Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Olive Fruit Samples
2.3. Olive Washing
2.4. Olive Oil Extraction
2.5. Analytical Determinations
2.5.1. Fruit Characterization
- -
- Weight of 100 fruits: In each of the samples, the fruit weight was determined as the weight of 100 drupes randomly picked from aliquots of samples that had been previously homogenized.
- -
- Ripening index (RI): The method followed to determine the RI was that proposed by Ferreira et al. [32], which consists of classifying the fruit into eight classes according to the color of the skin and pulp.
2.5.2. Analytical Indices
2.5.3. Oxidative Stability
2.6. Sensory Analysis
2.7. Determination of the Volatile Profile
2.8. Statistical Analysis
3. Results and Discussion
3.1. Influence of Washing and Time of Harvesting on Agronomic and Physicochemical Parameters
3.2. Assessment of Sensory and Volatile Profile Results
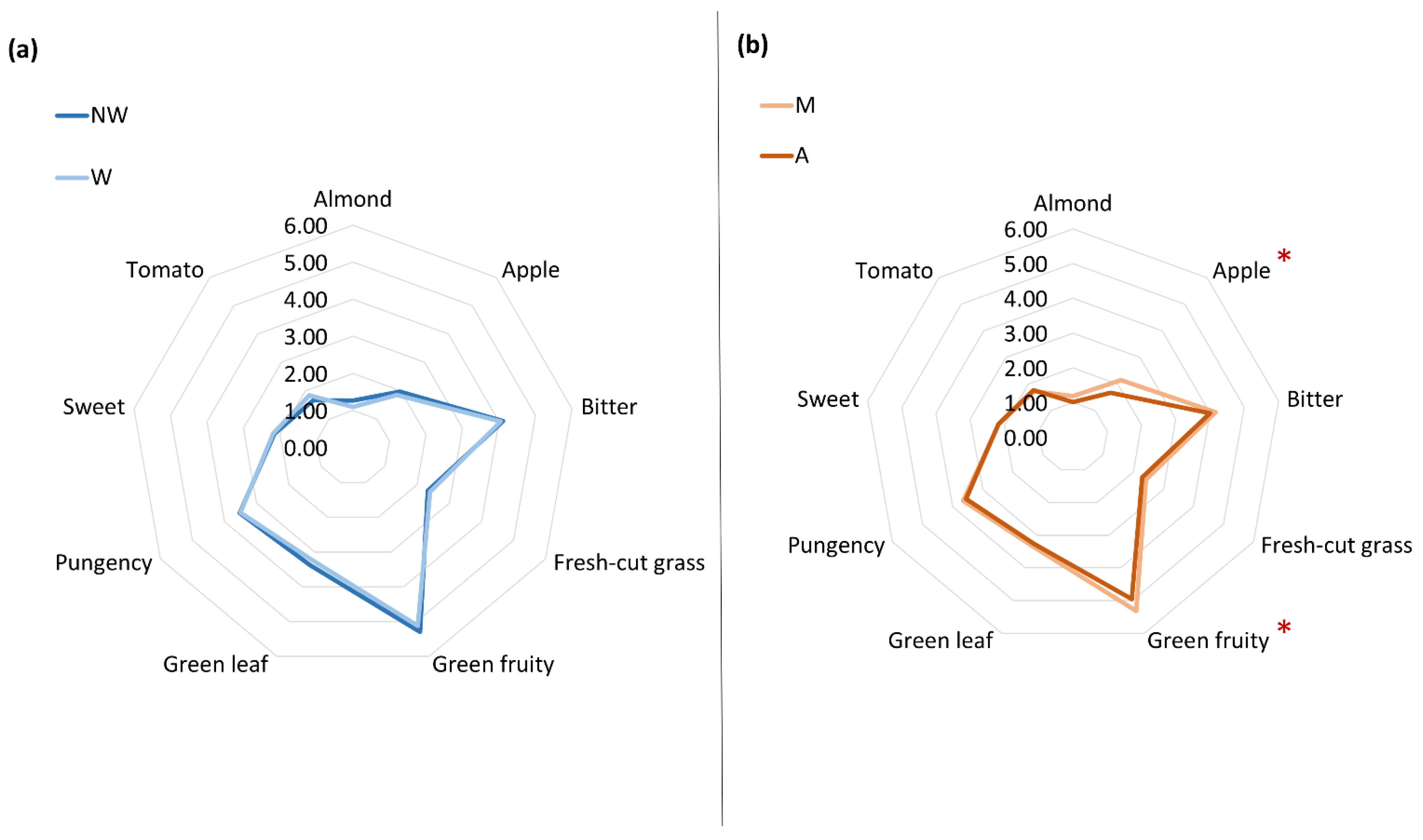
3.2.1. Sensory Profile
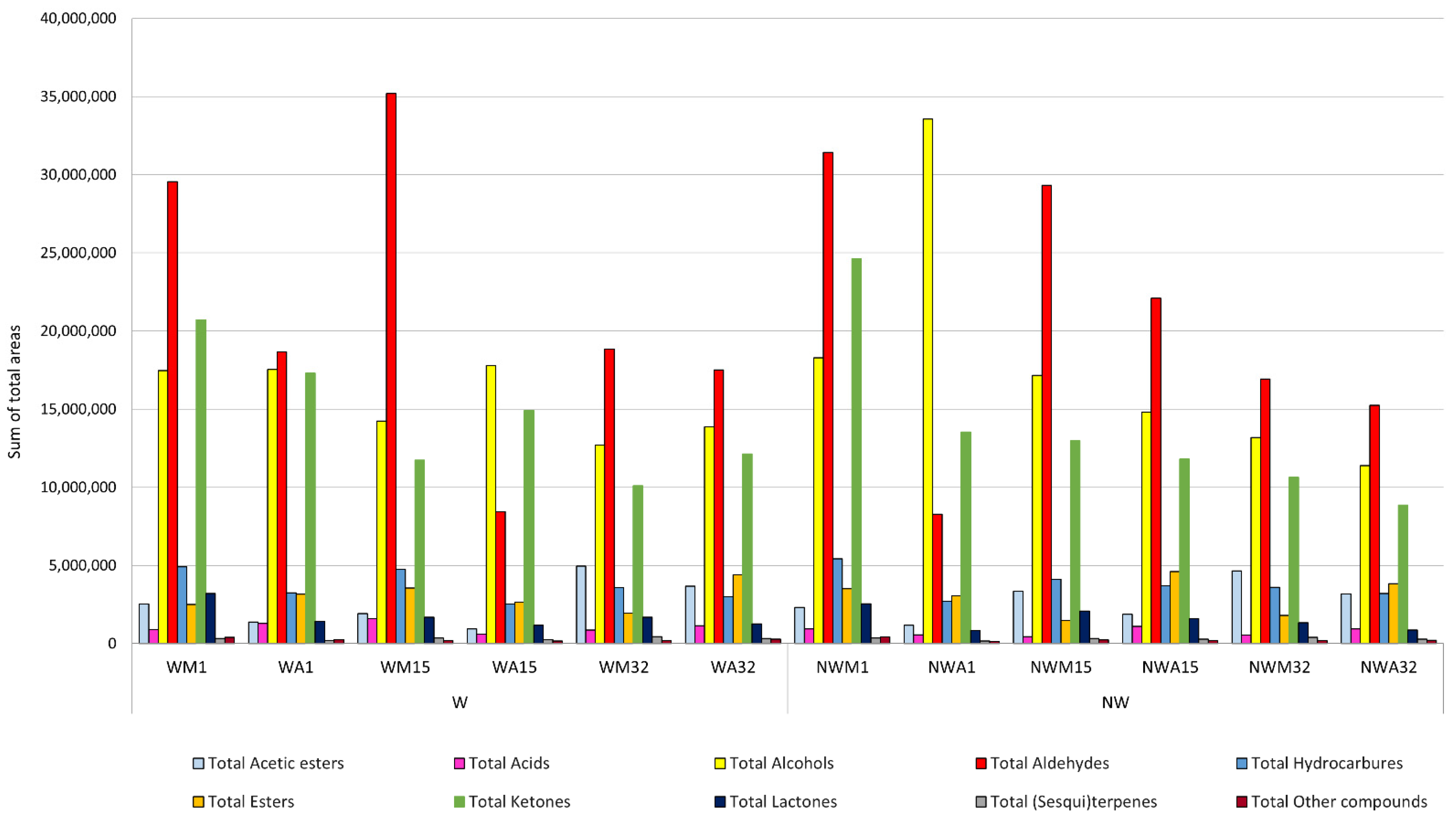
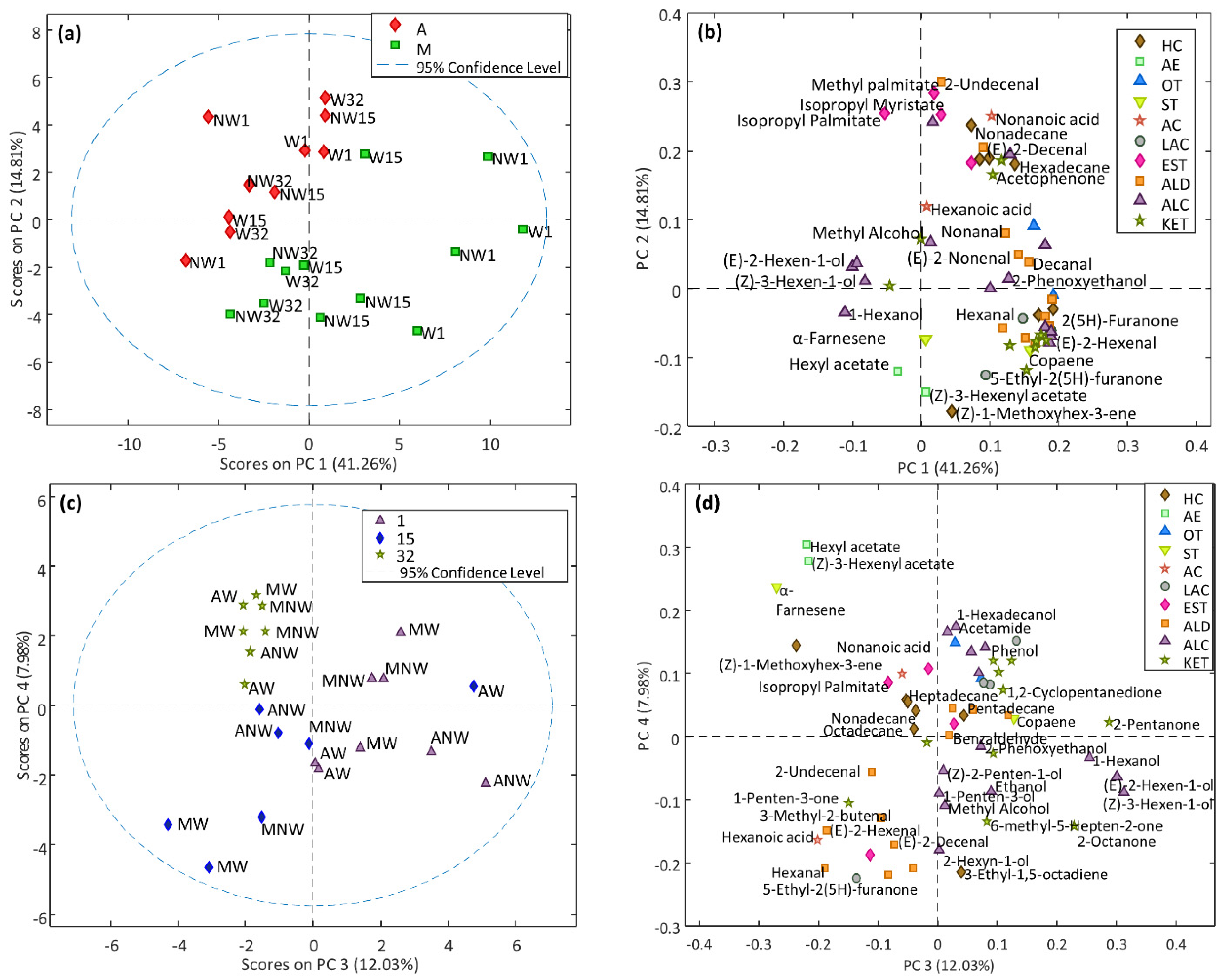
