Modulation of Postprandial Plasma Concentrations of Digestive Hormones and Gut Microbiota by Foods Containing Oat ß-Glucans in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Food Products
2.3. Short-Term Study
2.3.1. Experimental Design
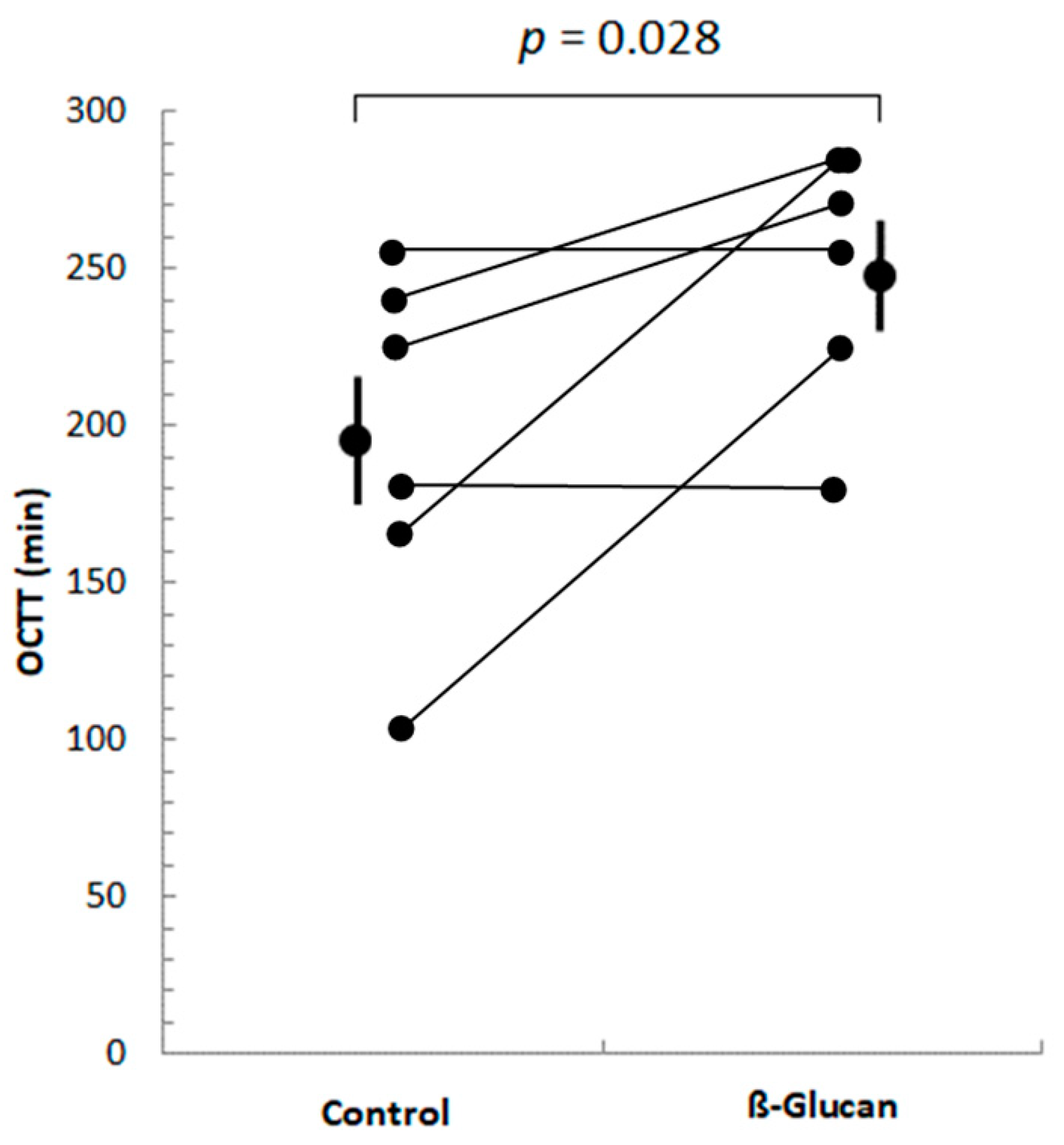
2.3.2. Orocecal Transit Time Determination
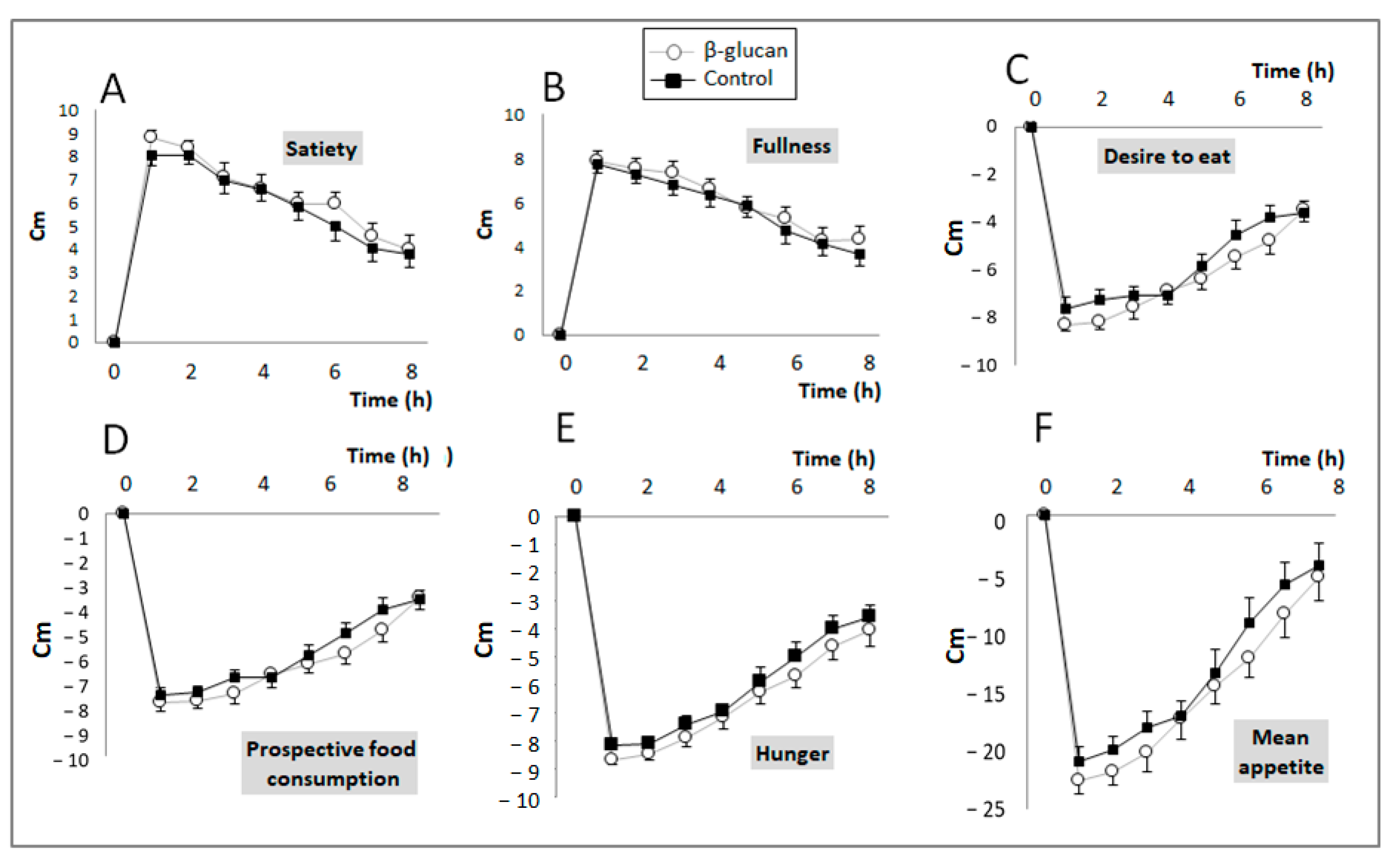
2.3.3. Satiety Index
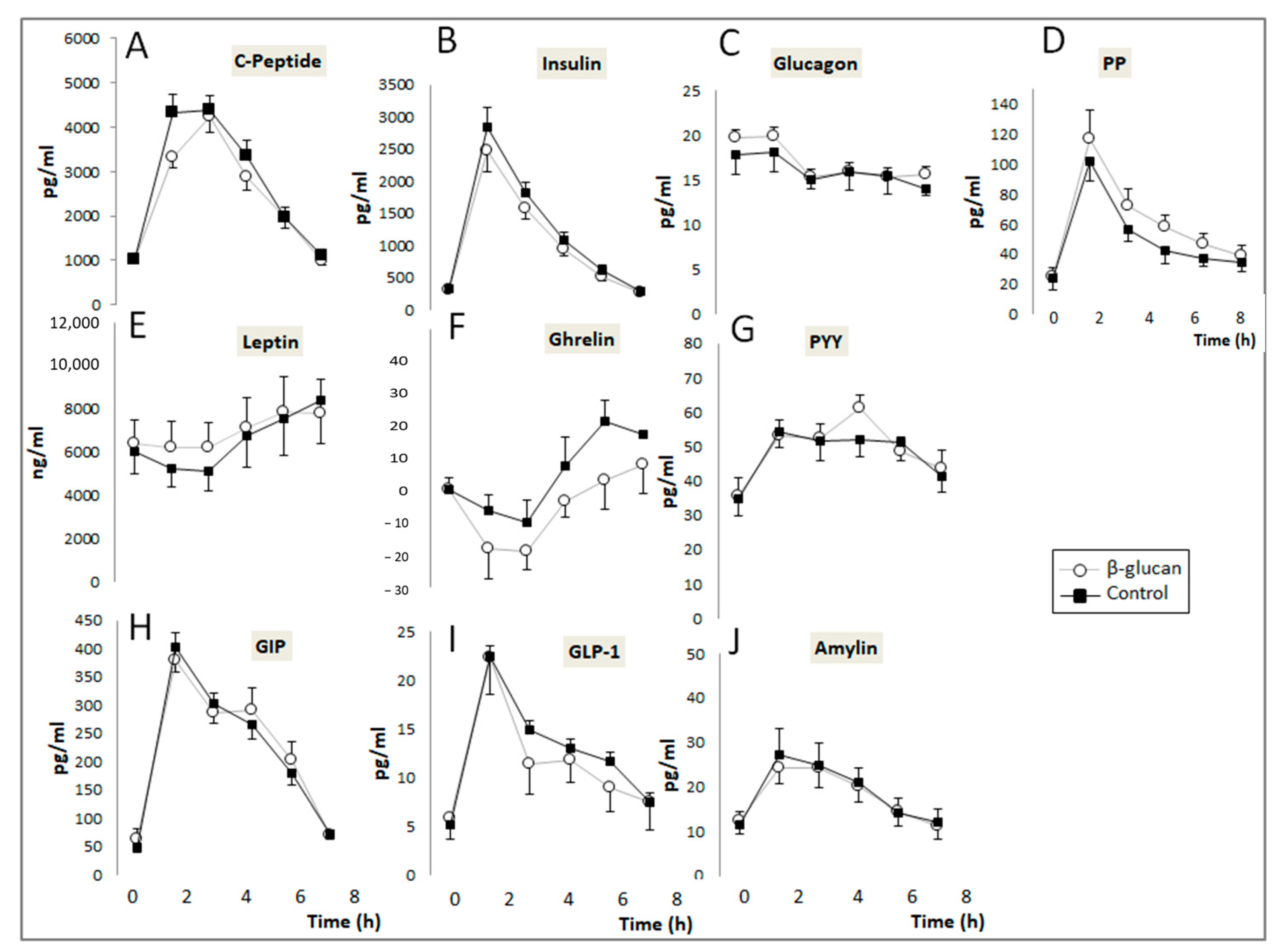
2.3.4. Plasma Hormones Determination
2.4. Long-Term Study
2.4.1. Experimental Design
2.4.2. Digestive Symptoms
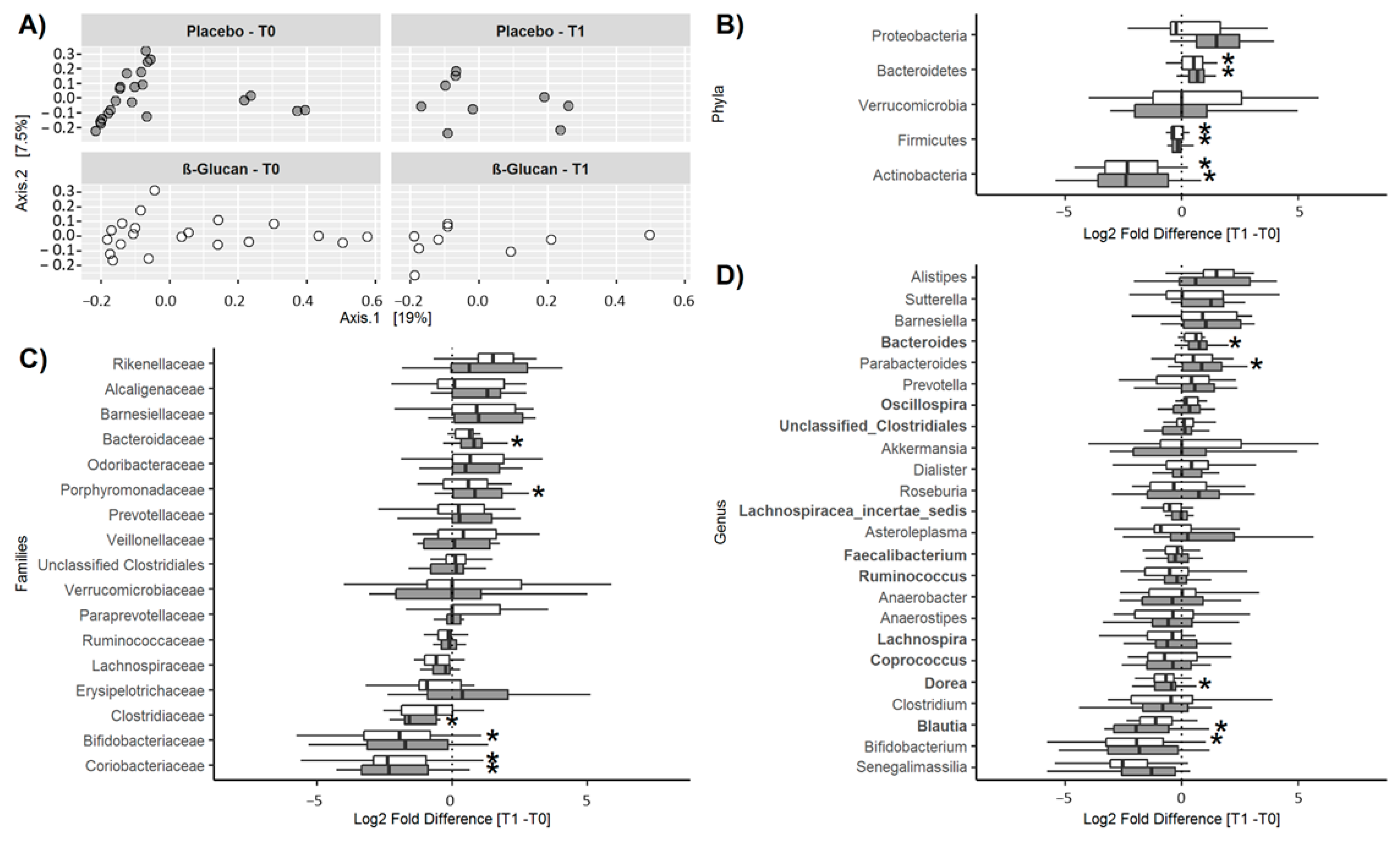
2.4.3. Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Short-Term Study
3.2. Long-Term Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of health-beneficial properties of oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Wolever, T.M.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Summo, C.; De Angelis, D.; Difonzo, G.; Caponio, F.; Pasqualone, A. Effectiveness of oat-hull-based ingredient as fat replacer to produce low fat burger with high beta-glucans content. Foods 2020, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahlany, S.; Al-Kaabi, W.; Al-Manhel, A.; Niamah, A.; Altemimi, A.; Al-wafi, H.; Cacciola, F. Effects of β-glucan extracted from Saccharomyces cerevisiae on the quality of bio-yoghurts: In vitro and in vivo evaluation. J. Food Meas. Charact. 2022, 16, 3607–3617. [Google Scholar] [CrossRef]
- Chiozzi, V.; Eliopoulos, C.; Markou, G.; Arapoglou, D.; Agriopoulou, S.; El Enshasy, H.A.; Varzakas, T. Biotechnological Addition of β-Glucans from Cereals, Mushrooms and Yeasts in Foods and Animal Feed. Processes 2021, 9, 1889. [Google Scholar] [CrossRef]
