Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
3. Use of EO for Metabolic Syndrome-Related Disorders Management
3.1. Anti-Obesogenic Potential
| Essential Oil (EO) | Study Details | Experimental Findings | Reference |
|---|---|---|---|
| 29 different EOs (lemon balm, Spanish sage, rosemary, marjoram, peppermint, lavender, thyme, basil, orange, bergamot, lemon, mandarin, grapefruit, tea tree, Niaouli nerolidol, eucalyptus, cypress, cedarwood, juniper-berry, black pepper, frankincense, ginger, geranium-rose, fennel, chamomile-roman, pine, and even Sara) | In vitro—3T3-L1 preadipocytes were differentiated. Oil-Red O (ORO) stain assay was done to assess lipid accumulation. 3T3-L1 adipocytes were treated with all samples at 60 µL/mL concentration for six days. | Lemon balm, peppermint, lavender, bergamot, cypress, niaouli nerolidol, geranium-rose, and revensara inhibited lipid accumulation by 53–90% compared to the control. Spanish sage, rosemary, marjoram, orange, eucalyptus, cedarwood, black pepper, and ginger increased lipid accumulation (110–167%). Thyme, lemon, tea tree, fennel, chamomile-roman, pine, basil, mandarin, grapefruit, juniper-berry, and frankincense did not show effects on lipid metabolism (90–110%). | [1] |
| Garlic EO (GEO) | In vivo—Six-week-old male C57BL/6J mice were fed a standard or high-fat diet (HFD) with and without GEO for 12 weeks. GEO concentrations were 25, 50, or 100 mg/kg. Blood, liver, subcutaneous, epididymal, and perirenal fats were collected. | GEO at 50 mg/kg concentration prevented the increment of subcutaneous, epididymal, and perirenal fat pads in mice fed with HFD, and reduced their elevated glucose levels, insulin, free fatty acids, and triglycerides. | [24] |
| Seven different EOs (lemon grass, ginger, black pepper, long pepper, turmeric, cassumunar ginger, and kaffir lime) | In vitro—3T3-L1 preadipocytes were differentiated. ORO stain assay was done to assess lipid accumulation. Total triglyceride content was determined using a triglyceride assay kit. | All EO inhibited or decreased lipid accumulation, adipogenesis, and triglyceride content. | [18] |
| Artemisia annua L. EO | In vitro—3T3-L1 preadipocytes were differentiated. ORO stain assay was done to assess lipid accumulation. | Inhibited adipogenesis. | [20] |
| Calamus EO | In vitro—After 3T3-L1 differentiation, a total triglycerides assay, ORO, RT-PCR, and western blot analyses (for analysis of p-ERK1/2, C/EBPβ, C/EBPα, and PPARγ protein) were conducted. | Reduction of intracellular triglyceride content and adipogenesis inhibition was detected. | [19] |
| Pinus koraiensis EO (PKEO) | In vitro—ORO staining, triglycerides content, and expression levels of adipogenic factors were measured in 3T3-L1 differentiated cells treated with PKEO. | In vitro results showed a reduction of intracellular triglyceride content and downregulation of adipogenic transcription factors expression. | [21] |
| In vivo—Male Sprague-Dawley rats, at four weeks of age, treated with high-fat diets, whose body weights, retroperitoneal and epididymal fats, and serum lipid metabolites (HDL, LDL, triglycerides) were assessed during six weeks. | In vivo results demonstrated that PKEO treatment prevented weight gain and suppressed serum triglyceride, total cholesterol, and LDL cholesterol. | ||
| Lime EO | In vivo—Fifty-six male mice weighing 25–30 g were divided into seven groups for 45 days. Males were subcutaneously treated with normal saline (0.1 mL/mice), DMSO (0.02 mL/mice), ketotifen dissolved in 0.1 mL of normal saline (32 mg/kg), lime EO dissolved in 0.02 mL of DMSO (125, 250, 500 mg/kg), and a mixture of ketotifen and lime EO (32 mg/kg, and 125 mg/kg, respectively) properly dissolved in normal saline and DMSO, respectively. Food intake and body weight changes were studied. | Mice treated with lime EO exhibited both body weight loss and food intake reduction. | [26] |
| Cinnamon EO (CEO) | In vitro—3T3-L1 cells were differentiated with the EO, and their major components, S-(+)-linalool, and R-(-)-linalool. After differentiation, the ORO assay was performed. | In vitro results exhibited that treatment with cinnamon EO reduced the accumulation of lipid droplets, S-(+)-linalool, and R-(-)-linalool compared with the control group. Higher doses (100 µg/mL) improved the inhibition effect more than lower ones (10 µg/mL). | [22] |