3.2.2. Volatile Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MAPAMA. General Subdirectorate for Differentiated Quality and Ecological Agriculture, Ministry of Agriculture and Fisheries, Food and Environment. 2019. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2019/default.aspx (accessed on 18 June 2022).
- Reganold, J.; Wachter, J. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Tres, A.; O’Neill, R.; van Ruth, S.M. Fingerprinting of fatty acid composition for the verification of the identity of organic eggs. Lipid Technol. 2011, 23, 40–42. [Google Scholar] [CrossRef]
- Cozzolino, D.; Holdstock, M.; Dambergs, R.G.; Cynkar, W.U.; Smith, P.A. Mid infrared spectroscopy and multivariate analysis: A tool to discriminate between organic and non-organic wines grown in Australia. Food Chem. 2009, 116, 761–765. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No. 834/2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32018R0848&from=ES (accessed on 25 June 2022).
- García-González, D.L.; Aparicio Ruiz, R.; Morales, M.T. Chemical characterization of organic and non-organic virgin olive oils. OCL Oilseeds Fats Crops Lipids 2014, 21, 12. [Google Scholar] [CrossRef]
- International Olive Oil Council (IOOC). Trade Standard Applying to Olive Oil and Olive-Pomace Oils. COI/T.15/NC No. 3/Rev. 8. 2015. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2020/07/Trade-standard-T15-NC3-Rev15-EN.pdf (accessed on 20 June 2022).
- Implementing Regulation (EU) No. 299/2013 of the Commission. That modifies Regulation (EEC) No. 2568/91 Relative to the Characteristics of Olive Oils and Olive-Pomace Oils and Their Methods of Analysis. Off. J. Eur. Union 2013, L90, 52–70. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:090:0052:0070:EN:PDF (accessed on 30 June 2022).
- Implementing Regulation (EU) No. 1348/2013 of the Commission. That Modifies Regulation (EEC) No. 2568/91 Relative to the Characteristics of Olive Oils and Olive-Pomace Oils and Their Methods of Analysis. Off. J. Eur. Union 2013, L338, 31–67. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013R1348&from=GA (accessed on 30 June 2022).
