Design and Characterization of a Cheese Spread Incorporating Osmundea pinnatifida Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Seaweed Extract Preparation
2.2. Cheese Spread Production
2.3. Nutritional, pH, and Microbiological Parameters Evaluation
2.4. Prebiotic Activity Determination
2.5. Sample Preparation for Antidiabetic, Antihypertensive, and Antioxidant Activities
2.6. Antidiabetic Activity Determination
2.7. Antihypertensive Activity Determination
2.8. Antioxidant Activity Determination
2.8.1. ABTS Radical Scavenging Assay
2.8.2. DPPH Free Radical Scavenging Assay
2.8.3. Hydroxyl Radical Scavenging Assay
2.9. Stastical Analysis
3. Results and Discussion
3.1. Nutritional, pH, and Microbiological Parameters of Cheese Spreads
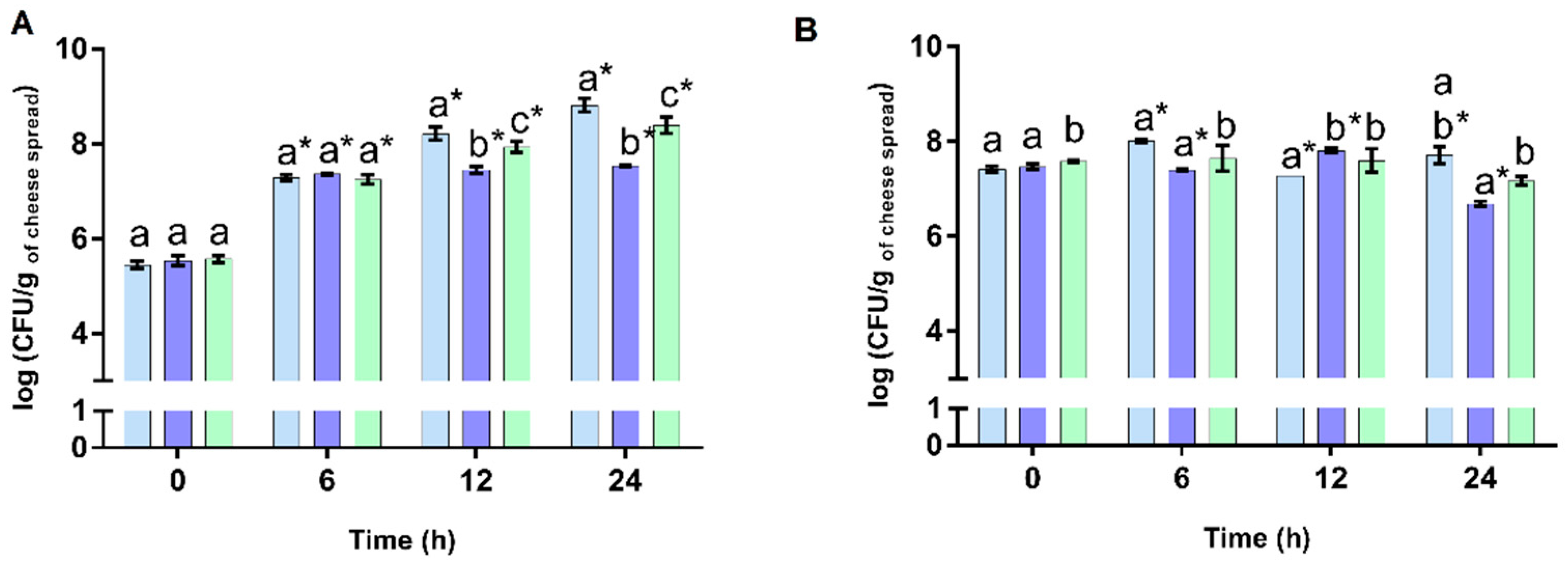
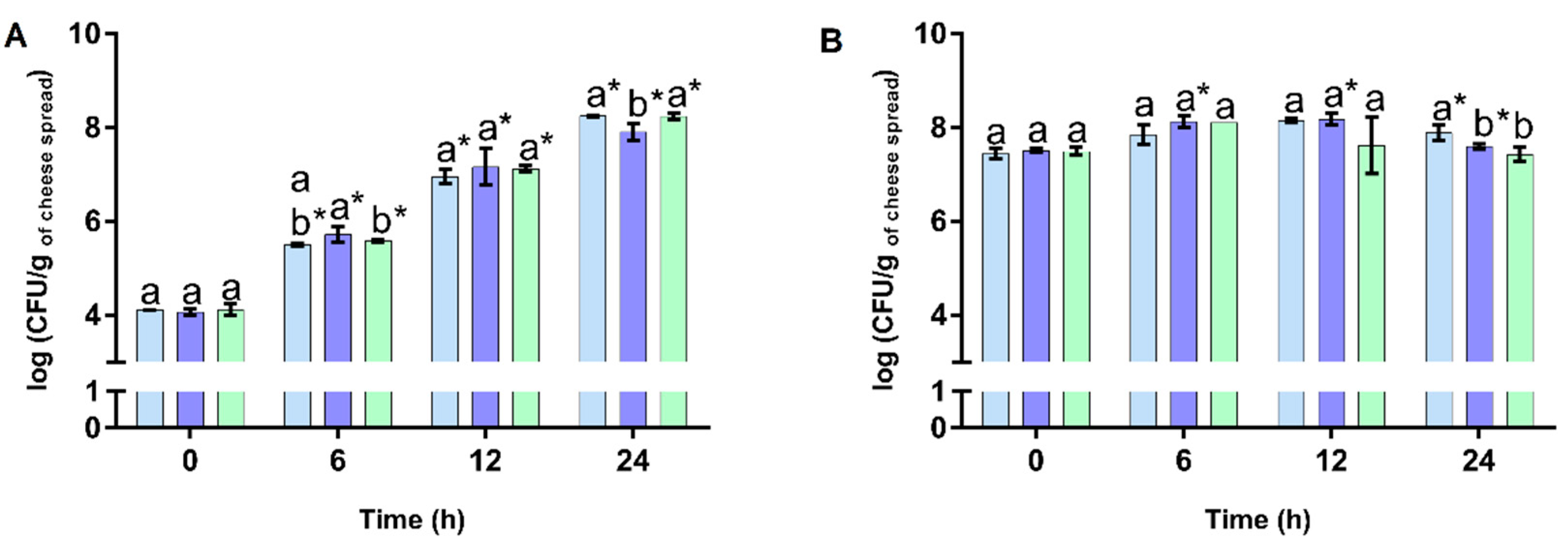
3.2. Prebiotic Activity of Cheese Spreads
3.3. Antidiabetic Activity of Cheese Spreads
3.4. Antihypertensive Activity of Cheese Spreads
3.5. Antioxidant Activity of Cheese Spreads
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Cardoso, S.; Pereira, O.; Seca, A.; Pinto, D.; Silva, A. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.; da Costa, J.P.; Silva, A.; Duarte, A.C.; et al. Sargassum muticum and Osmundea pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical Composition of Red, Brown and Green Macroalgae from Buarcos Bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of Enzyme- and Ultrasound-Assisted Extraction Methods on Biological Properties of Red, Brown, and Green Seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Biancacci, C.; McDougall, G.J.; Day, J.G.; Stanley, M.S. Cultivation of Osmundea pinnatifida (Hudson) Stackhouse in the Algem® Photobioreactor System. J. Appl. Phycol. 2022, 34, 3095–3105. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Gyawali, R.; Feng, X.; Chen, Y.P.; Lorenzo, J.M.; Ibrahim, S.A. A Review of Factors Influencing the Quality and Sensory Evaluation Techniques Applied to Greek Yogurt. J. Dairy Res. 2022, 89, 213–219. [Google Scholar] [CrossRef]
- Garcia, M.M.E.; Pereira, C.J.D.; Freitas, A.C.; Gomes, A.M.P.; Pintado, M.M.E. Development and Characterization of a Novel Sustainable Probiotic Goat Whey Cheese Containing Second Cheese Whey Powder and Stabilized with Thyme Essential Oil and Sodium Citrate. Foods 2022, 11, 2698. [Google Scholar] [CrossRef]
