Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction Pilot Plant
- Defoliator and washing machine (Mori TEM, Tavarnelle Val di Pesa, Italy);
- Hammer crusher mod. TEM 200 (Mori TEM, Tavarnelle Val di Pesa, Italy) equipped with a flow meter with a sensitivity of ±0.05 L/min only usable for oxygen gas (TEKNOM a/m 145, Figline e Incisa Valdarno, Italy) for oxygen measurement and addition in crushing;
- TCM ViscoLineTM XS, a viscous paste heat exchanger for rapid thermoconditioning of olive paste (Alfa Laval Corporate AB, Monza, Italy);
- Malaxer module, 200 kg (Mori TEM, Tavarnelle Val di Pesa, Italy);
- Decanter oliomio (Mori TEM, Tavarnelle Val di Pesa, Italy) at 300 kg/h;
- Vertical centrifuge separator Alfa Laval UVPX 305 AGT 14 (Alfa Laval S.p.A., Tavarnelle Val di Pesa, Italy).
2.2. Analysis of Legal Quality Parameters
2.3. Analysis of Phenolic Compounds
2.4. Analysis of Volatile Compounds
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Legal Quality Parameters
3.2. Phenolic Compounds
3.3. Volatile Compounds
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caracciolo, F.; Cavallo, C.; del Giudice, T.; Panico, T.; Vecchio, R.; Cicia, G. Consumers (dis)preference for bitterness in extra virgin olive oil: A field experiment. Int. J. Food Syst. Dyn. 2020, 11, 14–25. [Google Scholar] [CrossRef]
- Cavallo, C.; Caracciolo, F.; Cicia, G.; Del Giudice, T. Extra-virgin olive oil: Are consumers provided with the sensory quality they want? A hedonic price model with sensory attributes. J. Sci. Food Agric. 2018, 98, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Guinard, J.-X. How do consumer hedonic ratings for extra virgin olive oil relate to quality ratings by experts and descriptive analysis ratings? Food Qual. Prefer. 2011, 22, 213–225. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Panico, T.; del Giudice, T.; Caracciolo, F. Quality dimensions and consumer preferences: A choice experiment in the italian extra-virgin olive oil market. Agric. Econ. Rev. 2014, 15, 100–112. [Google Scholar]
- Roselli, L.; Cicia, G.; Cavallo, C.; Del Giudice, T.; Carlucci, D.; Clodoveo, M.L.; De Gennaro, B.C. Consumers’ willingness to buy innovative traditional food products: The case of extra-virgin olive oil extracted by ultrasound. Food Res. Int. 2018, 108, 482–490. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar influence on the volatile components of olive oil formed in the lipoxygenase pathway. LWT 2021, 147, 111485. [Google Scholar] [CrossRef]
- López-Yerena, A.; Ninot, A.; Jiménez-Ruiz, N.; Lozano-Castellón, J.; Pérez, M.; Escribano-Ferrer, E.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Influence of the Ripening Stage and Extraction Conditions on the Phenolic Fingerprint of ‘Corbella’ Extra-Virgin Olive Oil. Antioxidants 2021, 10, 877. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T. Characterization of Olive Ripeness by Green Aroma Compounds of Virgin Olive Oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Baccouri, O.; Guerfel, M.; Baccouri, B.; Cerretani, L.; Bendini, A.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008, 109, 743–754. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Rharrabti, Y. Influence of ripening index and water regime on the yield and quality of “Moroccan Picholine” virgin olive oil. OCL Oilseeds Fats Crops Lipids 2020, 27, 19. [Google Scholar] [CrossRef]
- Polari, J.J.; Mori, M.; Wang, S.C. Virgin Olive Oils from Super-High-Density Orchards in California: Impact of Cultivar, Harvest Time, and Crop Season on Quality and Chemical Composition. Eur. J. Lipid Sci. Technol. 2021, 123, 2000180. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation Effects on Quality, Phenolic Composition, and Selected Volatiles of Virgin Olive Oils Cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of different irrigation volumes during fruit development on quality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef]
- Di Giovacchino, L. Technological Aspects. In Handbook of Olive Oil; Aspen Publication: Gaithersburg, MD, USA, 2013; ISBN 9781461477778. [Google Scholar]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Di Maio, I.; Sordini, B.; Servili, M. Cooling treatment of olive paste during the oil processing: Impact on the yield and extra virgin olive oil quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef]
- Manganiello, R.; Pagano, M.; Nucciarelli, D.; Ciccoritti, R.; Tomasone, R.; Di Serio, M.G.; Giansante, L.; Del Re, P.; Servili, M.; Veneziani, G. Effects of Ultrasound Technology on the Qualitative Properties of Italian Extra Virgin Olive Oil. Foods 2021, 10, 2884. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-Virgin Olive Oil Extracted Using Pulsed Electric Field Technology: Cultivar Impact on Oil Yield and Quality. Front. Nutr. 2019, 6, 134. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Minnocci, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Sebastiani, L.; Servili, M. High vacuum-assisted extraction affects virgin olive oil quality: Impact on phenolic and volatile compounds. Food Chem. 2021, 342, 128369. [Google Scholar] [CrossRef]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of Low Temperature during the Malaxation Phase of Virgin Olive Oil Mechanical Extraction Processes of Three Different Italian Cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G. Influence of the Decrease in Oxygen during Malaxation of Olive Paste on the Composition of Volatiles and Phenolic Compounds in Virgin Olive Oil. J. Agric. Food Chem. 2008, 56, 10048–10055. [Google Scholar] [CrossRef] [PubMed]
- Luaces, P.; Pérez, A.G.; Sanz, C. Effect of the blanching process and olive fruit temperature at milling on the biosynthesis of olive oil aroma. Eur. Food Res. Technol. 2006, 224, 11–17. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Minnocci, A.; Sebastiani, L.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Olive Oil Mechanical Extraction Process: Impact on the Structural Modifications of Pastes and Oil Quality. J. Agric. Food Chem. 2013, 61, 4953–4960. [Google Scholar] [CrossRef] [PubMed]