- Regulation (EU) No. 1308/2013 of the European Parliament and of the Council. Establishing the Common Organization of the Markets for Agricultural Products and Repealing Regulations (EEC) No. 922/72, (EEC) No. 234/79, (EC) No. 1037/2001 and (CE) No. 1234/2007. Off. J. Eur. Union 2013, L347, 671–854. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R1308 (accessed on 30 June 2022).
- Regulation (EEC) No. 2568/91 of the Commission. Relating to the Characteristics of Olive oils and Olive-Pomace Oils and Their Methods of Analysis. 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31991R2568&qid=1659094060252 (accessed on 30 June 2022).
- Commission Regulation (EC), No. 656/95 of 28 March 1995 Amending Regulation (EEC) No. 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis and Council Regulation (EEC) No. 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31995R0656&qid=1659094098054 (accessed on 30 June 2022).
- Callejón, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the typical aroma of sherry vinegar: Sensory and chemical approach. J. Agric. Food Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef]
- Jiménez, B.; Callejón, R.; Sánchez-Ortiz, A.; Ortega, E.; Lorenzo, M.L.; Rivas, A. Agronomic parameters, quality indices, and sensory attributes of virgin olive oils from Hojiblanca and Picudo varieties from three successive crop years. Eur. J. Lipid Sci. Technol. 2014, 116, 1647–1653. [Google Scholar] [CrossRef]
- Patumi, M.; D’andria, R.; Marsilio, V.; Fontanazza, G.; Morelli, G.; Lanza, B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L.; cv. Kalamata) in different irrigation regimes. Food Chem. 2002, 77, 27–34. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Pérez, A.G.; Sanz, C. Cultivar Differences on Nonesterified Polyunsaturated Fatty Acid as a Limiting Factor for the Biogenesis of Virgin Olive Oil Aroma. J. Agric. Food Chem. 2007, 55, 7869–7873. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sillero, A.; García, J.M.; Torres-Ruiz, J.M.; Monteo, A.; Sánchez-Ortiz, A.; Fernández, J.E. Is the productive performance of olive trees under localized irrigation affected by leaving some roots in drying soil? Agric. Water Manag. 2013, 123, 79–92. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Rivas, A. Influence of the malaxation time and olive ripening stage on oil quality and phenolic compounds of virgin olive oils. Int. J. Food Sci. Technol. 2014, 49, 2521–2527. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Effect of organic cultivation of Picual and Hojiblanca olive varieties on the quality of virgin olive oil at four ripening stages. Eur. J. Lipid Sci. Technol. 2014, 116, 1634–1646. [Google Scholar] [CrossRef]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Sola-Guirado, R.R.; Castro-García, S.; Blanco-Roldán, G.L.; Jiménez-Jiménez, F.; Castillo-Ruiz, F.J.; Gil-Ribes, J.A. Traditional olive tree response to oil olive harvesting technologies. Biosyst. Eng. 2014, 118, 186–193. [Google Scholar] [CrossRef]
- Hermoso, M.; González, J.; Uceda, M.; García-Ortiz, A.; Morales, J.; Frías, L.; Fernández, A. Production of quality oil. In Obtained by the Two-Phase System; Ministry of Agriculture and Fisheries, Junta de Andalucía: Cordoba, Spain, 1996; ISBN 84-87564-17-8. [Google Scholar]
- Capuano, E.; Boerrigter-Eenling, R.; Van der Veer, G.; Van Ruth, S.M. Analytical Authentication of Organic Products: An Overview of Markers. J. Sci. Food Agric. 2013, 93, 12–28. [Google Scholar] [CrossRef]
- Jiménez, B.; Carpio, A. The Oil Tasting. Virgin Olive Oil. Organoleptic Characteristics and Sensory Analysis; Agricultural and Fisheries Research and Training Institute, Junta de Andalucía: Cordoba, Spain, 2008; Volume 145. [Google Scholar]
- De Torres Sánchez, A. Influence of Technological Factors on the Quality and Antioxidant Content of Virgin Olive Oil. Doctoral Thesis, University of Jaén, Jaén, Spain, 2013. [Google Scholar]
- Uceda, M.; Jiménez, A.; Beltrán, G. Trends in olive oil production. Fats Oils 2006, 57, 25–31. [Google Scholar]
- Cabellos, P.J.; García, M.; Martínez, M.; Hernández, B.; García, A. Manual de Aplicación del Sistema APPCC en Industrias de Aceites Vegetales Comestibles de Castilla-La Mancha; Junta de Comunidades de Castilla-La Mancha: Castile La Mancha, Spain, 2001; ISBN 8495690047, 978849569004.
- Vallesquino-Laguna, P.; Puentes-Campos, A.J.; Puentes-Campos, J.G.; Vergillos-Moreno, M.; Sánchez-Marín, R.; Jiménez-Herrera, B. Influencia del lavado de frutos sobre determinados parámetros de calidad de un aceite de oliva virgen extra ecológico. In Actas del XVII Simposio Científico Técnico—Expoliva; Fundación del Olivar: Jaén, España, 2015; Volume IND–11, pp. 1–8. Available online: http://hdl.handle.net/10396/26334 (accessed on 18 October 2025).