| In vivo—Six-week-old male ICR mice were orally treated with corn oil as control, 250 and 500 mg/kg of CEO, 500 mg/kg of S-(+)-linalool, and 500 mg/kg of R-(-)-linalool, for 14 days. Body weight changes and blood biochemical parameters (glucose, total cholesterol (TC), triglyceride levels (TG)) were monitored. | In vivo results demonstrated that the body weight change rate was lower than the control group for those mice treated with CEO and S-(+)-linalool. As well as this, blood glucose, TC, and TG were decreased. | ||
| Citronella EO | Clinical trial—A randomized, double-blind, placebo-controlled clinical trial was conducted with 78 overweight subjects aged between 18 and 60. Participants were divided into three groups: (1) treated with 100 mg EO of Cumin cyminum L. capsule; (2) treated with orlistat120 capsule, and (3) treated with placebo. Treatments were taken three times per day for eight weeks. Anthropometric measures and fasting blood samples were taken at baseline and after treatments. | Participants who were treated with EO of Cumin cyminum L. capsule exhibited a decrease in weight and body mass index compared to orlistat120 and placebo. Likewise, cumin EO capsules reduced serum insulin levels. | [28] |
| Ginger EO (GgEO) | In vivo—Eight-week-old male C57BL/6J mice were fed a standard diet or HFD for 12 weeks with orally administrated GgEO or citral (its main chemical compound). They were divided into four groups: (1) positive control with a standard diet with 13.5% kcal fat content; (2) negative control with an HFD with 60% kcal fat content; (3) HFD + GEO (12.5, 62.5, or 125 mg/kg) and (4) HFD + citral (2.5 or 25 mg/kg). Food intake and body weight were monitored. Serum biochemical parameters (glucose, insulin, free fatty acids, cholesterol, and triglycerides) were assessed. Liver, subcutaneous, epididymal, and perirenal adipose tissue were collected. | GgEO and citral treatments reduced average body weight by preventing the HFD-treated mice increasing their amount of subcutaneous, epididymal, and perirenal fat pads in a dose-dependent manner. These same treatments considerably decreased the results of serum biochemical levels in a dose-dependent manner. | [25] |
| Grapefruit EO (GpEO) | In vivo—Male Wistar rats (250–300 g) and male C57BL/6J mice were subjected to olfactory stimulation with GpEO. Autonomic nerve activities were examined electro-physiologically by placing the nose of the anesthetized rat inside a beaker that contained filter paper soaked in GpEO or water. To assess the effects of GpEO on food intake and body and tissue weights, a gauze soaked in GpEO was placed above the animal cage for 15 min, three times a week, for six weeks. | Sympathetic white and brown adipose tissue nerve was increased with GpEO inhalation treatment. GpEO reduced food intake, body weight, and organs and adipose tissue weights. | [31] |
| Patchouli EO (PEO) | In vivo—Four-week-old male Sprague Dawley rats were divided into four groups: (1) standard diet fed control + 30-min inhalation of distilled water (DW); (2) HFD fed control + 30-min inhalation of DW; (3) and (4) HFD + 0.3% and 1% PEO 30-min inhalation, respectively. All treatments lasted 12 weeks. Body weight, food intake, and serum biochemical parameters (TC, HDL cholesterol, and TG) were measured for all groups. Brain, heart, kidney, liver, white adipose tissue (WAT), and brown adipose tissue (BAT) were extracted. | Groups subjected to PEO inhalation treatments exhibited a decrement in food intake and body weight. BAT weight was decreased. HDL cholesterol was increased while LDL was decreased. | [15] |
| Sweet orange EO (SOEO) | In vivo—Four- to six-week-old male Sprague Dawley rats (190–210 g) were divided into six groups: (1) HFD + 2 mL of normal saline; (2) HFD + 2 mL of β-cyclodextrin; (3) HFD + 19 mg of SOEO + 2 mL of normal saline; (4) HFD + 2 mL suspension of SOEO microcapsules (microcapsules were made with SOEO + β-cyclodextrin); (5) HFD + 2 mL suspension of orlistat powder and (6) rats treated with a low-fat diet. Rats were subjected to treatments for 15 days. Body weight and food intake were assessed every two days. Serum biochemical analysis was done. | SOEO microcapsules significantly lowered body weight gain and fat rate compared to HFD-fed rats. Furthermore, SOEO microcapsules decreased total cholesterol and LDL cholesterol levels in serum. | [29] |
