Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Procedures
2.1.1. Sample Preparation
2.1.2. Ultrasound-Assisted Extraction (UAE)
2.2. Film Forming Solutions (FFS) and Film Preparation
2.3. Determination of Total Phenolic Content (TPC)
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
2.6. Migration of Bioactive Compounds
2.7. Physico-Chemical Properties of Films
2.7.1. Thickness
2.7.2. Water Vapour Permeability (WVP)
2.7.3. Colour and Opacity
2.7.4. Moisture Content and Water Solubility
2.8. Mechanical Properties
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Scanning Electron Microscopy (SEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield of Extracts
3.2. Total Phenolic Compounds and Antioxidant Activity of Extracts
3.3. Antimicrobial Activity of the Extracts
3.4. Antimicrobial Activity of Films
3.5. Migration of Bioactive Compounds
3.6. Physico-Chemical Properties of the Films
3.7. Mechanical Properties
3.8. Fourier Transform Infrared Spectroscopy (FTIR)
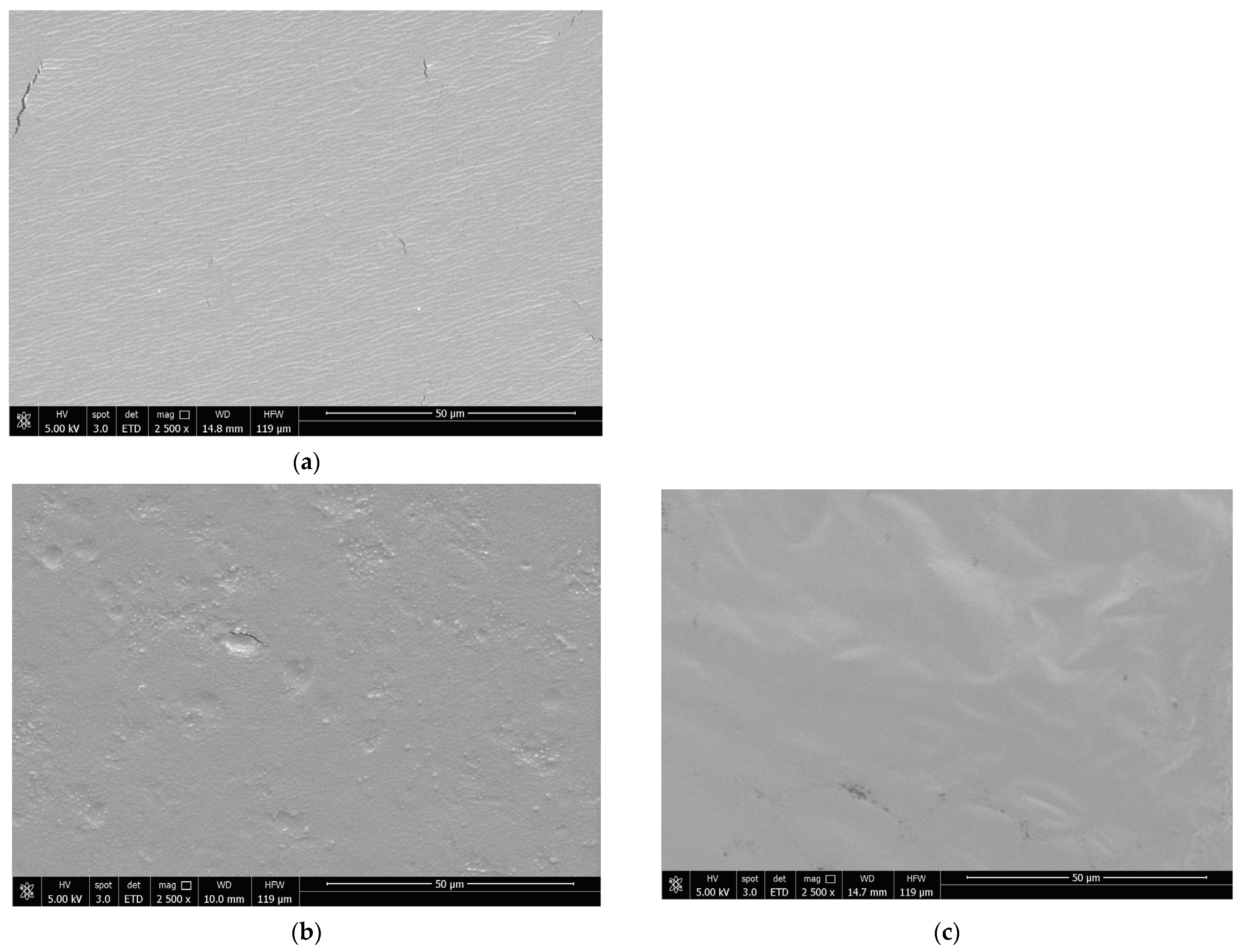
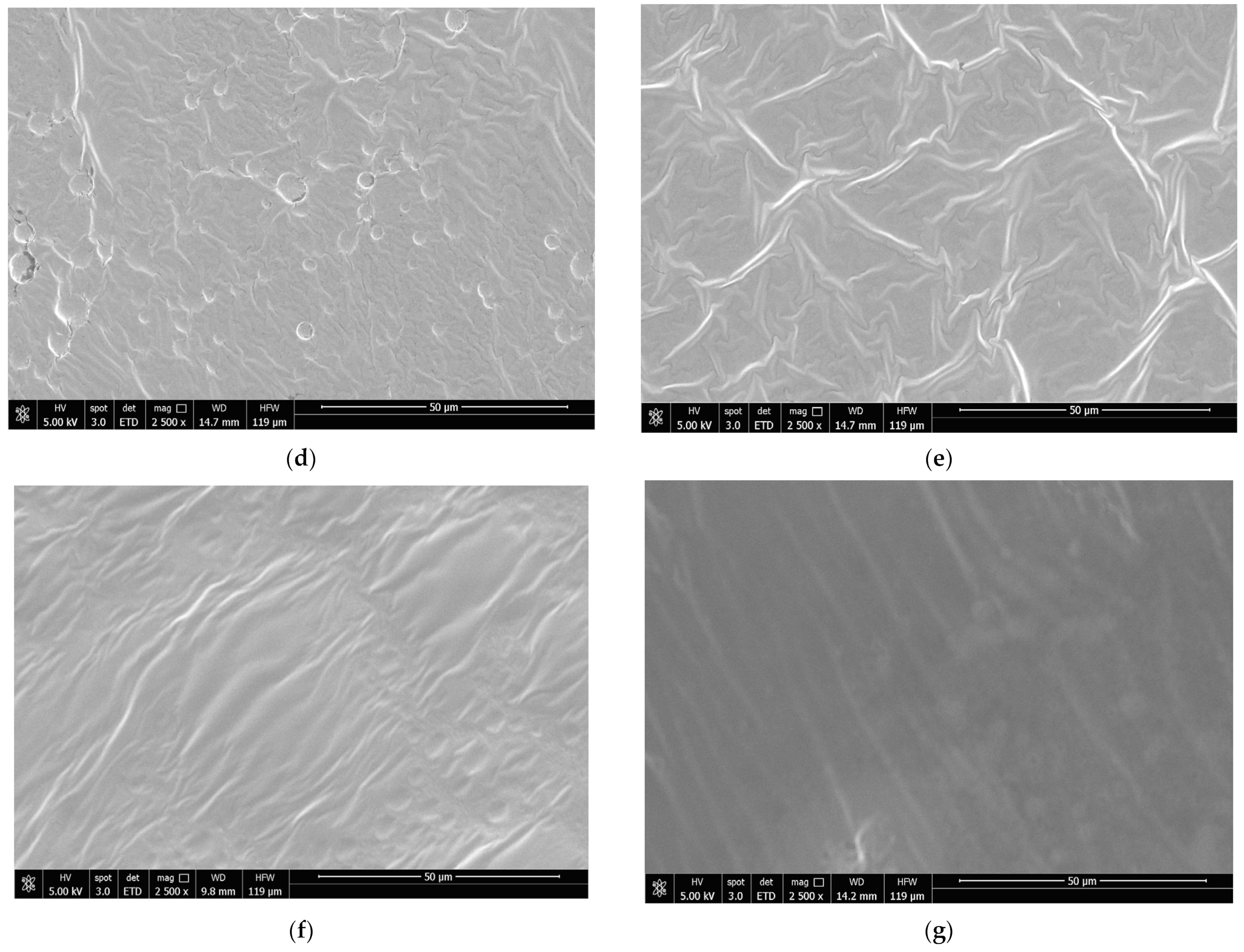
3.9. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Kola, V.; Carvalho, I.S. Plant extracts as additives in biodegradable films and coatings in active food packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Active Edible Packaging. Encyclopedia 2021, 1, 360–370. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Code for Federal Regulations Title 21 Part 184—Direct Food Substances Affirmed as Generally Recognized as Safe. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1724 (accessed on 20 July 2023).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA J. 2017, 15, e05049. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Capar, T.D. Characterization of sodium alginate-based biodegradable edible film incorporated with Vitis vinifera leaf extract: Nano-scaled by ultrasound-assisted technology. Food Packag. Shelf Life 2023, 37, 101068. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E. Developing multifunctional edible coatings based on alginate for active food packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef]