- Vallesquino-Laguna, P.; Puentes-Campos, A.J.; Puentes-Campos, J.G.; Jiménez-Herrera, B. Fruit washing influence on extra virgin olive oil quality: A sensory perspective. In Actas del XIX Simposio Científico–Técnico del Aceite de Oliva—Expoliva; Fundación del Olivar: Jaén, España, 2019; Volume IND–7, pp. 1–7. Available online: http://hdl.handle.net/10396/26332 (accessed on 18 October 2025).
- Council Regulation (EC) No 1804/1999 of 19 July 1999 Supplementing Regulation (EEC) No 2092/91 on Organic Production of Agricultural Products and Indications Referring Thereto on Agricultural Products and Foodstuffs to Include Livestock Production. Available online: https://eur-lex.europa.eu/eli/reg/1999/1804/1999-08-24 (accessed on 18 October 2025).
- Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Available online: https://eur-lex.europa.eu/eli/reg/2008/889/oj/eng (accessed on 18 October 2025).
- Ferreira, J. Collaborating Olive Farms No. 5; Ministry of Agriculture: Madrid, Spain, 1979.
- Commission Regulation (EC) No. 640/2008 of 4 July 2008 amending Regulation (EEC) No. 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32008R0640&from=ES (accessed on 30 June 2022).
- Gutiérrez Rosales, F.; González-Quijano, R. Quality parameters of olive oil. In Proceedings of the III National Symposium of Olive Oil. Expoliva-89, Jaén, Spain, 17 May 1989. [Google Scholar]
- Ríos-Reina, R.; Camacho, F.; Morales, M.L.; Jiménez-Herrera, B.; Callejón, R.M. Influence of Irrigation Modalities (Irrigation Management and Dryland), Fruit Ripening, and Cultivation Modality (Organic and Conventional) on Quality and Chemosensory Profile of Hojiblanca and Picual Extra Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2021, 123, 2000375. [Google Scholar] [CrossRef]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef]
- Civantos, L. When to start harvesting olives in Jaén? Agric. Rev. Agropecu. Year LXXII 2003, 851, 338–343. [Google Scholar]
- Morales, J.; García-Ortíz, C. The olive harvest and its influence on the quality of the oil. Agric. Agric. Mag. 2003, 857, 840–845. [Google Scholar]
- Jiménez, B.; Rivas, A.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Úbeda, M.; Callejón, R.M.; Ortega, E. Influence of the fruit ripening process on the sensory quality of virgin olive oils of the Picual, Hojiblanca and Picudo varieties. Fats Oils 2012, 63, 403–410. [Google Scholar]
- Franco, M.N.; Galeano-Díaz, T.; Sánchez, J.; De Miguel, C.; Martín-Vertedor, D. Antioxidant capacity of the phenolic fraction and its effect on the oxidative stability of olive oil varieties grown in the southwest of Spain. Fats Oils 2014, 65, e004. [Google Scholar]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality a study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Cerretani, L.; Gómez, A.M.; Bendini, A. Technological aspects of olive oil production. In The Virgin Olive Oil: Treasure of Andalusia; Publications Service of the Unicaja Foundation: Málaga, Spain, 2010; Volume 6, pp. 171–193. [Google Scholar]
- Smaoui, S.; Ben Hssine, K.; Kammoun, N.G. Physico-chemical characteristics and oxidative stability of virgin olive oils in relation to mixing temperature, ripening index and storage conditions. In Proceedings of the XVI Scientific-Technical Symposium on Olive Oil—Expoliva 2013, IND 49, Jaén, Spain, 8–11 May 2013. [Google Scholar]
- Pardo, J.E.; Serrano-Díaz, J.; Sena, E.; Carmona, M.; Álvarez-Ortí, M.; López, E.; Alonso, G. Influence of storage temperature on the quality and stability of virgin olive oils flavored with saffron. In Proceedings of the XVI Scientific-Technical Symposium of Olive Oil—Expoliva 2013, Jaén, Spain, 8–11 May 2013. [Google Scholar]
- Beltran, G.; Hueso, A.; Bejaoui, M.A.; Gila, A.M.; Costales, R.; Sánchez-Ortiz, A.; Jimenez, A. How olive washing and storage affect fruit ethanol and virgin olive oil ethanol, ethyl esters and composition. J. Sci. Food Agric. 2021, 101, 3714–3722. [Google Scholar] [CrossRef]