- Tamborrino, A.; Taticchi, A.; Romaniello, R.; Perone, C.; Esposto, S.; Leone, A.; Servili, M. Assessment of the olive oil extraction plant layout implementing a high-power ultrasound machine. Ultrason. Sonochem. 2021, 73, 105505. [Google Scholar] [CrossRef]
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-frequency, high-power ultrasound treatment at different pressures for olive paste: Effects on olive oil yield and quality. Ultrason. Sonochem. 2019, 59, 104747. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined industrial olive oil extraction plant using ultrasounds, microwave, and heat exchange: Impact on olive oil quality and yield. J. Food Eng. 2019, 245, 124–130. [Google Scholar] [CrossRef]
- Aydar, A.Y. Physicochemical characteristics of extra virgin olive oils obtained by ultrasound assisted extraction from different olive cultivars. Int. J. Sci. Technol. Res. 2018, 4, 3. [Google Scholar]
- Nardella, M.; Moscetti, R.; Chakravartula, S.S.N.; Bedini, G.; Massantini, R. A Review on High-Power Ultrasound-Assisted Extraction of Olive Oils: Effect on Oil Yield, Quality, Chemical Composition and Consumer Perception. Foods 2021, 10, 2743. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Juliano, P.; Bainczyk, F.; Swiergon, P.; Supriyatna, M.I.M.; Guillaume, C.; Ravetti, L.; Canamasas, P.; Cravotto, G.; Xu, X.-Q. Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochem. 2017, 38, 104–114. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Dugo, G. Oxygen concentration control during olive oil extraction process: A new system to emphasize the organoleptic and healthy properties of virgin olive oil. Acta Hortic. 2012, 949, 473–480. [Google Scholar] [CrossRef]
- Olías, J.M.; Pérez, A.G.; Ríos, J.J.; Sanz, L.C. Aroma of virgin olive oil:biogenesis of the “green” odor notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G.F. Effect of Olive Stoning on the Volatile and Phenolic Composition of Virgin Olive Oil. J. Agric. Food Chem. 2007, 55, 7028–7035. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Summo, C.; Pasqualone, A. Influence of the type of olive-crusher used on the quality of extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2003, 105, 201–206. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Taticchi, A.; Veneziani, G.; Genovese, A.; Sacchi, R. Estrazione, conservazione, packaging e qualità. In Oleum; Conte, L., Servili, M., Eds.; Edagricole: Bologna, Italy, 2022; pp. 102–165. ISBN 978-88-506-5617-2. [Google Scholar]
- Nucciarelli, D.; Esposto, S.; Veneziani, G.; Daidone, L.; Urbani, S.; Taticchi, A.; Selvaggini, R.; Servili, M. The Use of a Cooling Crusher to Reduce the Temperature of Olive Paste and Improve EVOO Quality of Coratina, Peranzana, and Moresca Cultivars: Impact on Phenolic and Volatile Compounds. Food Bioprocess Technol. 2022, 15, 1988–1996. [Google Scholar] [CrossRef]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the Temperature and Oxygen Concentration Conditions in the Malaxation during the Oil Mechanical Extraction Process of Four Italian Olive Cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Synthesis of Volatile Compounds of Virgin Olive Oil Is Limited by the Lipoxygenase Activity Load during the Oil Extraction Process. J. Agric. Food Chem. 2012, 60, 812–822. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Fregapane, G.; Salvador, M.D. Effect of crushing on olive paste and virgin olive oil minor components. Eur. Food Res. Technol. 2011, 232, 441–451. [Google Scholar] [CrossRef]
- Soldo, B.; Šprung, M.; Mušac, G.; Pavela-Vrančić, M.; Ljubenkov, I. Evaluation of Olive Fruit Lipoxygenase Extraction Protocols on 9- and 13-Z,E-HPODE Formation. Molecules 2016, 21, 506. [Google Scholar] [CrossRef] [PubMed]
- Masella, P.; Angeloni, G.; Guerrini, L.; Spadi, A.; Corti, F.; Parenti, A. Pumping contribution to dissolved oxygen in virgin olive oil during processing. Chem. Eng. Trans. 2021, 87, 307–312. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Romero, C.; Pérez, A.G.; Sanz, C. Oxygen Concentration Affects Volatile Compound Biosynthesis during Virgin Olive Oil Production. J. Agric. Food Chem. 2008, 56, 4681–4685. [Google Scholar] [CrossRef]
- Rastogi, H.; Bhatia, S. Future prospectives for enzyme technologies in the food industry. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 845–860. ISBN 9780128132807. [Google Scholar]
- Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile Compounds and Phenolic Composition of Virgin Olive Oil: Optimization of Temperature and Time of Exposure of Olive Pastes to Air Contact during the Mechanical Extraction Process. J. Agric. Food Chem. 2003, 51, 7980–7988. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; Basti, C.; Vito, R. Virgin Olive Oil Volatile Compounds from Lipoxygenase Pathway and Characterization of Some Italian Cultivars. J. Agric. Food Chem. 1999, 47, 836–839. [Google Scholar] [CrossRef]
- Morales, M.T.; Angerosa, F.; Aparicio, R. Efecto de las condiciones de extracción del aceite de oliva virgen en la ruta de la li-poxigenasa: Implicaciones químicas y sensoriales. Grasas Aceites 1999, 50, 114–121. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Pérez, A.G.; Sanz, C. Synthesis of aroma compounds of virgin olive oil: Significance of the cleavage of polyunsaturated fatty acid hydroperoxides during the oil extraction process. Food Res. Int. 2013, 54, 1972–1978. [Google Scholar] [CrossRef]
- Cerezo, S.; Hernández, M.L.; Palomo-Ríos, E.; Gouffi, N.; García-Vico, L.; Sicardo, M.D.; Sanz, C.; Mercado, J.A.; Pliego-Alfaro, F.; Martínez-Rivas, J.M. Modification of 13-hydroperoxide lyase expression in olive affects plant growth and results in altered volatile profile. Plant Sci. 2021, 313, 111083. [Google Scholar] [CrossRef]
- Lorenzi, V.; Maury, J.; Casanova, J.; Berti, L. Purification, product characterization and kinetic properties of lipoxygenase from olive fruit (Olea europaea L.). Plant Physiol. Biochem. 2006, 44, 450–454. [Google Scholar] [CrossRef]
- Beltrán, G.; Uceda, M.; Jiménez, A.; Aguilera, M.P. Olive oil extractability index as a parameter for olive cultivar characterisation. J. Sci. Food Agric. 2003, 83, 503–506. [Google Scholar] [CrossRef]
- COI/T. 20/Doc. No 34/Rev. 1; Determination of Free Fatty Acids, Cold Method. International Olive Council: Madrid, Spain, 2017.