- Aala, J.; Ahmadi, M.; Golestan, L.; Shahidi, S.-A.; Shariatifar, N. Effect of multifactorial free and liposome-coated of bay laurel (Laurus nobilis) and rosemary (Salvia rosmarinus) extracts on the behavior of Listeria monocytogenes and Vibrio parahaemolyticus in silver carp (Hypophthalmichthys molitrix) stored at 4 °C. Environ. Res. 2023, 216, 114478. [Google Scholar] [CrossRef]
- Akcan, T.; Estévez, M.; Serdaroğlu, M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis. LWT 2017, 77, 323–331. [Google Scholar] [CrossRef]
- Sales, H.; Nunes, J.; Vaz Patto, M.C. Achievements and Challenges towards a Sustainable Conservation and Use of ‘Galega vulgar’ Olea europaea Variety. Agronomy 2020, 10, 1467. [Google Scholar] [CrossRef]
- De la Casa, J.A.; Bueno, J.S.; Castro, E. Recycling of residues from the olive cultivation and olive oil production process for manufacturing of ceramic materials. A comprehensive review. J. Clean. Prod. 2021, 296, 126436. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Chalapud, M.C.; Carrín, M.E. Ultrasound-assisted extraction of oilseeds—Sustainability processes to obtain traditional and non-traditional food ingredients: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2161–2196. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active edible films with plant extracts: A updated review of their types, preparations, reinforcing properties, and applications in muscle foods packaging and preservation. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2018, 109, 1219–1231. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of active films utilizing antioxidant compounds obtained from tomato and lemon by-products for use in food packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- ASTM E96/E96M-10; American Society for Testing and Materials: Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2013. [CrossRef]
- ASTM D882; Standard Test Methods of Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002.