- Thondre, P.S.; Shafat, A.; Clegg, M.E. Molecular weight of barley β-glucan influences energy expenditure, gastric emptying and glycaemic response in human subjects. Br. J. Nutr. 2013, 110, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, L.; Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: An ileostomy study. Eur. J. Clin. Nutr. 2007, 61, 938–945. [Google Scholar] [CrossRef]
- Abumweis, S.S.; Jew, S.; Ames, N.P. Oat β-glucan from barley and its lipid-lowering capacity: A meta-analysis of randomized, controlled trials. Eur. J. Clin. Nutr. 2010, 64, 1472–1480. [Google Scholar] [CrossRef]
- Biörklund, M.; Holm, J.; Önning, G. Serum lipids and postprandial glucose and insulin levels in hyperlipidemic subjects after consumption of an oat β-Glucan-containing ready meal. Ann. Nutr. Metab. 2008, 52, 83–90. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2011, 9, 2207. [Google Scholar]
- Beck, E.J.; Tosh, S.M.; Batterham, M.J.; Tapsell, L.C.; Huang, X.F. Oat β-glucan increases postprandial cholecystokinin levels, decreases insulin response and extends subjective satiety in overweight subjects. Mol. Nutr. Food Res. 2009, 53, 1343–1351. [Google Scholar] [CrossRef]
- Juvonen, K.R.; Purhonen, A.K.; Salmenkallio-Marttila, M.; Lähteenmäki, L.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef]
- Juvonen, K.R.; Salmenkallio-Marttila, M.; Lyly, M.; Liukkonen, K.H.; Lähteenmäki, L.; Laaksonen, D.E.; Uusitupa, M.I.; Herzig, K.H.; Poutanen, K.S.; Karhunen, L.J. Semisolid meal enriched in oat bran decreases plasma glucose and insulin levels, but does not change gastrointestinal peptide responses or short-term appetite in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 748–756. [Google Scholar] [CrossRef]