| Lemongrass EO (LGEO) | In vitro—ORO staining, triglycerides content, and expression levels of adipogenic factors were measured in 3T3-L1 differentiated cells treated with LGEO and its major constituents: citral and citral diethyl acetal. | LGEO and its major constituents decreased lipid accumulation via adipogenesis inhibition, increased lipolysis, and decreased lipid uptake. | [23] |
| Mix of EO (MEO) composed of thyme (50%), orange peel (25%), bay leaf (12.5%), and eucalyptus (12.5%) EO | In vivo—15-day-old Japanese quails were divided into three groups and exposed to a low ambient temperature. Treatments were: (1) basal-diet; (2) basal diet + 50 ppm of MEO; and (3) basal-diet + 100 ppm of MEO. Serum biochemical parameters were measured. | MEO decreased serum glucose, TG, and TC compared to the control group. | [27] |
3.2. Antidiabetic Potential
3.2.1. Postprandial Hyperglycemia
3.2.2. Starch and Digestive Enzymes Activity
3.2.3. Diabetes Pharmacological Therapy
3.2.4. EO as an Alternative to Pharmaceutical Drugs
| Essential Oil (EO) | Study Details | Experimental Findings | Reference |
|---|---|---|---|
| Clove, thyme, oregano, and sweet orange | Enzymatic assay—EO were extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were assessed. Experimental concentrations for each EO were 250 mg/mL. Acarbose was used as a positive control. | α-amylase inhibition Clove, thyme, oregano, sweet orange EO, and acarbose inhibited 93.1, 81.3, 81.4, 95.4, and 73.5% of α-amylase activity, respectively. α-glucosidase inhibition Clove, thyme, oregano, sweet orange EO, and acarbose inhibited 75.5, 98.9, 50.5, 37.3, and 34.5% of α-glucosidase activity, respectively. | [49] |
| Wild mint (Mentha longifolia var. calliantha) | Enzymatic assay—EO was extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition assays were assessed using 3,5-dinitrosalisylic acid (DNS) and p-nitrophenyl-α-D-glucopyranoside (pNPG) methods, respectively. Enzymes’ inhibitory activity was expressed as equivalents of acarbose (ACEs). | α-amylase inhibitory activity: 2.74 mmol ACEs/g EO α-glucosidase inhibitory activity: 5.62 mmol ACEs/g EO | [58] |
| Hertia cheirifolia | Enzymatic assay—EO from leaves, flower buds, flowers, and fruits were extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase inhibition assay was assessed using the DNS method. Results were expressed as equivalent acarbose per gram of EO (ACEs). | α-amylase inhibitory activities in different plant organs: Leaves: 8.32 mg ACEs/g EO Flower buds: 2.75 mg ACEs/g EO Flowers: 5.85 mg ACEs/g EO Fruits: 8.84 mg ACEs/g EO | [50] |
| Nepeta curviflora | Enzymatic assay—EO was extracted utilizing a microwave ultrasonic apparatus. α-amylase and α-glucosidase inhibition assays were assessed using DNS and pNPG, respectively. Experimental concentrations for α-amylase assay were 10, 50, 70, 100, and 500 μg/mL, while for α-glucosidase they were 100, 200, 300, 400, and 500 μg/mL. | The highest inhibitory percentage for α-amylase was 65.8%, achieved with a concentration of 500 μg/mL. However, at the same concentration, acarbose presented a higher inhibitory activity (72.54%). Nepeta curviflora EO IC50 in this assay was 45.7 μg/mL. In comparison, acarbose IC50 was 28.84 μg/mL. In the case of α-glucosidase, the highest inhibitory percentage was 92.72% with a concentration of 500 μg/mL. It had a slightly higher inhibitory activity than acarbose at the same concentration (92.28%). Nepeta curviflora EO IC50 in this assay was 54.9 μg/mL. In comparison, acarbose IC50 was 37.15 μg/mL. | [53] |
| Oliveria decumbens (OD), Thymus kotschyanus (TK), Trachyspermum ammi (TA), and Zataria multiflora (ZM) EO | Enzymatic assay—EOs were extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were assessed. | α-amylase inhibition OD IC50: 223 μg/mL TK IC50: 229 μg/mL TA IC50: 218 μg/mL ZM IC50: 216 μg/mL Acarbose IC50: 126 μg/mL α-glucosidase inhibition OD IC50: 220 μg/mL TK IC50: 238 μg/mL TA IC50: 212 μg/mL ZM IC50: 219 μg/mL Acarbose IC50: 139 μg/mL For both assays, all EOs similarly inhibited enzymes but at lower levels than acarbose. | [54] |
