characterization of pectin from grapefruit peels. MOJ Food Process. Technol. 2016, 2, 29. [Google Scholar] [CrossRef]
- Kute, A.B.; Mohapatra, D.; Kotwaliwale, N.; Giri, S.K.; Sawant, B.P. Characterization of pectin extracted from orange peel powder using microwave-assisted and acid extraction methods. Agric. Res. 2020, 9, 241–248. [Google Scholar] [CrossRef]
- Grassino, A.N.; Halambek, J.; Djaković, S.; Brnčić, S.R.; Dent, M.; Grabarić, Z. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Lane, L.A.; Qian, X.; Smith, A.M.; Nie, S. Physical chemistry of nanomedicine: Understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015, 66, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Barar, J. Bioimpacts of nanoparticle size: Why it matters? Bioimpacts 2015, 5, 113–115. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Mello, D.F.; Trevisan, R.; Rivera, N.; Geitner, N.K.; Di Giulio, R.T.; Wiesner, M.R.; Hsu-Kim, H.; Meyer, J.N. Caveats to the use of MTT, neutral red, Hoechst and Resazurin to measure silver nanoparticle cytotoxicity. Chem. Interact. 2020, 315, 108868. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Liu, L.; Tao, W.; Wang, G.; Sun, W.; Pei, X.; Xiao, Z.; Jin, Y.; Wang, M. Prooxidation and cytotoxicity of selenium nanoparticles at nonlethal level in sprague-dawley rats and buffalo rat liver cells. Oxidat. Med. Cell Longev. 2020, 2020, 7680276. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, H.; Bi, Y.; Fan, X. Preparation and antioxidant activity of selenium nanoparticles decorated by polysaccharides from Sargassum Fusiforme. J. Food Sci. 2021, 86, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Selenium nanoparticles: Synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol. Rep. 2021, 30, e00615. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. The influence of synthesis conditions on the antioxidant activity of selenium nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. Antioxidant properties of selenium nanoparticles synthesized using tea and herb water extracts. Appl. Sci. 2023, 13, 1071. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The folin-ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Čepo, D.V.; Radić, K.; Turčić, P.; Anić, D.; Komar, B.; Šalov, M. Food (matrix) effects on bioaccessibility and intestinal permeability of major olive antioxidants. Foods 2020, 9, 1831. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.; Almahri, A.; Ahmed, M.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97. [Google Scholar] [CrossRef]
| Sample | Na2SeO3 (0.1 M) (mL) | L-Ascorbic Acid (1 M) (mL) | OPE (1%) (mL) | Raw Pectin (0.05%) (mg) | Purified Pectin (0.05%) (mg) | Ultrapure Water (mL) |
|---|---|---|---|---|---|---|
| M | 1 | 1 | 0 | 15 | 0 | 28 |
| Mpr | 1 | 1 | 0 | 0 | 15 | 28 |
| Mf | 1 | 1 | 5 | 15 | 0 | 23 |
| Mprf | 1 | 1 | 5 | 0 | 15 | 23 |
| Yield (%) | EM (g/mol) | MC (%) | DE (%) | GLA (%) | |
|---|---|---|---|---|---|
| Raw pectin | 12.8 ± 0.6 a | 780.0 ± 4.1 a | 9.1 ± 0.1 ab | 69.1 ± 2.4 a | 74.8 ± 0.8 a |
| Purified pectin | 7.9 ± 0.5 b | 2018.8 ± 10.2 b | 10.6 ± 0.6 b | 86.6 ± 3.5 b | 69.2 ± 0.7 b |
| Commercial pectin (reference) | / | 1954.6 ± 52.6 b | 8.7 ± 0.2 b | 72.5 ± 1.3 a | 59.0 ± 1.2 c |
| Sample | Average Diameter (nm) | Zeta Potential (mV) | Polydispersity Index | pH |
|---|---|---|---|---|
| M | 211.83 ± 2.47 a | −22.58 ± 0.91 a | 0.25 ± 0.00 a | 4.02 |
| Mpr | 171.33 ± 2.29 b | −22.47 ± 0.90 a | 0.20 ± 0.02 a | 3.87 |
| Mf | 216.87 ± 1.20 c | −23.09 ± 0.83 a | 0.19 ± 0.03 b | 4.11 |
| Mprf | 178.93 ± 1.03 d | −22.27 ± 0.70 a | 0.20 ± 0.02 a | 3.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golub, N.; Galić, E.; Radić, K.; Jagodić, A.-M.; Predović, N.; Katelan, K.; Tesla, L.; Pedisić, S.; Vinković, T.; Vitali Čepo, D. Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes. Foods 2023, 12, 1117. https://doi.org/10.3390/foods12051117
Golub N, Galić E, Radić K, Jagodić A-M, Predović N, Katelan K, Tesla L, Pedisić S, Vinković T, Vitali Čepo D. Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes. Foods. 2023; 12(5):1117. https://doi.org/10.3390/foods12051117
Chicago/Turabian StyleGolub, Nikolina, Emerik Galić, Kristina Radić, Ana-Maria Jagodić, Nela Predović, Kristina Katelan, Lucija Tesla, Sandra Pedisić, Tomislav Vinković, and Dubravka Vitali Čepo. 2023. "Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes" Foods 12, no. 5: 1117. https://doi.org/10.3390/foods12051117
APA StyleGolub, N., Galić, E., Radić, K., Jagodić, A.-M., Predović, N., Katelan, K., Tesla, L., Pedisić, S., Vinković, T., & Vitali Čepo, D. (2023). Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes. Foods, 12(5), 1117. https://doi.org/10.3390/foods12051117