- Weickert, M.O.; Spranger, J.; Holst, J.J.; Otto, B.; Koebnick, C.; Möhlig, M.; Pfeiffer, A.F. Wheat-fibre- induced changes of postprandial peptide YY and ghrelin responses are not associated with acute alterations of satiety. Br. J. Nutr. 2006, 96, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.L.; Keung Cheung, P.K. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Aguilera, G.; Cárcamo, C.; Soto-Alarcón, S.; Gotteland, M. Improvement in lactose tolerance in hypolactasic subjects consuming ice creams with high or low concentrations of Bifidobacterium bifidum 900791. Foods 2021, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Anderson, G.H.; Catherine, N.L.; Woodend, D.M.; Wolever, T.M. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am. J. Clin. Nutr. 2002, 76, 1023–1030. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Skou Hedemann, M.; Nygaard Lærke, H.; Bach Knudsen, K.E. Obesity development and signs of metabolic abnormalities in young Göttingen minipigs consuming energy dense diets varying in carbohydrate quality. Nutrients 2021, 13, 1560. [Google Scholar] [CrossRef]
- Gälman, C.; Miquel, J.F.; Pérez, R.M.; Einarsson, C.; Ståhle, L.; Marshall, G.; Nervi, F.; Rudling, M. Bile acid synthesis is increased in Chilean Hispanics with gallstones and in gallstone high-risk Mapuche Indians. Gastroenterology 2004, 126, 741–748. [Google Scholar] [CrossRef]
- Morales, P.; Fujio, S.; Navarrete, P.; Ugalde, J.A.; Magne, F.; Carrasco-Pozo, C.; Tralma, K.; Quezada, M.; Hurtado, C.; Covarrubias, N.; et al. Impact of dietary lipids on colonic function and microbiota: An experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin. Transl. Gastroenterol. 2016, 7, e161. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, J.; Chen, D.G. Statistical Analysis of Microbiome Data with R; ICSA Book Series in Statistics; Springer: Singapore, 2018. [Google Scholar]