| Cedrus libani | Enzymatic assay—EO from wood, leaves, and cones was extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase inhibition colorimetric assay (DNS) was performed. Experimental concentrations range from 1 mg/mL to 0.1 mg/mL. | Wood EO IC50: 0.14 mg/mL Cone EO IC50: >1 mg/mL Leaves did not demonstrate inhibition. | [59] |
| Orange and lemon peels EO | Enzymatic assay—EOs were extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were assessed. Experimental concentrations for each EO were 0–16 μg/mL. | α-amylase inhibition Orange peel IC50: 11.51 μg/mL Lemon peel IC50: 8.16 μg/mL Acarbose IC50: 7.45 μg/mL α-glucosidase inhibition Orange peel IC50: 11.53 μg/mL Lemon peel IC50: 7.56 μg/mL Acarbose IC50: 8.44 μg/mL Lemon peel EO exhibited the highest inhibitory effects in both enzymes. The α-glucosidase inhibition assay has a higher inhibitory effect than acarbose. | [55] |
| Black pepper | Enzymatic assay—EO was extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were performed. Experimental concentrations for each EO were 0–120 mL/L. | α-amylase inhibition IC50: 86.06 mL/L α-glucosidase inhibition IC50: 68.29 mL/L Black pepper EO showed more potent inhibitory activity in α-glucosidase than in α-amylase. | [60] |
| Peppermint (Mentha piperita L.) | Enzymatic assay—different extraction methods for EO: conventional hydrodistillation (HD); microwave-assisted hydrodistillation (MWHD); soxhlet extraction (SOX); ultrasound-assisted extraction (UAE); microwave-assisted extraction (MAE); and supercritical fluid extraction (SFE). α-amylase and α-glucosidase inhibition colorimetric assays were assessed. | α-amylase inhibitory activity range: 1.24–1.76 mmol ACEs/g α-glucosidase inhibitory activity range: 57.96–58.89 mmol ACEs/g | [51] |
| Lavender | In vivo—15-weeks-old adult male Wistar rats (220–230 g) were divided into four groups: (1) control (nondiabetic rats) treated with 0.9% NaCl; (2) alloxan-induced diabetic rats treated with 0.9% NaCl; (3) nondiabetic rats treated with EO (50 mg/kg body weight); and (4) alloxan-induced diabetic rats treated with EO (50 mg/kg body weight). Treatments lasted 15 days. Serum biochemical parameters were determined. EO was extracted by hydrodistillation for 3 h using a Clevenger apparatus. | There was a significant increase in blood glucose levels within alloxan-induced diabetic rat groups; however, treatment with EO significantly reduced this parameter. | [61] |
| Origanum vulgare subsp. vulgare and subsp. hirtum | Enzymatic assay—EO were extracted by hydrodistillation for 5 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were performed. | For α-amylase inhibitory activity, Origanum vulgare subsp. vulgare and subsp. hirtum exhibited similar activity (0.13 and 0.14 mmol ACEs/g oil). The highest α-glucosidase inhibitory activity was achieved by Origanum vulgare subsp. vulgare with 6.04 mmol ACEs/g oil. | [62] |
| Clove bud | Enzymatic assay—EO was extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-amylase and α-glucosidase inhibition colorimetric assays were performed. Experimental concentrations were 0, 40, 80, 120, and 160 µL/L. Acarbose was used as a positive control. | 35–78% inhibition of α-amylase. 58–90% inhibition of α-glucosidase. Clove bud oil EC50 for α-amylase: 88.89 µL/L Clove bud oil EC50 for α-glucosidase: 71.94 µL/L Acarbose EC50 for α-amylase: 18.63 µg/mL Acarbose EC50 for α-amylase: 21.1 µg/mL Acarbose exhibited higher inhibitory activity for both enzymes compared to clove bud EO. | [56] |
| Cinnamomum zeylanicum (CZ), Psiadia arguta (PA), Psiadia terebinthina (PT), Citrus grandis (CGp), Citrus hystrix (CH), and Citrus reticulata (CR) | Enzymatic assay—EO were extracted by hydrodistillation for 3 h using a Clevenger apparatus. α-glucosidase inhibition assay was assessed using the pNPG method. The inhibition type was determined using the Lineweaver-Burk linearization method. | Inhibition % at 500 µg/mL for CH, CR, CGp, CZ, PT, and PA was 85.49, 81.15, 83.19, 93.71, 40.12, and 76.45, respectively. IC50 (µg/mL) values are 276.7, 169.9, 240.6, 64.52, 14,584, and 313, respectively. In the case of inhibition %, all EO exhibited higher activity than acarbose (51.39%). CZ was demonstrated to be the most potent inhibitory activity compared to acarbose. For all EOs, the inhibition type was uncompetitive, except for CZ, which has a competitive inhibition type. | [63] |