- Vichi, S.; Boynuegri, P.; Caixach, J.; Romero, A. Quality losses in virgin olive oil due to washing and short-term storage before olive milling. EJOSAT 2015, 117, 2015–2022. [Google Scholar] [CrossRef]
- Rivas, A.; Sanchez-Ortiz, A.; Jimenez, B.; García-Moyano, J.; Lorenzo, M.L. Phenolic acid content and sensory properties of two Spanish monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2013, 115, 621–630. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterization of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Aguilera, E.; Guzmán, G.I.; Álvaro-Fuentes, J.; Infante-Amate, J.; García-Ruiz, R.; Carranza-Gallego, G.; de Molina, M.G. A historical perspective on soil organic carbon in Mediterranean cropland (Spain, 1900–2008). Sci. Total Environ. 2018, 621, 634–648. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavor development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Jiménez, B.; Rivas, A.; Lorenzo, M.L.; Sánchez-Ortiz, A. Chemosensory characterization of virgin olive oils obtained from organic and conventional practices during fruit ripening. Flavour Fragr. J. 2017, 32, 294–304. [Google Scholar] [CrossRef]
- Ouni, Y.; Flamini, G.; Zarrouk, M. The Chemical Properties and Volatile Compounds of Virgin Olive Oil from Oueslati Variety: Influence of Maturity Stages in Olives. J. Am. Oil Chem. Soc. 2016, 93, 1265–1273. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Romero, C.O.; Casadei, E.; García-González, D.L.; Servili, M.; Selvaggini, R.; Toschi, T.G. Collaborative peer validation of a harmonized SPME-GC-MS method for analysis of selected volatile compounds in virgin olive oils. Food Control 2022, 135, 108756. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio-Ruiz, R.; Aparicio, R. Chromatographic methodologies: Compounds for olive oil odor issues. In Handbook of Olive Oil; Springer: Boston, MA, USA, 2013; pp. 261–309. [Google Scholar]
- Jiménez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res. Int. 2013, 54, 1860–1867. [Google Scholar] [CrossRef]
- Dabbou, S.; Brahmi, F.; Selvaggini, R.; Chehab, H.; Dabbou, S.; Taticchi, A.; Hammami, M. Contribution of irrigation and cultivars to volatile profile and sensory attributes of selected virgin olive oils produced in Tunisia. Int. J. Food Sci. Technol. 2011, 46, 1964–1976. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Magagna, F.; Valverde-Som, L.; Ruíz-Samblás, C.; Cuadros-Rodríguez, L.; Reichenbach, S.E.; Bicchi, C.; Cordero, C. Combined untargeted and targeted fingerprinting with comprehensive two-dimensional chromatography for volatiles and ripening indicators in olive oil. Anal. Chim. Acta. 2016, 936, 245–258. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Kranjac, M.; Marijanović, Z.; Jerković, I.; Corell, M.; Moriana, A.; Hernández, F. Quality attributes and fatty acid, volatile and sensory profiles of “Arbequina” hydrosostainable olive oil. Molecules 2019, 24, 2148. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin olive oil volatile compounds: Composition, sensory characterization, analytical approaches, quality control, and authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Dorota, D.; Rupert, M.; Wołosiak, R.; Bzducha-Wróbel, A.; Ścibisz, I.; Matuszewska-Janica, A. Volatiles as markers of bioactive components found in Croatian extra virgin olive oils. LWT 2021, 139, 110532. [Google Scholar] [CrossRef]
- García-González, D.L.; Vivancos, J.; Aparicio, R. Mapping brain activity induced by olfaction of virgin olive oil aroma. J. Agric. Food Chem. 2011, 59, 10200–10210. [Google Scholar] [CrossRef]
- Da Silva, M.D.G.; Freitas, A.M.C.; Cabrita, M.J.; Garcia, R. Olive oil composition: Volatile compounds. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; Dimitrios, B., Ed.; Intechopen: London, UK, 2012; pp. 17–47. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Database. 2005. Available online: http://pubchem.ncbi.nlm.nih.gov/compound (accessed on 30 June 2022).