- Rebello, C.J.; Johnson, W.D.; Martin, C.K.; Xie, W.; O’Shea, M.; Kurilich, A.; Bordenave, N.; Andler, S.; van Klinken, B.J.W.; Chu, Y.-F.; et al. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: A randomized crossover trial. J. Am. Coll. Nutr. 2013, 32, 272–279. [Google Scholar] [CrossRef]
- Aoe, S.; Ikenaga, T.; Noguchi, H.; Kohashi, C.; Kakumoto, K.; Kohda, N. Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: A randomized controlled trial. Plant Foods Hum. Nutr. 2014, 69, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Pentikäinen, S.; Karhunen, L.; Flander, L.; Katina, K.; Meynier, A.; Aymard, P.; Vinoy, S.; Poutanen, K. Enrichment of biscuits and juice with oat β-glucan enhances postprandial satiety. Appetite 2014, 75, 150–156. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Stergiou, A.; Karavasilis, K.; Konstantopoulos, P.; Perrea, D.; Tentolouris, N.; Karathanos, V.T. Enrichment of bread with beta-glucans or resistant starch induces similar glucose, insulin and appetite hormone responses in healthy adults. Eur. J. Nutr. 2021, 60, 455–464. [Google Scholar] [CrossRef]
- Peters, H.P.; Boers, H.M.; Haddeman, E.; Melnikov, S.M.; Qvyjt, F. No effect of added beta-glucan or of fructooligosaccharide on appetite or energy intake. Am. J. Clin. Nutr. 2009, 89, 58–63. [Google Scholar] [CrossRef]
- Ames, N.; Blewett, H.; Storsley, J.; Thandapilly, S.J.; Zahradka, P.; Taylor, C. A double-blind randomised controlled trial testing the effect of a barley product containing varying amounts and types of fibre on the postprandial glucose response of healthy volunteers. Br. J. Nutr. 2015, 113, 1373–1383. [Google Scholar] [CrossRef]