| Lemon balm (Melissa officinalis) | In vivo—15-weeks-old male C57BL/KsJ-db/db (db/db) mice were fed with standard chow or chow supplemented with lemon balm EO. Treatments lasted for six weeks. Serum biochemical parameters were monitored. Oral glucose tolerance tests were assessed, and serum insulin was monitored. EO was extracted by steam distillation. | Plasma glucose levels were reduced (up to 64.6%). There was an improvement in glucose tolerance with lemon balm EO administration. Serum insulin was increased. Serum biochemical parameters (total cholesterol, TG and HDL-cholesterol) were reduced. | [52] |
| Phoebe bournei (Hemsl.) Yang | In vitro—3T3-L1 preadipocytes were differentiated with 40 µg/mL of leaf EO. After 24 h, glucose consumption activity was determined by measuring the medium glucose concentration. | Promotion of glucose uptake in adipocytes. | [64] |
| Rosemary | In vivo—15-weeks-old adult male Wistar rats (220–225 g) were divided into four groups: (1) nondiabetic rats treated with distilled water; (2) alloxan-induced diabetic rats treated with distilled water; (3) nondiabetic rats treated with EO; and (4) alloxan-induced diabetic rats treated with EO. Treatments lasted 15 days. Blood glucose level was measured. EO was extracted by hydrodistillation for 3 h using a Clevenger apparatus. | Blood glucose level was higher in alloxan-induced diabetic rats; however, treatments with EO corrected this hyperglycemia. | [65] |
| Rhaponticum acaule (L) DC | Enzymatic assay—EO was extracted by hydrodistillation for 5 h using a Clevenger apparatus. α-glucosidase inhibition assay was assessed using the pNPG method. The inhibition type was determined using the Lineweaver-Burk method. | Rhaponticum acaule EO IC50: 6.7 ± 0.10 μg/mL Acarbose IC50: 280 ± 0.10 μg/mL EO demonstrated high inhibition activity compared to acarbose. Mixed inhibition type. | [66] |
| Salvia officinalis L. | EO was extracted by hydrodistillation for 2 h using a Clevenger apparatus. Enzymatic assay—α-amylase inhibition assay was assessed using the CNPG3 method. Experimental concentrations were 50, 100, and 200 µg/mL. In vivo—Male Wistar rats (180–200 g) were induced into diabetes with alloxan and divided into five groups: (1) nondiabetic rats treated with water (control); (2) nondiabetic rats treated with EO; (3) alloxan-induced diabetic rats treated with water; (4) alloxan-induced diabetic rats treated with Glymepiride; and (5) alloxan-induced diabetic rats treated with EO. Fasting blood glucose, α-amylase, and hepatic glycogen content were measured. Treatments were daily and orally administered. | EO IC50: 38 μg/mL Acarbose IC50: 14.9 μg/mL EO exhibited less inhibition activity than acarbose. EO administration to diabetic rats reduced serum α-amylase activity and fasting blood glucose. Moreover, liver glycogen storage was enhanced by 44%. Langerhans islets were restored to normal size in diabetic rats. | [57] |
3.3. Other Bioactivities of EO Related to Metabolic Syndrome Comorbidities: Neuroprotection
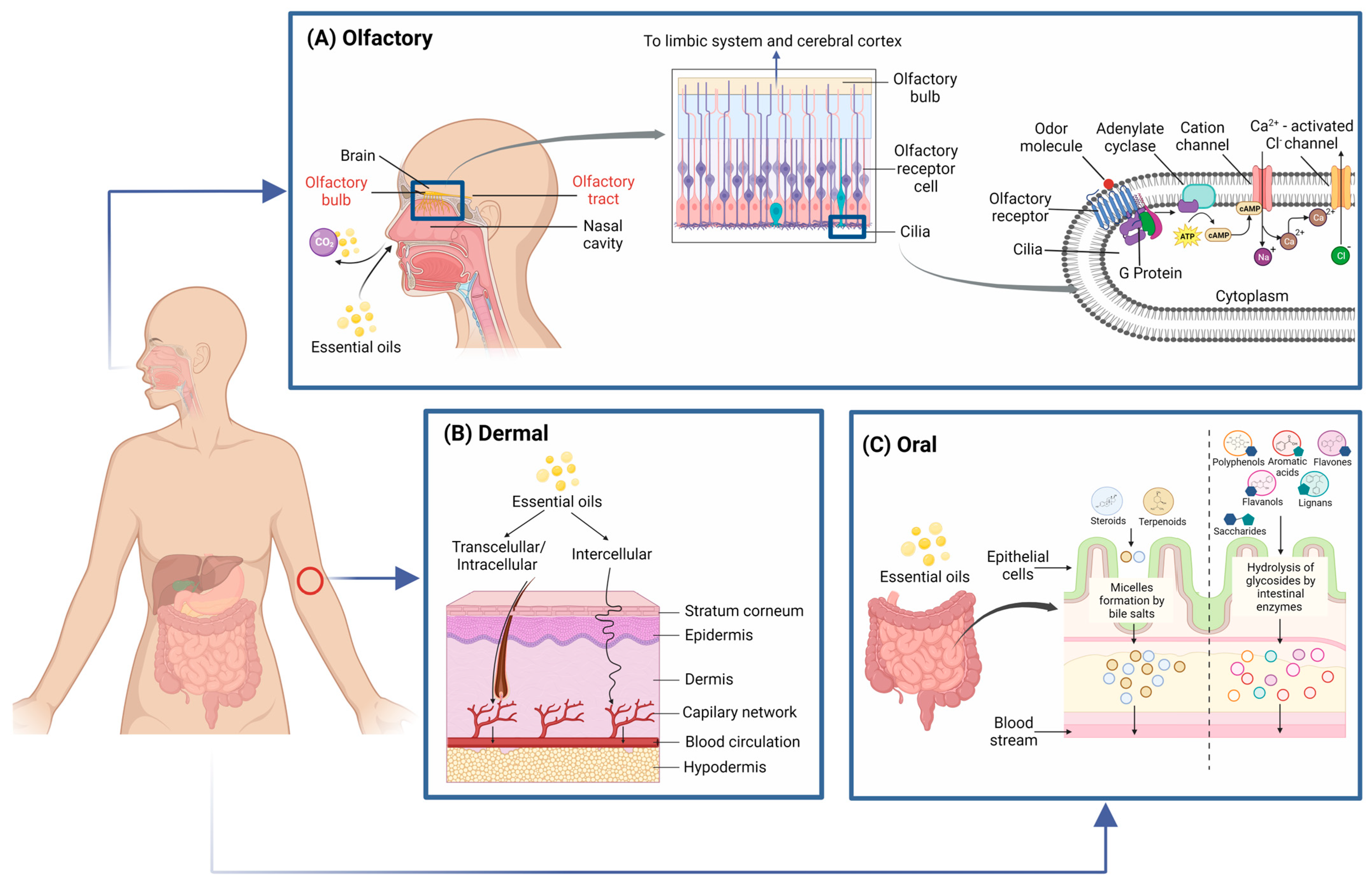
4. Bioavailability and Mechanisms of Action of EO
5. EO as Food Preservatives
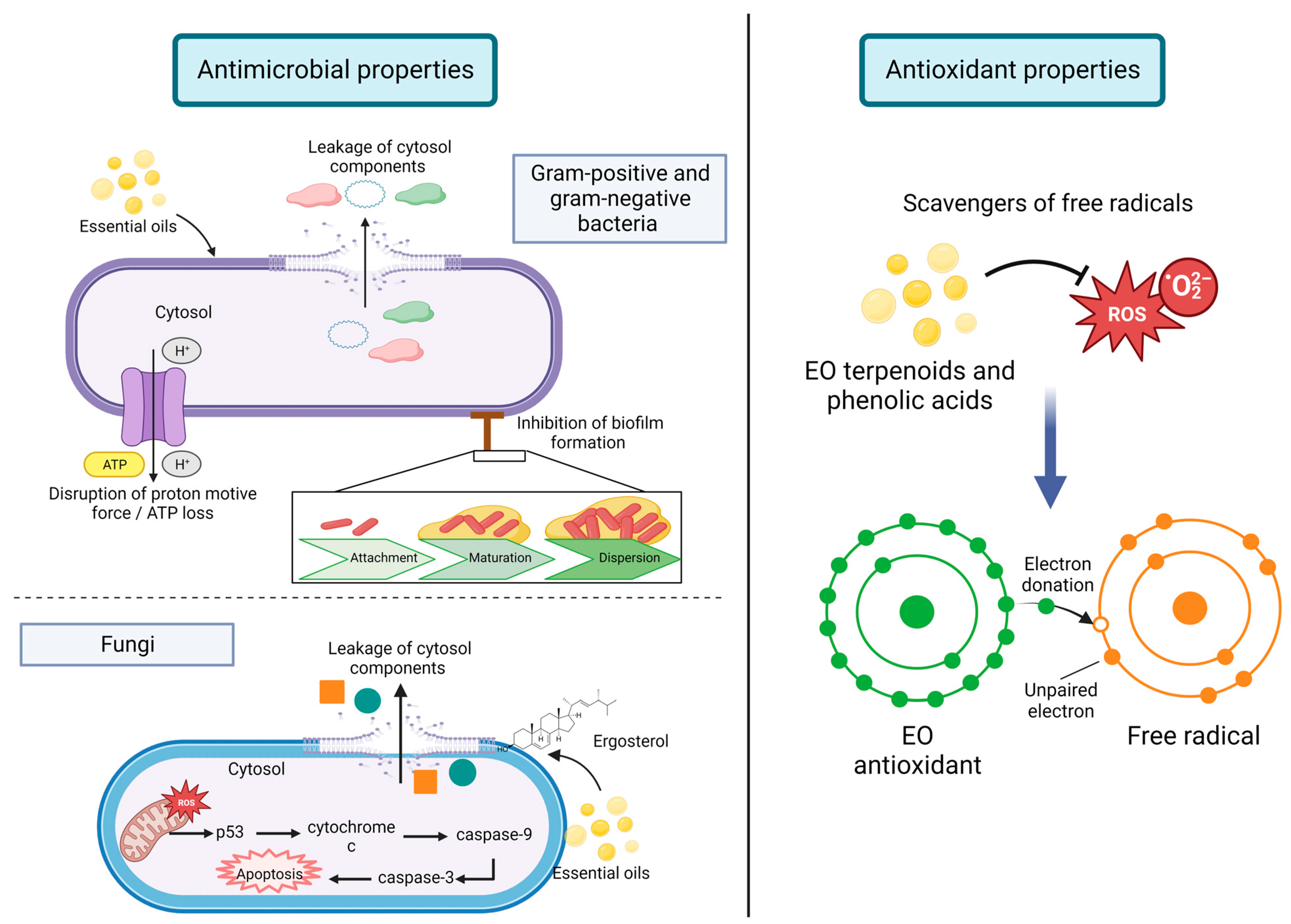
5.1. Antimicrobial Properties
5.2. Antioxidant Capacity
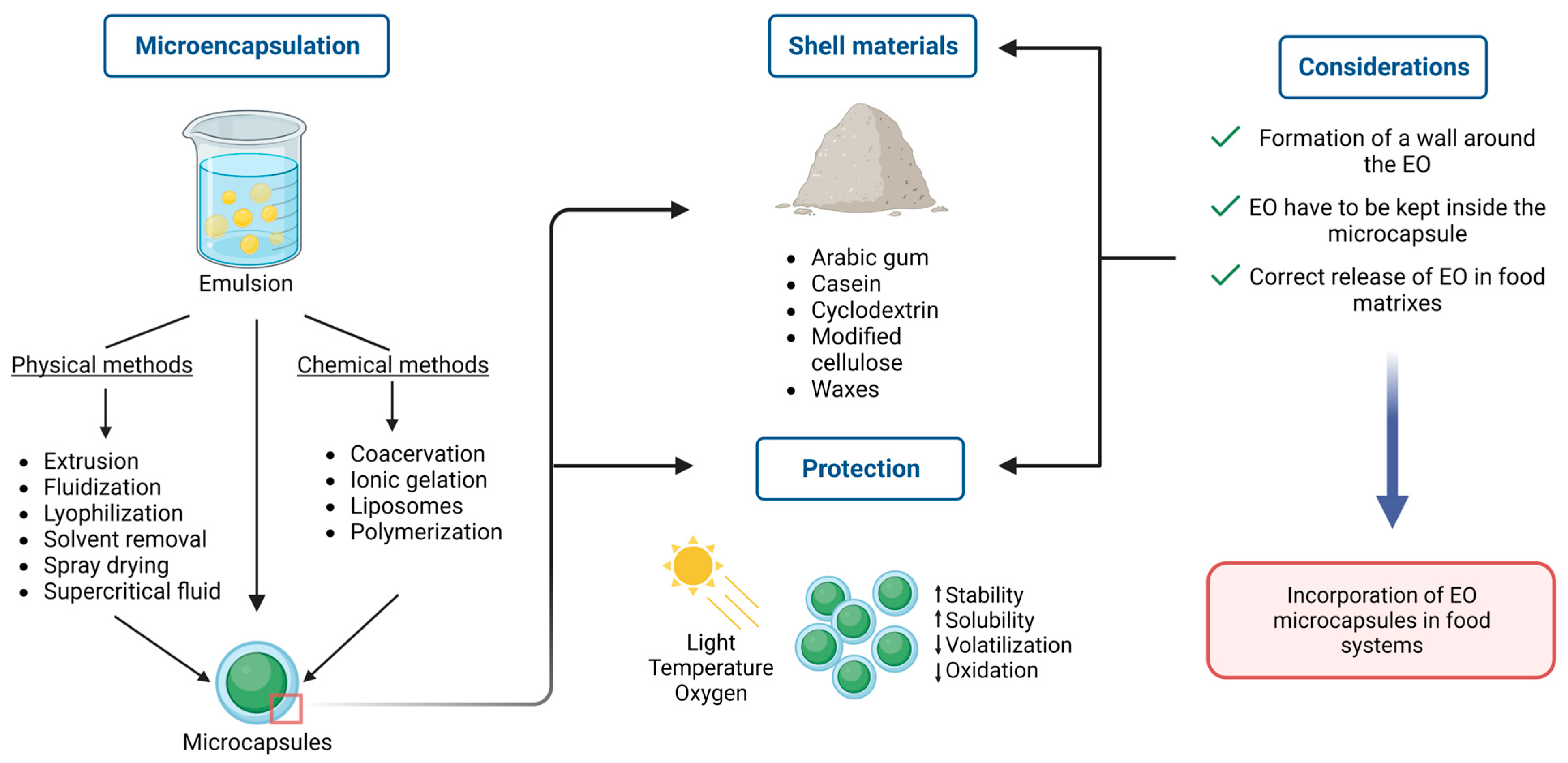
6. Stability and Formulation of EO
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Buttar, H.S.; Dicholkar, P.; Kaur, G.; Chintamaneni, M. Chapter 39-Role of nutraceuticals, functional foods, and spices in the management of metabolic syndrome and related disorders. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 583–601. [Google Scholar] [CrossRef]