- Pontonio, E.; Montemurro, M.; de Gennaro, G.V.; Miceli, V.; Rizzello, C.G. Antihypertensive Peptides from Ultrafiltration and Fermentation of the Ricotta Cheese Exhausted Whey: Design and Characterization of a Functional Ricotta Cheese. Foods 2021, 10, 2573. [Google Scholar] [CrossRef]
- Madureira, A.R.; Soares, J.C.; Pintado, M.E.; Gomes, A.M.P.; Freitas, A.C.; Malcata, F.X. Sweet Whey Cheese Matrices Inoculated with the Probiotic Strain Lactobacillus paracasei LAFTI® L26. Dairy Sci. Technol. 2008, 88, 649–665. [Google Scholar] [CrossRef]
- Madureira, A.R.; Soares, J.C.; Amorim, M.; Tavares, T.; Gomes, A.M.; Pintado, M.M.; Malcata, F.X. Bioactivity of Probiotic Whey Cheese: Characterization of the Content of Peptides and Organic Acids. J. Sci. Food Agric. 2013, 93, 1458–1465. [Google Scholar] [CrossRef]
- Pintado, M.E.; Malcata, F.X. Characterization of Whey Cheese Packaged under Vacuum. J. Food Prot. 2000, 63, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.; del Nobile, M.; Conte, A. Fruit and Vegetable By-Products to Fortify Spreadable Cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, O.; Argyri, K.; Loukas, T.; Magkoutis, A.; Biagki, T.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Bioactivity of a Spread Cheese Enriched with Mountain Tea and Orange Peel Extract in Plasma Oxidative Stress Status, Serum Lipids and Glucose Levels: An Interventional Study in Healthy Adults. Biomolecules 2021, 11, 1241. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In Vitro Studies of Eggplant (Solanum Melongena) Phenolics as Inhibitors of Key Enzymes Relevant for Type 2 Diabetes and Hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. A Rapid, Simple and Sensitive Fluorescence Method for the Assay of Angiotensin-I Converting Enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Quirós, A.; Contreras, M.d.M.; Ramos, M.; Amigo, L.; Recio, I. Stability to Gastrointestinal Enzymes and Structure-Activity Relationship of β-Casein-Peptides with Antihypertensive Properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese Medicinal Plants: Dependence of Final Antioxidant Capacity and Phenol Content on Extraction Features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, Purification and Preliminary Characterization of Sulfated Polysaccharides from Sargassum plagiophyllum and Its In Vitro Anticancer and Antioxidant Activity. Process Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Sudha, G.; Priya, M.S.; Shree, R.I.; Vadivukkarasi, S. In