- Almirall, M.; Francesch, M.; Perez-Vendrell, A.M.; Brufau, J.; Esteve-Garcia, E. The differences in intestinal viscosity produced by barley and beta-glucanase alter digesta enzyme activities and ileal nutrient digestibilities more in broiler chicks than in cocks. J. Nutr. 1995, 125, 947–955. [Google Scholar]
- Abbasi, N.N.; Purslow, P.P.; Tosh, S.M.; Bakovic, M. Oat β-glucan depresses SGLT1- and GLUT2-mediated glucose transport in intestinal epithelial cells (IEC-6). Nutr. Res. 2016, 36, 541–552. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Rubenstein, A.H. C-peptide as a measure of the secretion and hepatic extraction of insulin: Pitfalls and limitations. Diabetes 1984, 33, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Baisley, S.K.; Baldo, B.A. Amylin receptor signaling in the nucleus accumbens negatively modulates μ-opioid-driven feeding. Neuropsychopharmacology 2014, 39, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef]
- Barone Lumaga, R.; Azzali, D.; Fogliano, V.; Scalfi, L.; Vitaglione, P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Vitaglione, P.; Lumaga, R.B.; Stanzione, A.; Scalfi, L.; Fogliano, V. Beta-glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite 2009, 53, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, K.S.; Niskanen, L.K.; Liukkonen, K.H.; Poutanen, K.S.; Holst, J.J.; Mykkänen, H.M. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am. J. Clin. Nutr. 2002, 75, 254–262. [Google Scholar] [CrossRef]