- Nijhawan, P.; Behl, T. Nutraceuticals in the management of obesity. Obes. Med. 2020, 17, 100168. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Unusan, N. Essential oils and microbiota: Implications for diet and weight control. Trends Food Sci. Technol. 2020, 104, 60–71. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of Cytotoxicity of Some Essential Oils for Translation in Cancer Therapy. Evid.-Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Awadelkareem, A.M.; Ashraf, S.A. Recent insights into green extraction techniques as efficient methods for the extraction of bioactive components and essential oils from foods. CyTA-J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Pelvan, E.; Karaoğlu, Ö.; Fırat, E.Ö.; Kalyon, K.B.; Ros, E.; Alasalvar, C. Immunomodulatory effects of selected medicinal herbs and their essential oils: A comprehensive review. J. Funct. Foods 2022, 94, 105108. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Fan, M.; Qian, H.; Ying, H.; Li, Y.; Wang, L. Functional ingredients present in whole-grain foods as therapeutic tools to counteract obesity: Effects on brown and white adipose tissues. Trends Food Sci. Technol. 2021, 109, 513–526. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Dmytriv, T.R.; Melnychuk, A.V.; Strilets, N.V.; Storey, K.B.; Lushchak, V.I. Chamomile as a potential remedy for obesity and metabolic syndrome. EXCLI J. 2021, 20, 1261–1286. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, S.; Flati, V.; Toniato, E.; Di Gregorio, J.; Marino, A.; Pierdomenico, L.; Marchisio, M.; D’Orazi, G.; Cacciatore, I.; Robuffo, I. Carvacrol reduces adipogenic differentiation by modulating autophagy and ChREBP expression. PLoS ONE 2018, 13, e0206894. [Google Scholar] [CrossRef]
- Hong, S.J.; Cho, J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.-C. Inhalation of Patchouli (Pogostemon Cablin Benth.) Essential Oil Improved Metabolic Parameters in Obesity-Induced Sprague Dawley Rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Ngamdokmai, N.; Paracha, T.U.; Waranuch, N.; Chootip, K.; Wisuitiprot, W.; Suphrom, N.; Insumrong, K.; Ingkaninan, K. Effects of Essential Oils and Some Constituents from Ingredients of Anti-Cellulite Herbal Compress on 3T3-L1 Adipocytes and Rat Aortae. Pharmaceuticals 2021, 14, 253. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chen, Y.-Y.; Tsai, J.-W.; Wang, S.-C.; Watanabe, T.; Tsai, Y.-C. Inhibitory effect of β-asarone, a component of Acorus calamus essential oil, on inhibition of adipogenesis in 3T3-L1 cells. Food Chem. 2011, 126, 1–7. [Google Scholar] [CrossRef]
- Hwang, D.I.; Won, K.-J.; Kim, D.-Y.; Yoon, S.W.; Park, J.-H.; Kim, B.; Lee, H.M. Anti-adipocyte Differentiation Activity and Chemical Composition of Essential Oil from Artemisia annua. Nat. Prod. Commun. 2016, 11, 539–542. [Google Scholar] [CrossRef]
- Ko, H.-S.; Lee, H.-J.; Sohn, E.J.; Yun, M.; Lee, M.-H.; Kim, S.-H. Essential Oil of Pinus koraiensis Exerts Antiobesic and Hypolipidemic Activity via Inhibition of Peroxisome Proliferator-Activated Receptors Gamma Signaling. Evid.-Based Complement. Altern. Med. 2013, 2013, 947037. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Sheen, L.-Y.; Chang, S.-T. Hypolipidemic effects of S -(+)-linalool and essential oil from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2018, 8, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, S.; Woldemariam, T.; Kotchoni, S.; Elshabrawy, H.A.; Chaturvedi, L.S. Lemongrass essential oil and its major constituent citral isomers modulate adipogenic gene expression in 3T3-L1 cells. J. Food Biochem. 2022, 46, e14037. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-S.; Chen, W.-C.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Tseng, H.-C.; Lin, S.-Y.; Sheen, L.-Y. Garlic Essential Oil Protects against Obesity-Triggered Nonalcoholic Fatty Liver Disease through Modulation of Lipid Metabolism and Oxidative Stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Essential Oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef]
- Ciftci, M.; Simsek, U.G.; Dalkilic, B.; Erisir, Z.; Mutlu, S.I.; Azman, M.A.; Ozcelik, M.; Yilmaz, O.; Tonbak, F. Effects of Essential Oil Mixture Supplementation to Basal Diet on Fattening Performance, Blood Parameters and Antioxidant Status of Tissues in Japanese Quails Exposed to Low Ambient Temperature. J. Anim. Plant Sci. 2018, 28, 421–430. [Google Scholar]
- Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Effect of the cumin cyminum L. Intake on Weight Loss, Metabolic Profiles and Biomarkers of Oxidative Stress in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann. Nutr. Metab. 2015, 66, 117–124. [Google Scholar] [CrossRef]