- COI/T.20/Doc. No 35/Rev.1; Determination of Peroxide Value. International Olive Council: Madrid, Spain, 2017.
- COI/T. 20/Doc. No 19/Rev. 5; Spectrophotometric Investigation in the Ultraviolet. International Olive Council: Madrid, Spain, 2019.
- Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union L 2022, 284, 1–22.
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- COI/T. 20/Doc. No 14/Rev. 7; Sensory Analysis of Olive Oil Standard Guide for the Selection, Training and Quality Control of Virgin Olive Oil Tasters—Qualifications of Tasters, Panel Leaders and Trainers. International Olive Council: Madrid, Spain, 2021.
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future—An overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Taticchi, A.; Urbani, S.; Di Maio, I.; Veneziani, G.; Selvaggini, R. New approaches to virgin olive oil quality, technology, and by-products valorization. Eur. J. Lipid Sci. Technol. 2015, 117, 1882–1892. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Zagaria, R.; Tamborrino, A. Development of a prototype malaxer to investigate the influence of oxygen on extra-virgin olive oil quality and yield, to define a new design of machine. Biosyst. Eng. 2014, 118, 95–104. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Bejaoui, M.A.; Herrera, M.P.A.; Márquez, A.J.; Maza, G.B. Application of oxygen during olive fruit crushing impacts on the characteristics and sensory profile of the virgin olive oil. Eur. J. Lipid Sci. Technol. 2016, 118, 1018–1029. [Google Scholar] [CrossRef]
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servili, M. Quality evolution of extra-virgin olive oils according to their chemical composition during 22 months of storage under dark conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of light exposure on the quality of extra virgin olive oils according to their chemical composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef]