- Beck, E.J.; Tapsell, L.C.; Batterham, M.J.; Tosh, S.M.; Huang, X.F. Increases in peptide Y-Y levels following oat beta-glucan ingestion are dose-dependent in overweight adults. Nutr. Res. 2009, 29, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, M.L.; Gregersen, S.; Lærke, H.N.; Holst, J.J.; Bach Knudsen, K.E.; Hermansen, K. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2014, 68, 84–90. [Google Scholar] [CrossRef]
- Beck, E.J.; Tapsell, L.C.; Batterham, M.J.; Tosh, S.M.; Huang, X.F. Oat beta-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br. J. Nutr. 2010, 103, 1212–1222. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Berr, F.; Beuers, U.; Fischer, S.; Paumgartner, G. Serum concentrations of 7alpha-hydroxy-4-cholesten-3-one reflect bile acid synthesis in humans. Hepatology 1996, 24, 123–126. [Google Scholar] [CrossRef]
- Andersson, M.; Ellegård, L.; Andersson, H. Oat bran stimulates bile acid synthesis within 8 h as measured by 7alpha-hydroxy-4-cholesten-3-one. Am. J. Clin. Nutr. 2002, 76, 1111–1116. [Google Scholar] [CrossRef]
- Zhao, J.; Cheung, P.C. Fermentation of β-glucans derived from different sources by bifidobacteria: Evaluation of their bifidogenic effect. J. Agric. Food Chem. 2011, 59, 5986–5992. [Google Scholar] [CrossRef]
- Shen, R.L.; Dang, X.Y.; Dong, J.L.; Hu, X.Z. Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. J. Agric. Food Chem. 2012, 60, 11301–11308. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived β-glucans by human fecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef]