- Li, D.; Wu, H.; Dou, H. Weight loss effect of sweet orange essential oil microcapsules on obese SD rats induced by high-fat diet. Biosci. Biotechnol. Biochem. 2019, 83, 923–932. [Google Scholar] [CrossRef]
- Batubara, I.; Suparto, I.H.; Sa’Diah, S.; Matsuoka, R.; Mitsunaga, T. Effects of Inhaled Citronella Oil and Related Compounds on Rat Body Weight and Brown Adipose Tissue Sympathetic Nerve. Nutrients 2015, 7, 1859–1870. [Google Scholar] [CrossRef]
- Shen, J.; Niijima, A.; Tanida, M.; Horii, Y.; Maeda, K.; Nagai, K. Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005, 380, 289–294. [Google Scholar] [CrossRef]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Blaslov, K.; Naranđa, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Aisa, H.A.; Gao, Y.; Yili, A.; Ma, Q.; Cheng, Z. Beneficial Role of Chickpea (Cicer arietinum L.) Functional Factors in the Intervention of Metabolic Syndrome and Diabetes Mellitus. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 615–627. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J. Biomed. Res. 2019, 33, 1–16. [Google Scholar] [CrossRef]
- Beręsewicz, A. NADPH oxidases, nuclear factor kappa B, NF-E2-related factor2, and oxidative stress in diabetes. In Diabetes, Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R., Ed.; Elsevier, Academic Press: Cambridge, MA, USA, 2020; pp. 129–137. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.-L.; Shen, L.-H.; Feng, L.-J.; Zhou, Q. Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) against α-amylase and α-glucosidase. Food Chem. 2021, 359, 129934. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic Potential of Monoterpenes: A Case of Small Molecules Punching above Their Weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef]
- Hanefeld, M.; Mertes, G. Treatment: Alpha Glucosidase Inhibitors. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; pp. 238–244. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Sun, W.; Wang, Y.; Liu, S.; Kang, L. Metformin combined with acarbose vs. single medicine in the treatment of type 2 diabetes: A meta-analysis. Exp. Ther. Med. 2017, 13, 3137–3145. [Google Scholar] [CrossRef] [PubMed]
- Al-Asri, J.; Fazekas, E.; Lehoczki, G.; Perdih, A.; Görick, C.; Melzig, M.F.; Gyémánt, G.; Wolber, G.; Mortier, J. From carbohydrates to drug-like fragments: Rational development of novel α-amylase inhibitors. Bioorg. Med. Chem. 2015, 23, 6725–6732. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 989. [Google Scholar] [CrossRef]
- Radünz, M.; Camargo, T.M.; dos Santos Hackbart, H.C.; Alves, P.I.C.; Radünz, A.L.; Gandra, E.A.; Zavareze, E.D.R. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Rahali, N.; Mehdi, S.; Younsi, F.; Boussaid, M.; Messaoud, C. Antioxidant, α-amylase, and acetylcholinesterase inhibitory activities of Hertia cheirifolia essential oils: Influence of plant organs and seasonal variation. Int. J. Food Prop. 2017, 20, 1637–1651. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Cho, S.-Y.; Bhuiyan, M.J.H.; Kim, K.H.; Lee, S.-J. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind. Crop. Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G.; Shakeri, R. In vitro antioxidant and antidiabetic activity of essential oils encapsulated in gelatin-pectin particles against sugar, lipid and protein oxidation and amylase and glucosidase activity. Food Sci. Nutr. 2020, 8, 6457–6466. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Inhibition of enzymes linked to type-2 diabetes and hypertension by essential oils from peels of orange and lemon. Int. J. Food Prop. 2017, 20, S586–S594. [Google Scholar] [CrossRef]
- Oboh, G.; Akinbola, I.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential Oil from Clove Bud (Eugenia aromatica Kuntze) Inhibit Key Enzymes Relevant to the Management of Type-2 Diabetes and Some Pro-oxidant Induced Lipid Peroxidation in Rats Pancreas in vitro. J. Oleo Sci. 2015, 64, 775–782. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hammami, M.; Ben Hadj, A.; Boudawara, T.; Dammak, M.; Zouari, S.; El Feki, A. Metabolic impairments and tissue disorders in alloxan-induced diabetic rats are alleviated by Salvia officinalis L. essential oil. Biomed. Pharmacother. 2018, 108, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Zengin, G.; Bahadori, M.B.; Abbas-Mohammadi, M.; Dinparast, L. Amylase, glucosidase, tyrosinase, and cholinesterases inhibitory, antioxidant effects, and GC-MS analysis of wild mint (Mentha longifolia var. calliantha) essential oil: A natural remedy. Eur. J. Integr. Med. 2018, 22, 44–49. [Google Scholar] [CrossRef]