- Barr, D.P.; Aust, S.D. On the Mechanism of Peroxidase-Catalyzed Oxygen Production. Arch. Biochem. Biophys. 1993, 303, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Notario, A.; Sánchez, R.; Luaces, P.; Sanz, C.; Pérez, A.G. The Infestation of Olive Fruits by Bactrocera oleae (Rossi) Modifies the Expression of Key Genes in the Biosynthesis of Volatile and Phenolic Compounds and Alters the Composition of Virgin Olive Oil. Molecules 2022, 27, 1650. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, O.; Dminić, I.; Kosić, U.; Majetić, V.; Godena, S.; Valenčič, V. Dynamics of oil quality parameters changes related to olive fruit fly attack. Eur. J. Lipid Sci. Technol. 2010, 112, 1033–1040. [Google Scholar] [CrossRef]
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular Modeling of Peroxidase and Polyphenol Oxidase: Substrate Specificity and Active Site Comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The influence of the malaxation temperature on the activity of polyphenoloxidase and peroxidase and on the phenolic composition of virgin olive oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; Rallo, L.; Luque de Castro, M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Calderón-Santiago, M.; Priego-Capote, F. Metabolic patterns in the lipoxygenase pathway associated to fruitiness attributes of extra virgin olive oil. J. Food Compos. Anal. 2022, 109. [Google Scholar] [CrossRef]
- Da Costa, J.R.O.; Dal Bosco, S.M.; Ramos, R.C.D.S.; Machado, I.C.K.; Garavaglia, J.; Villasclaras, S.S. Determination of volatile compounds responsible for sensory characteristics from Brazilian extra virgin olive oil using HS-SPME/GC-MS direct method. J. Food Sci. 2020, 85, 3764–3775. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin Olive Oil Volatile Compounds: Composition, Sensory Characteristics, Analytical Approaches, Quality Control, and Authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Lioupi, A.; Sampsonidis, I.; Virgiliou, C.; Papoti, V.T.; Zinoviadou, K.G.; Spyros, A.; Theodoridis, G. Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils. Metabolites 2022, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- de los Angeles Fernandez, M.; Assof, M.; Jofre, V.; Silva, M.F. Volatile Profile Characterization of Extra Virgin Olive Oils from Argentina by HS-SPME/GC-MS and Multivariate Pattern Recognition Tools. Food Anal. Methods 2014, 7, 2122–2136. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Raffo, A.; Bucci, R.; D’Aloise, A.; Pastore, G. Combined effects of reduced malaxation oxygen levels and storage time on extra-virgin olive oil volatiles investigated by a novel chemometric approach. Food Chem. 2015, 182, 257–267. [Google Scholar] [CrossRef]
- Da Ros, A.; Masuero, D.; Riccadonna, S.; Bubola, K.B.; Mulinacci, N.; Mattivi, F.; Lukić, I.; Vrhovsek, U. Complementary Untargeted and Targeted Metabolomics for Differentiation of Extra Virgin Olive Oils of Different Origin of Purchase Based on Volatile and Phenolic Composition and Sensory Quality. Molecules 2019, 24, 2896. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
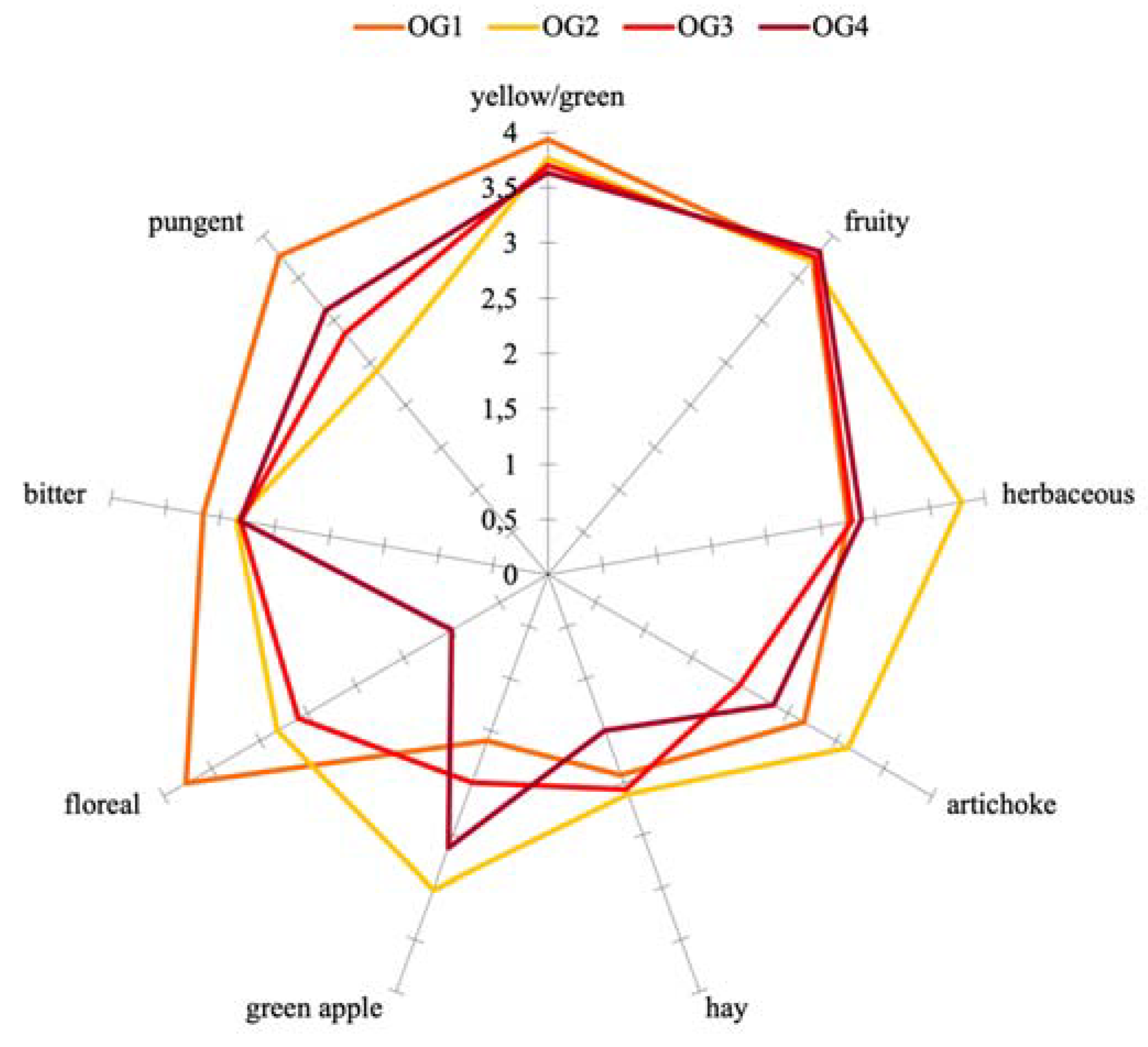
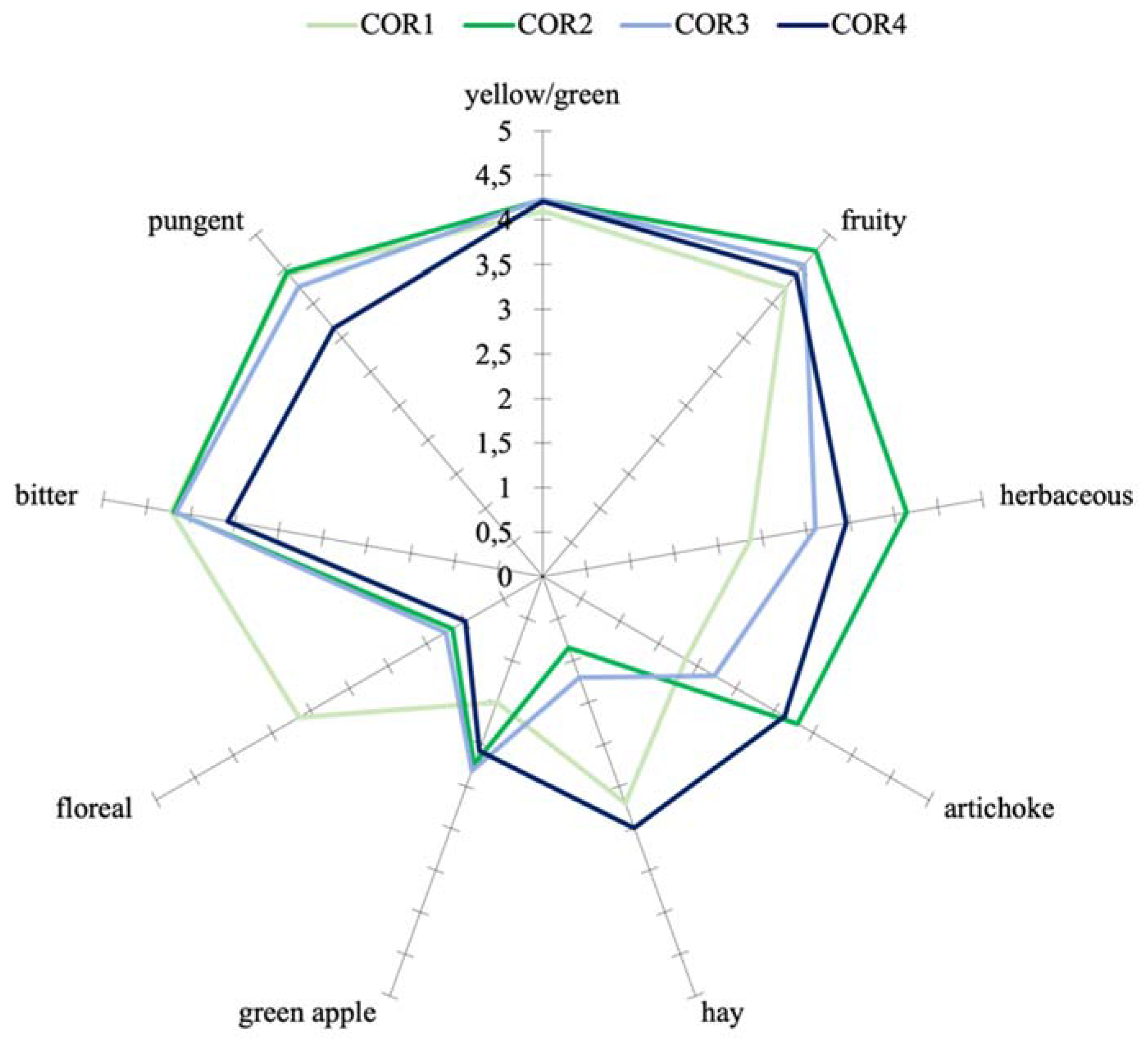
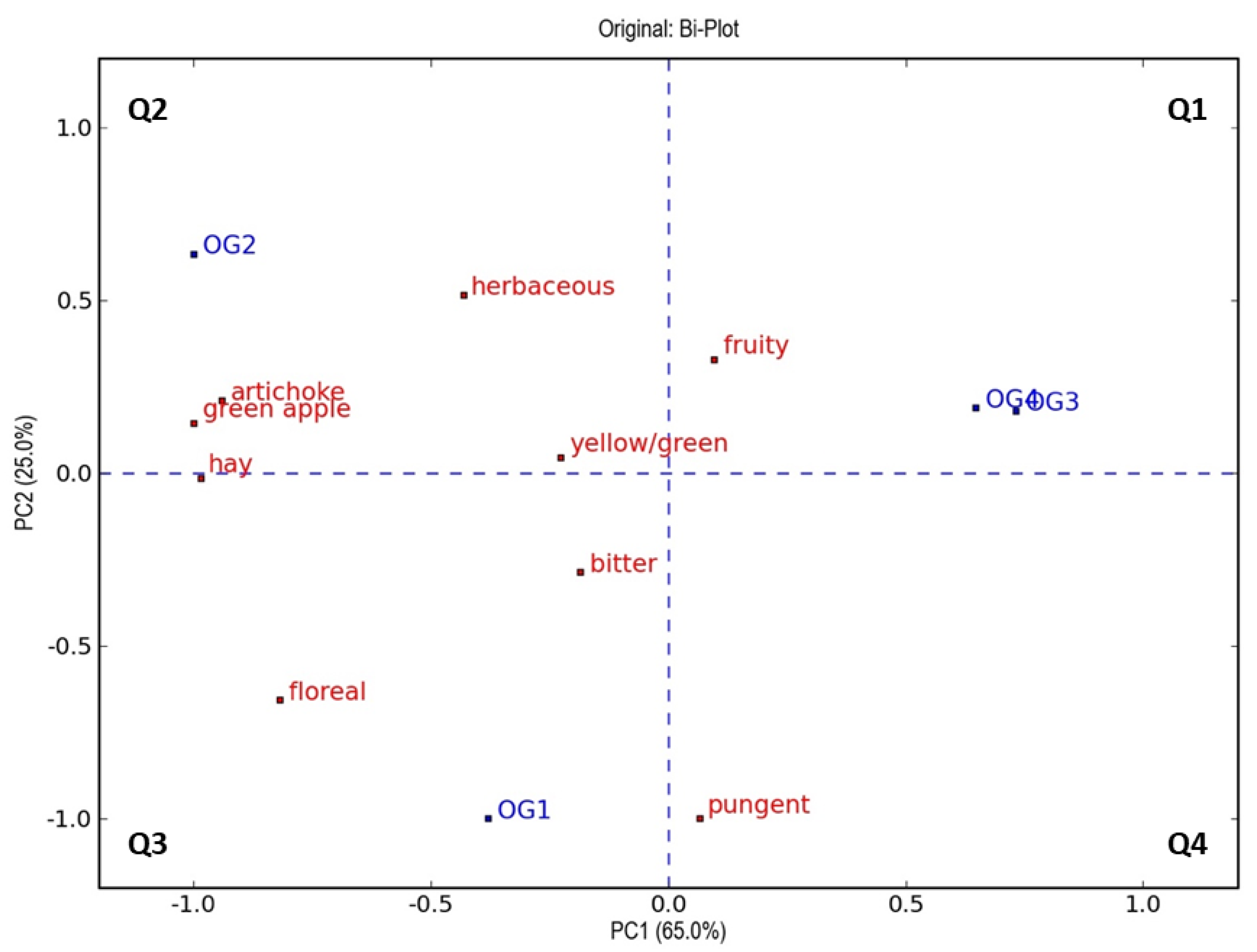
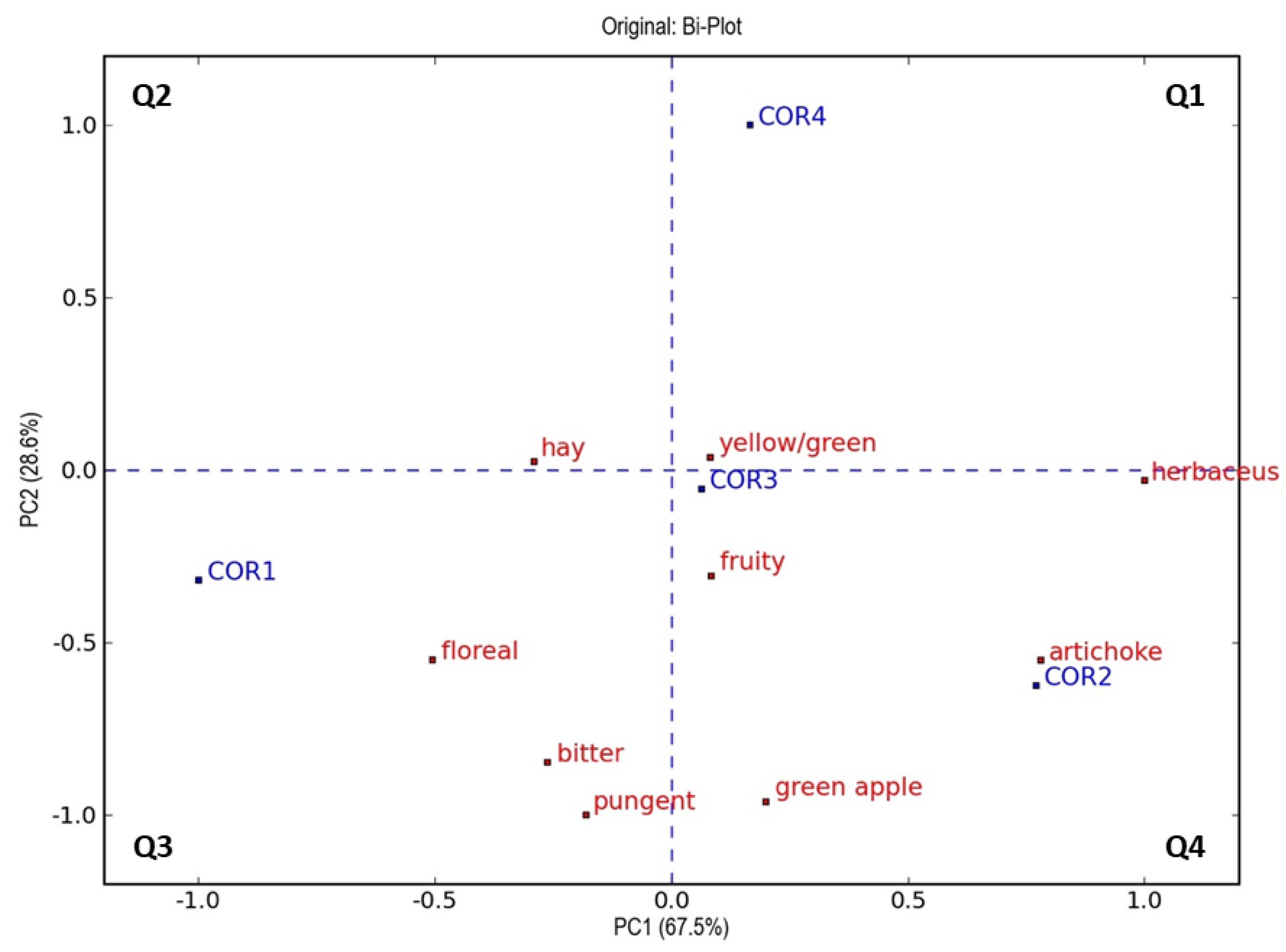
| Parameters | Ogliarola | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| OG1 (No O2 Supply) | OG2 (O2 0.2 L/min) | OG3 (O2 0.4 L/min) | OG4 (O2 0.8 L/min) | COR1 (No O2 Supply) | COR2 (O2 0.2 L/min) | COR3 (O2 0.4 L/min) | COR4 (O2 0.8 L/min) | |
| Acidity (% oleic ac.) | 0.35 ± 0.04 a | 0.31 ± 0.00 a | 0.33 ± 0.00 a | 0.32 ± 0.01 a | 0.22 ± 0.01 a | 0.25 ± 0.02 a | 0.22 ± 0.01 a | 0.23 ± 0.02 a |
| Peroxide value (meq O2/kg of oil) | 7.6 ± 0.6 a | 7 ± 0.3 a | 6.8 ± 0.1 a | 7.5 ± 0.1 a | 4.65 ± 0.8 a | 4.15 ± 0.07 a | 4.65 ± 0.2 a | 4.2 ± 0.3 a |
| K232 | 1.79 ± 0.00 b | 1.78 ± 0.02 b | 1.71 ± 0.03 c | 1.84 ± 0.06 a | 1.62 ± 0.06 a | 1.56 ± 0.04 a | 1.61 ± 0.02 a | 1.61 ± 0.08 a |
| K270 | 0.13 ± 0.00 a | 0.15 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.00 a | 0.15 ± 0.00 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a |