- Turunen, K.; Tsouvelakidou, E.; Nomikos, T.; Mountzouris, K.C.; Karamanolis, D.; Triantafillidis, J.; Kyriacou, A. Impact of beta-glucan on the faecal microbiota of polypectomized patients: A pilot study. Anaerobe 2011, 17, 4003–4006. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Panopoulou, K.; Turunen, K.; Spiliotis, V.; Kyriacou, K. Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical study. Food Res. Int. 2010, 43, 1086–1092. [Google Scholar] [CrossRef]
- Nilsson, U.; Johansson, M.; Nilsson, A.; Björck, I.; Nyman, M. Dietary supplementation with beta-glucan enriched oat bran increases faecal concentration of carboxylic acids in healthy subjects. Eur. J. Clin. Nutr. 2008, 62, 978–984. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
| Control | ß-Glucan | |
|---|---|---|
| Energy (kcal) | 393 | 396 |
| Proteins (g) | 9.5 | 11.6 |
| Fat (g) | 8.6 | 11.2 |
| Available Carbohydrates (g) | 69.5 | 62.1 |
| Sugar (g) | 12.7 | 11 |
| Oat β-glucan (g) | 0 | 5.2 |
| AUC (cm·min) | Control (n = 15) | β-Glucan (n = 15) | p Value (Paired t-Test) |
|---|---|---|---|
| Satiety | 47.1 ± 13.6 | 49.2 ± 12.9 | 0.19 |
| Fullness | 44.8 ± 12.6 | 46.8 ± 13.2 | 0.19 |
| Desire to eat | −46.1 ± 12.4 | −49.6 ± 11.0 | 0.05 |
| Prospective food consumption | −45.0 ± 8.7 | −48.3 ± 10.9 | 0.033 |
| Hunger | −47.2 ± 9.0 | −50.7 ± 9.1 | 0.04 |
| Mean appetite score | −107 ± 39 | −121 ± 39 | 0.014 |
| Post-Prandial AUC of Plasma Digestive Hormones (pg·h/mL) | Control (n = 14) | β-Glucan (n = 14) | p Value (Wilcoxon Paired Test) |
|---|---|---|---|
| Ghrelin | 27 (−30–58) | −13.6 (−91–18) | 0.030 |
| Leptin | 27,502 (13,502–38,172) | 24,206 (16,115–47,404) | 0.68 |
| GIP | 1204 (952–1513) | 1329 (1161–1504) | 0.035 |
| GLP-1 | 60.9 (34.5–84.9) | 53.1 (32.3–80.3) | 0.47 |
| PYY | 231 (186–280) | 240 (204–288) | 0.64 |
| PP | 237 (179–342) | 282 (181–473) | 0.018 |
| Glucagon | 77.3 (62.4–89.1) | 81.4 (65.9–98.2) | 0.30 |