Vitro Free Radical Scavenging Activity of Raw Pepino Fruit (Solanum muricatum Aiton). Int. J. Curr. Pharm. Res. 2011, 3, 137–140. [Google Scholar]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.S.; Kabisch, J.; Böhnlein, C.; Franz, C.M.A.P. Microbial Quality and Safety of Milk and Milk Products in the 21st Century. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2013–2049. [Google Scholar] [CrossRef]
- Soni, A.; Samuelsson, L.M.; Loveday, S.M.; Gupta, T.B. Applications of Novel Processing Technologies to Enhance the Safety and Bioactivity of Milk. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4652–4677. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; del Carmen Mondragon, A.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In Vitro Fermentation and Prebiotic Potential of Novel Low Molecular Weight Polysaccharides Derived from Agar and Alginate Seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of Type 2 Diabetes: An Update and Future Directions. Metabolism 2022, 137, 155332. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Paul Ross, R. The α-Amylase and α-Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-Glucosidase Activity by Selected Edible Seaweeds and Fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Fu, X. Screening α-Glucosidase Inhibitors from Four Edible Brown Seaweed Extracts by Ultra-Filtration and Molecular Docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Lee, K.-W.; Jeon, Y.-J. Screening of Extracts from Red Algae in Jeju for Potentials MarineAngiotensin—I Converting Enzyme (ACE) Inhibitory Activity. Algae 2006, 21, 343–348. [Google Scholar] [CrossRef]
- Cho, C.H.; Lu, Y.A.; Kim, M.Y.; Jeon, Y.J.; Lee, S.H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, Y.-S.; Jeon, Y.-J. Seaweeds and Their Natural Products for Preventing Cardiovascular Associated Dysfunction. Mar. Drugs 2021, 19, 507. [Google Scholar] [CrossRef]
- Olivares-Molina, A.; Fernández, K. Comparison of Different Extraction Techniques for Obtaining Extracts from Brown Seaweeds and Their Potential Effects as Angiotensin I-Converting Enzyme (ACE) Inhibitors. J. Appl. Phycol. 2016, 28, 1295–1302. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring Bioactive Fraction of Sargassum wightii: In Vitro Elucidation of Angiotensin-I-Converting Enzyme Inhibition and Antioxidant Potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Antioxidative Sulphated Polygalactans from Marine Macroalgae as Angiotensin-I Converting Enzyme Inhibitors. Nat. Prod. Res. 2018, 32, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and Functional Bioactivity Value of Selected Azorean Macroalgae: Ulva Compressa, Ulva Rigida, Gelidium Microdon, and Pterocladiella Capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant Content and Activity of the Seaweed Saccharina Latissima: A Seasonal Perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–221. [Google Scholar]


| Samples | ACE Inhibition Percentage (%) 1 |
|---|---|
| O. pinnatifida extract | 68.04 ± 3.70 |
| CSExt at day 0 of storage | 57.40 ± 1.52 |
| CSExt after 21 days of storage | 50.50 ± 0.28 |
| Cheese Spread | Storage (Days) | ABTS Radical Scavenging Activity (%) 1 | DPPH Free Radical Scavenging Activity (%) 1 | Hydroxyl Radical Scavenging Activity (%) 1 |
|---|---|---|---|---|
| CSExt | 0 | 14.34 ± 0.94 a | 4.47 ± 0.53 a | 74.71 ± 0.75 a |
| 21 | 14.35 ± 0.46 a | 3.68 ± 0.58 a,* | 61.47 ± 1.90 a,* | |
| CSFos | 0 | 9.91 ± 0.16 b | 3.20 ± 0.33 b | 70.59 ± 1.57 c |
| 21 | 10.81 ± 0.17 b | 4.85 ± 0.51 b,* | 44.56 ± 0.01 c,* | |
| CS | 0 | 10.15 ± 0.64 b | 2.78 ± 0.41 b | 68.07 ± 0.60 b |
| 21 | 10.99 ± 0.36 b | 4.53 ± 0.24 b | 42.09 ± 0.41 b,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustino, M.; Machado, D.; Rodrigues, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Design and Characterization of a Cheese Spread Incorporating Osmundea pinnatifida Extract. Foods 2023, 12, 611. https://doi.org/10.3390/foods12030611
Faustino M, Machado D, Rodrigues D, Andrade JC, Freitas AC, Gomes AM. Design and Characterization of a Cheese Spread Incorporating Osmundea pinnatifida Extract. Foods. 2023; 12(3):611. https://doi.org/10.3390/foods12030611
Chicago/Turabian StyleFaustino, Margarida, Daniela Machado, Dina Rodrigues, José Carlos Andrade, Ana Cristina Freitas, and Ana Maria Gomes. 2023. "Design and Characterization of a Cheese Spread Incorporating Osmundea pinnatifida Extract" Foods 12, no. 3: 611. https://doi.org/10.3390/foods12030611
APA StyleFaustino, M., Machado, D., Rodrigues, D., Andrade, J. C., Freitas, A. C., & Gomes, A. M. (2023). Design and Characterization of a Cheese Spread Incorporating Osmundea pinnatifida Extract. Foods, 12(3), 611. https://doi.org/10.3390/foods12030611