- Liang, H.Y.; Chen, J.Y.; Reeves, M.; Han, B.Z. Aromatic and sensorial profiles of young Cabernet Sauvignon wines fermented by different Chinese autochthonous Saccharomyces cerevisiae strains. Food Res. Int. 2013, 51, 855–865. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.; Portolés, T.; Johnsen, L.G.; Danielsen, M.; Beltran, J. Olive oil quality classification and measurement of its organoleptic attributes by untargeted GC–MS and multivariate statistical-based approach. Food Chem. 2019, 271, 488–496. [Google Scholar] [CrossRef] [PubMed]
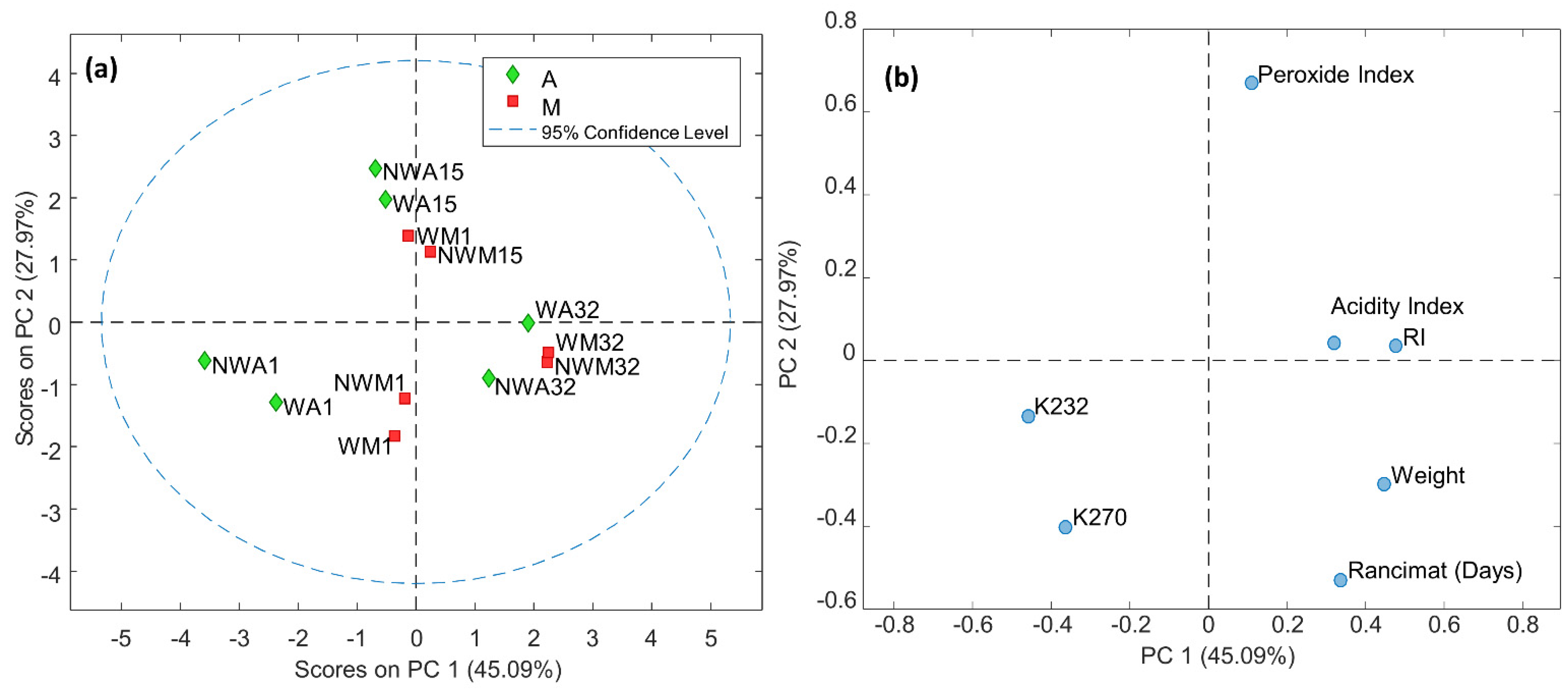
| Sampling Day | Average Weight (100 g) | Ripening Index (RI) | Acidity Index | Peroxide Index | K232 | K270 | Rancimat (Days) | ||
|---|---|---|---|---|---|---|---|---|---|
| Non-washed (NW) | Morning (M) | 1 | 320 | 0.71 | 0.23 | 2.28 | 1.78 | 0.17 | 8.60 |
| 3 | 291 | 1.1 | 0.29 | 2.46 | 2.25 | 0.18 | 8.32 | ||
| 8 | 294 | 1.74 | 0.23 | 2.89 | 1.70 | 0.16 | 6.78 | ||
| 15 | 318 | 1.82 | 0.22 | 2.85 | 1.59 | 0.16 | 6.13 | ||
| 25 | 340 | 3.22 | 0.28 | 3.71 | 2.36 | 0.10 | 7.31 | ||
| 29 | 318 | 4.08 | 0.29 | 2.46 | 1.66 | 0.12 | 7.55 | ||
| 32 | 324 | 3.22 | 0.26 | 2.48 | 1.68 | 0.15 | 9.54 | ||
| Afternoon (A) | 1 | 300 | 0.82 | 0.22 | 2.20 | 3.19 | 0.20 | 6.82 | |
| 3 | 302 | 0.49 | 0.27 | 4.51 | 1.74 | 0.20 | 8.11 | ||
| 8 | 296 | 1.58 | 0.25 | 3.33 | 1.62 | 0.16 | 9.57 | ||
| 15 | 298 | 1.45 | 0.26 | 3.31 | 1.83 | 0.16 | 5.93 | ||
| 25 | 346 | 3.37 | 0.26 | 3.71 | 2.32 | 0.11 | 7.58 | ||
| 29 | 298 | 4.04 | 0.27 | 2.47 | 1.78 | 0.12 | 8.24 | ||
| 32 | 324 | 3.22 | 0.22 | 2.50 | 1.61 | 0.17 | 8.79 | ||
| Washed (W) | Morning (M) | 1 | 320 | 0.71 | 0.22 | 2.15 | 1.66 | 0.19 | 9.04 |
| 3 | 291 | 1.1 | 0.20 | 2.91 | 2.16 | 0.23 | 8.31 | ||
| 8 | 294 | 1.74 | 0.20 | 3.26 | 1.63 | 0.17 | 6.98 | ||
| 15 | 318 | 1.82 | 0.17 | 2.85 | 1.81 | 0.13 | 6.49 | ||
| 25 | 340 | 3.22 | 0.24 | 3.33 | 2.40 | 0.09 | 7.13 | ||
| 29 | 318 | 4.08 | 0.22 | 2.49 | 1.64 | 0.13 | 7.13 | ||
| 32 | 324 | 3.22 | 0.26 | 2.48 | 1.58 | 0.14 | 9.21 | ||
| Afternoon (A) | 1 | 300 | 0.82 | 0.18 | 2.05 | 2.28 | 0.18 | 8.04 | |
| 3 | 302 | 0.49 | 0.23 | 2.88 | 2.74 | 0.18 | 7.50 | ||
| 8 | 296 | 1.58 | 0.22 | 3.71 | 1.65 | 0.18 | 6.68 | ||
| 15 | 298 | 1.45 | 0.22 | 2.87 | 1.64 | 0.14 | 6.39 | ||