| Insulin | 6155 (4389–7935) | 4999 (3563–8407) | 0.064 |
| C-Peptide | 16,277 (13,373–17,707) | 14,491 (11,037–17,817) | 0.001 |
| Amylin | 79.7 (46.3–107.6) | 74.1 (58.9–109.2) | 0.40 |
| Glycaemia | 367 (341–425) | 332 (315–358) | 0.0063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotteland, M.; Zazueta, A.; Pino, J.L.; Fresard, A.; Sambra, V.; Codoceo, J.; Cires, M.J.; López, X.; Vivanco, J.P.; Magne, F. Modulation of Postprandial Plasma Concentrations of Digestive Hormones and Gut Microbiota by Foods Containing Oat ß-Glucans in Healthy Volunteers. Foods 2023, 12, 700. https://doi.org/10.3390/foods12040700
Gotteland M, Zazueta A, Pino JL, Fresard A, Sambra V, Codoceo J, Cires MJ, López X, Vivanco JP, Magne F. Modulation of Postprandial Plasma Concentrations of Digestive Hormones and Gut Microbiota by Foods Containing Oat ß-Glucans in Healthy Volunteers. Foods. 2023; 12(4):700. https://doi.org/10.3390/foods12040700
Chicago/Turabian StyleGotteland, Martin, Alejandra Zazueta, José Luis Pino, Andrea Fresard, Verónica Sambra, Juana Codoceo, María José Cires, Ximena López, Juan Pablo Vivanco, and Fabien Magne. 2023. "Modulation of Postprandial Plasma Concentrations of Digestive Hormones and Gut Microbiota by Foods Containing Oat ß-Glucans in Healthy Volunteers" Foods 12, no. 4: 700. https://doi.org/10.3390/foods12040700
APA StyleGotteland, M., Zazueta, A., Pino, J. L., Fresard, A., Sambra, V., Codoceo, J., Cires, M. J., López, X., Vivanco, J. P., & Magne, F. (2023). Modulation of Postprandial Plasma Concentrations of Digestive Hormones and Gut Microbiota by Foods Containing Oat ß-Glucans in Healthy Volunteers. Foods, 12(4), 700. https://doi.org/10.3390/foods12040700