- Loizzo, M.; Saab, A.; Statti, G.; Menichini, F. Composition and α-amylase inhibitory effect of essential oils from Cedrus libani. Fitoterapia 2007, 78, 323–326. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Odubanjo, O.V.; Akinbola, I.A. Antioxidative Properties and Inhibition of Key Enzymes Relevant to Type-2 Diabetes and Hypertension by Essential Oils from Black Pepper. Adv. Pharmacol. Sci. 2013, 2013, 926047. [Google Scholar] [CrossRef]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; van Staden, A.B.; Hosenally, M.; Mahomoodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef]
- Ding, W.; Liping, N.; Xing, H.; Wei, Z.; Zhoua, Q.; Nong, R.; Chen, J. Essential oil extracted from leaf of Phoebe bournei (Hemsl.) yang: Chemical constituents, antitumor, antibacterial, hypoglycemic activities. Nat. Prod. Res. 2020, 34, 2524–2527. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Rosemary (Rosmarinus officinalis) essential oil components exhibit anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects in experimental diabetes. Pathophysiology 2017, 24, 297–303. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Kammoun, J.; Hlila, M.B.; Louati, H.; Hammami, S.; Flamini, G.; Achour, L.; Selmi, B. Rhaponticum acaule (L) DC essential oil: Chemical composition, in vitro antioxidant and enzyme inhibition properties. BMC Complement. Altern. Med. 2018, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res.-Mod. Chin. Med. 2022, 3, 100075. [Google Scholar] [CrossRef]
- García-Ayllón, M.-S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Nutrition, Well-Being and Health; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, C.; van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef]
- Sherwood, L. Human Physiology: From Cells to Systems; Cengage Learning: Belmont, CA, USA, 2012. [Google Scholar]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Cal, K. Skin Penetration of Terpenes from Essential Oils and Topical Vehicles. Planta Med. 2006, 72, 311–316. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef]
- Varman, R.M.; Singh, S. Investigation of Effects of Terpene Skin Penetration Enhancers on Stability and Biological Activity of Lysozyme. AAPS PharmSciTech 2012, 13, 1084–1090. [Google Scholar] [CrossRef]
- Sendra, E. Essential Oils in Foods: From Ancient Times to the 21st Century. Foods 2016, 5, 43. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Li, Y.-X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Shellie, R.; Keast, R.; Palombo, E.; Jadhav, S. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Stoleru, E.; Brebu, M. Stabilization Techniques of Essential Oils by Incorporation into Biodegradable Polymeric Materials for Food Packaging. Molecules 2021, 26, 6307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Chen, X.-W.; Yang, X.-Q. Characterization of Orange Oil Powders and Oleogels Fabricated from Emulsion Templates Stabilized Solely by a Natural Triterpene Saponin. J. Agric. Food Chem. 2019, 67, 2637–2646. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef]
- Doost, A.S.; Van Camp, J.; Dewettinck, K.; Van der Meeren, P. Production of thymol nanoemulsions stabilized using Quillaja Saponin as a biosurfactant: Antioxidant activity enhancement. Food Chem. 2019, 293, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Delgado, E.L.; Jacobo-Velázquez, D.A. Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome. Foods 2023, 12, 1079. https://doi.org/10.3390/foods12051079
Chávez-Delgado EL, Jacobo-Velázquez DA. Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome. Foods. 2023; 12(5):1079. https://doi.org/10.3390/foods12051079
Chicago/Turabian StyleChávez-Delgado, Emily L., and Daniel A. Jacobo-Velázquez. 2023. "Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome" Foods 12, no. 5: 1079. https://doi.org/10.3390/foods12051079
APA StyleChávez-Delgado, E. L., & Jacobo-Velázquez, D. A. (2023). Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome. Foods, 12(5), 1079. https://doi.org/10.3390/foods12051079