| 25 | 346 | 3.37 | 0.19 | 3.30 | 4.97 | 0.09 | 6.25 | ||
| 29 | 298 | 4.04 | 0.20 | 2.47 | 1.81 | 0.13 | 5.96 | ||
| 32 | 324 | 3.22 | 0.24 | 2.43 | 1.64 | 0.13 | 8.09 |
| Day | Almond | Apple | Bitter | Fresh-Cut Grass | Green Fruity | Green Leaf | Pungency | Sweet | Tomato | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NW | M | 1 | 1.09 | 1.06 | 4.71 | 2.54 | 5.80 | 3.62 | 3.20 | 1.68 | 2.07 |
| 15 | 2.43 | 2.77 | 4.77 | 2.99 | 5.51 | 3.06 | 3.6 | 2.6 | 0 | ||
| 32 | 0 | 0 | 3.44 | 1.84 | 5.19 | 3.42 | 4.28 | 1.97 | 1.91 | ||
| A | 1 | 0 | 1.41 | 4.4 | 2.3 | 5.61 | 3.65 | 3.25 | 2.11 | 1.96 | |
| 15 | 2.04 | 2.04 | 3.54 | 2.43 | 4.91 | 2.83 | 2.80 | 2.43 | 0 | ||
| 32 | 1.4 | 0 | 3.49 | 2.35 | 5.87 | 3.46 | 4.44 | 1.99 | 1.76 | ||
| W | M | 1 | 1.79 | 2.11 | 4.18 | 2.36 | 5.77 | 3.2 | 3.84 | 2.04 | 2.22 |
| 15 | 0 | 2.27 | 4.42 | 1.55 | 5.23 | 3.03 | 3.6 | 2.57 | 0 | ||
| 32 | 2.21 | 0 | 3.33 | 2.47 | 5.19 | 2.83 | 4.57 | 2.28 | 2.57 | ||
| A | 1 | 2 | 1.99 | 4.05 | 2.37 | 5.05 | 3.36 | 3.87 | 2.11 | 1.56 | |
| 15 | 1.14 | 0 | 3.02 | 2.13 | 4.21 | 1.99 | 2.17 | 2.89 | 2.94 | ||
| 32 | 0 | 0 | 3.6 | 2.96 | 5.56 | 4.47 | 5.55 | 1.84 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura-Borrego, M.P.; Ríos-Reina, R.; Puentes-Campos, A.J.; Puentes-Campos, J.G.; Jiménez-Herrera, B.; Vallesquino-Laguna, P.; Callejón, R.M. Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils. Foods 2022, 11, 3004. https://doi.org/10.3390/foods11193004
Segura-Borrego MP, Ríos-Reina R, Puentes-Campos AJ, Puentes-Campos JG, Jiménez-Herrera B, Vallesquino-Laguna P, Callejón RM. Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils. Foods. 2022; 11(19):3004. https://doi.org/10.3390/foods11193004
Chicago/Turabian StyleSegura-Borrego, M. Pilar, Rocío Ríos-Reina, Antonio J. Puentes-Campos, Juan G. Puentes-Campos, Brígida Jiménez-Herrera, Pedro Vallesquino-Laguna, and Raquel M. Callejón. 2022. "Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils" Foods 11, no. 19: 3004. https://doi.org/10.3390/foods11193004
APA StyleSegura-Borrego, M. P., Ríos-Reina, R., Puentes-Campos, A. J., Puentes-Campos, J. G., Jiménez-Herrera, B., Vallesquino-Laguna, P., & Callejón, R. M. (2022). Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils. Foods, 11(19), 3004. https://doi.org/10.3390/foods11193004