| Phenolic Compounds | Ogliarola | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| OG1 | OG2 | OG3 | OG4 | COR1 | COR2 | COR3 | COR4 | |
| (No O2 Supply) | (O2 0.2 L/min) | (O2 0.4 L/min) | (O2 0.8 L/min) | (No O2 Supply) | (O2 0.2 L/min) | (O2 0.4 L/min) | (O2 0.8 L/min) | |
| Hydroxytyrosol | 1.6 ± 0.1 b | 3.2 ± 0.8 a | 1.7 ± 0 b | 1.7 ± 0.2 b | 1 ± 0.0 b | 1.3 ± 0.1 a | 1.2 ± 0.0 a | 1 ± 0.1 b |
| Tyrosol | 4.4 ± 0.6 a | 4.2 ± 0.3 a | 4.3 ± 0.2 a | 4.3 ± 0.0 a | 2 ± 0.1 b | 2.4 ± 0.2 ab | 2.5 ± 0.3 a | 2 ± 0.0 b |
| Vanillic acid | 0.4 ± 0.1 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.4 ± 0.0 a | 0.5 ± 0.1 ab | 0.6 ± 0.1 a | 0.6 ± 0.1 a | 0.4 ± 0.0 b |
| p-Coumaric acid | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | n.d. | n.d. | n.d. | n.d. |
| Oleacein | 384.1 ± 20.4 a | 375.4 ± 23.2 a | 364.8 ± 31.4 a | 330.4 ± 15.6 a | 555.3 ± 8.6 a | 527.1 ± 3.2 a | 472.3 ± 6.1 b | 268.1 ± 37.8 c |
| Oleocanthal | 71.1 ± 4.4 a | 69.1 ± 8.8 a | 66 ± 5.2 a | 59.8 ± 2.9 a | 105 ± 5.8 a | 106.3 ± 0.7 a | 105.3 ± 5.7 a | 107.4 ± 3.1 a |
| (+)-1-acetoxypinoresinol | 42.6 ± 1.1 a | 41 ± 1.6 a | 42.6 ± 1.7 a | 41.6 ± 0.3 a | 33.4 ± 0.2 a | 34.2 ± 0.3 a | 33.5 ± 1 a | 34.3 ± 3.3 a |
| (+)-pinoresinol | 19.1 ± 0.7 a | 19.4 ± 0.8 a | 19.8 ± 2.2 a | 19.5 ± 0.7 a | 15.2 ± 0.3 a | 15.4 ± 0.4 a | 15 ± 0.7 a | 13.9 ± 1.6 a |
| Oleuropein aglycone | 103.6 ± 5.5 a | 101.1 ± 6.4 a | 93.5 ± 4.4 ab | 87 ± 0.2 b | 126.5 ± 7.0 b | 128.5 ± 2.3 b | 112.7 ± 1.4 b | 146.5 ± 10.4 a |
| Ligstroside aglycone | 17.5 ± 1.6 a | 16.4 ± 1.0 a | 17.6 ± 0.4 a | 16.7 ± 0.8 a | 17.4 ± 2.2 b | 21.3 ± 1.7 ab | 22.2 ± 1.3 a | 17.6 ± 0.6 b |
| Sum of Oleuropein derivatives | 489.3 ± 21.1 a | 459.9 ± 24.1 ab | 479.6 ± 31.7 ab | 419 ± 15.6 b | 682.9 ± 11.1 a | 657 ± 3.9 a | 586.2 ± 6.2 b | 415.6 ± 39.2 c |
| Sum of ligstroside derivatives | 582.3 ± 4.7 a | 547.8 ± 8.8 a | 569.4 ± 5.2 b | 499.9 ± 3.0 c | 124.4 ± 6.2 a | 127 ± 3.1 a | 130 ± 5.9 a | 129.9 ± 1.8 a |
| Sum of lignans derivatives | 61.7 ± 1.3 a | 62.4 ± 1.8 a | 60.4 ± 2.7 a | 61.1 ± 0.8 a | 48.6 ± 0.4 a | 49.6 ± 0.5 a | 48.5 ± 1.2 a | 48.3 ± 3.7 a |
| Total phenol | 644.8 ± 22.3 a | 630.4 ± 26.5 a | 610.8 ± 32.5 ab | 561.7 ± 15.9 b | 856.4 ± 14.6 a | 837.1 ± 4.9 a | 765.2 ± 8.8 b | 591.2 ± 40.8 c |
| Volatile Compounds | Ogliarola | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| OG1 (No O2 Supply) | OG2 (O2 0.2 L/min) | OG3 (O2 0.4 L/min) | OG4 (O2 0.8 L/min) | COR1 (No O2 Supply) | COR2 (O2 0.2 L/min) | COR3 (O2 0.4 L/min) | COR4 (O2 0.8 L/min) | |
| Aldehydes | ||||||||
| Pentanal | 8 ± 1 a | 8 ± 0 a | 6 ± 1 a | 10 ± 4 a | 13 ± 0 a | 17 ± 0 a | 18 ± 4 a | 12 ± 11 a |
| (E)-2-Pentenal | 44 ± 2 a | 47 ± 3 a | 38 ± 2 b | 36 ± 1 c | 60 ± 9 a | 54 ± 3 a | 55 ± 1 a | 35 ± 24 a |
| Hexanal | 908 ± 20 a | 1009 ± 97 a | 820 ± 14 b | 774 ± 19 c | 2298 ± 7 a | 2051 ± 144 b | 1954 ± 39 b | 1960 ± 111 b |
| (Z)-3-Hexenal | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| (E)-2-Hexenal | 16,772 ± 444 c | 20,007 ± 537 a | 17,137 ± 239 b | 16,470 ± 386 d | 44,408 ± 1906 b | 48,329 ± 599 a | 45,871 ± 1211 a | 39,504 ± 1545 c |
| (E,E)-2,4-Hexadienal | 66 ± 1 a | 75 ± 7 a | 58 ± 3 b | 61 ± 3 ab | 172 ± 1 a | 158 ± 4 ab | 162 ± 0 a | 140 ± 16 b |
| Sum of aldehydes at C5 and C6 | 17,798 ± 444 b | 21,146 ± 545 a | 18,059 ± 239 b | 17,351 ± 386 b | 46,950 ± 1906 b | 50,609 ± 616 a | 48,059 ± 1212 a | 41,651 ± 1549 b |
| Alcohols | ||||||||
| 1-Pentanol | 27 ± 4 ab | 31 ± 0 a | 23 ± 1 b | 23 ± 1 b | 41 ± 0 a | 42 ± 0 a | 41 ± 1 a | 37 ± 5 a |
| 1-Penten-3-ol | 210 ± 14 a | 170 ± 11 b | 172 ± 8 b | 172 ± 5 b | 621 ± 54 a | 491 ± 23 b | 509 ± 2 b | 484 ± 13 b |
| (E)-2-Penten-1-ol | 28 ± 1 b | 34 ± 2 a | 24 ± 1 c | 23 ± 0 c | 57 ± 10 a | 48 ± 2 a | 47 ± 1 a | 41 ± 9 a |
| (Z)-2-Penten-1-ol | 201 ± 15 a | 222 ±24 a | 162 ± 2 b | 162 ± 0 b | 661 ± 105 a | 559 ± 21 a | 565 ± 9 a | 538 ± 25 a |
| 1-Hexanol | 748 ± 55 a | 577 ± 21 b | 547 ± 46 b | 514 ± 3 b | 649 ± 113 a | 497 ± 33 b | 596 ± 19 b | 463 ± 47 b |
| (E)-2-Hexen-1-ol | 669 ± 37 a | 518 ± 29 b | 401 ± 4 c | 392 ± 8 d | 1280 ± 38 a | 977 ± 41 c | 1152 ± 22 b | 935 ± 16 c |
| (Z)-2-Hexen-1-ol | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| (E)-3-Hexen-1-ol | 15 ± 4 a | 11 ± 1 a | 10 ± 0 a | 10 ± 1 a | 0 ± 0 | 0 ± 0 | 5 ± 0 | 0 ± 0 |
| (Z)-3-Hexen-1-ol | 159 ± 25 a | 118 ± 15 b | 106 ± 9 b | 112 ± 7 b | 373 ± 37 a | 286 ± 9 b | 298 ± 22 b | 237 ± 29 b |
| Sum of alcohols at C5 and C6 | 2057 ± 74 a | 1681 ± 47 b | 1445 ± 48 c | 1407 ± 12 c | 3683 ± 172 a | 2898 ± 62 c | 3212 ± 39 b | 2735 ± 65 c |
| Esters | ||||||||
| Hexyl acetate | 43 ± 5 a | 27 ± 1 b | 25 ± 1 b | 27 ± 4 b | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| (Z)-3-Hexenyl acetate | 31 ± 4 a | 19 ± 1 b | 16 ± 0 b | 18 ± 1 b | 6 ± 1 a | 4 ± 0 b | 4 ± 0 b | 4 ± 0 b |
| Sum of esters at C6 | 74 ± 6 a | 46 ± 2 b | 42 ± 1 b | 45 ± 4 b | 6 ± 1 a | 4 ± 0 b | 4 ± 0 b | 4 ± 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneziani, G.; García-González, D.L.; Esposto, S.; Nucciarelli, D.; Taticchi, A.; Boudebouz, A.; Servili, M. Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils. Foods 2023, 12, 612. https://doi.org/10.3390/foods12030612
Veneziani G, García-González DL, Esposto S, Nucciarelli D, Taticchi A, Boudebouz A, Servili M. Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils. Foods. 2023; 12(3):612. https://doi.org/10.3390/foods12030612
Chicago/Turabian StyleVeneziani, Gianluca, Diego L. García-González, Sonia Esposto, Davide Nucciarelli, Agnese Taticchi, Abdelaziz Boudebouz, and Maurizio Servili. 2023. "Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils" Foods 12, no. 3: 612. https://doi.org/10.3390/foods12030612
APA StyleVeneziani, G., García-González, D. L., Esposto, S., Nucciarelli, D., Taticchi, A., Boudebouz, A., & Servili, M. (2023). Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils. Foods, 12(3), 612. https://doi.org/10.3390/foods12030612