- Inés Molina, R.D.; Campos-Silva, R.; Díaz, M.A.; Macedo, A.J.; Blázquez, M.A.; Alberto, M.R.; Arena, M.E. Laurel extracts inhibit Quorum sensing, virulence factors and biofilm of foodborne pathogens. LWT 2020, 134, 109899. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Mechanochemical extraction of antioxidant phenolic compounds from Mediterranean and medicinal Laurus nobilis: A comparative study with other traditional and green novel techniques. Ind. Crops Prod. 2019, 141, 111805. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Oudjedi, K.; Manso, S.; Nerin, C.; Hassissen, N.; Zaidi, F. New active antioxidant multilayer food packaging films containing Algerian Sage and Bay leaves extracts and their application for oxidative stability of fried potatoes. Food Control 2019, 98, 216–226. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods 2019, 8, 579. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr Opin Microbiol 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Salama, N.R. The gram-negative bacterial periplasm: Size matters. PLoS Biol. 2018, 16, e2004935. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef]
- M26-A; Methods of Determining Bactericidal Activity of Antimicrobial Agents. CLSI, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999.
- Cui, Y.; Zhang, R.; Cheng, M.; Guo, Y.; Wang, X. Sustained release and antioxidant activity of active potato starch packaging films encapsulating thymol with MCM-41. LWT 2023, 173, 114342. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef]
- Saroat, R. Edible Films Incorporated with Active Compounds: Their Properties and Application. In Active Antimicrobial Food Packaging; Işıl, V., Sinan, U., Eds.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 5. [Google Scholar]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga–España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A novel biodegradable film based on κ-carrageenan activated with olive leaves extract. Food Sci. Nutr. 2020, 8, 3147–3156. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.-X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin films for enhancing the quality of cold-smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract. Polymers 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Oliveira, R.A.d.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Rosa, A.D.; Secco, M.C.; De Cezaro, A.M.; Fischer, B.; Cansian, R.L.; Junges, A.; Franceschi, E.; Backes, G.T.; Valduga, E. Encapsulation of olive leaf (Olea europaea) extract using solution-enhanced dispersion by supercritical fluids (SEDS) technique. J. Supercrit. Fluids 2023, 198, 105922. [Google Scholar] [CrossRef]
- Azar, F.A.N.; Pezeshki, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M.; Hamdipour, S.; Daliri, H. Pectin-sodium caseinat hydrogel containing olive leaf extract-nano lipid carrier: Preparation, characterization and rheological properties. LWT 2021, 148, 111757. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Oliveira, R.M.; Gehrmann Buchweitz, L.T.; Pereira, J.R.; Cristina dos Santos Hackbart, H.; Nalério, É.S.; Borges, C.D.; Zambiazi, R.C. Encapsulation of olive leaf extract (Olea europaea L.) in gelatin/tragacanth gum by complex coacervation for application in sheep meat hamburger. Food Control 2022, 131, 108426. [Google Scholar] [CrossRef]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Shama, S.; Zhao, Y.; Sameen, D.E.; He, L.; Liu, Y. Investigation of Ultrasonic Treatment on Physicochemical, Structural and Morphological Properties of Sodium Alginate/AgNPs/Apple Polyphenol Films and Its Preservation Effect on Strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Nascimento, K.M.; Cavalheiro, J.B.; Monge Netto, A.Á.; da Silva Scapim, M.R.; Bergamasco, R.d.C. Properties of alginate films incorporated with free and microencapsulated Stryphnodendron adstringens extract (barbatimão). Food Packag. Shelf Life 2021, 28, 100637. [Google Scholar] [CrossRef]
- Aydin, G.; Zorlu, E.B. Characterisation and Antibacterial Properties of Novel Biodegradable Films Based on Alginate and Roselle (Hibiscus sabdariffa L.) Extract. Waste Biomass Valorization 2022, 13, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
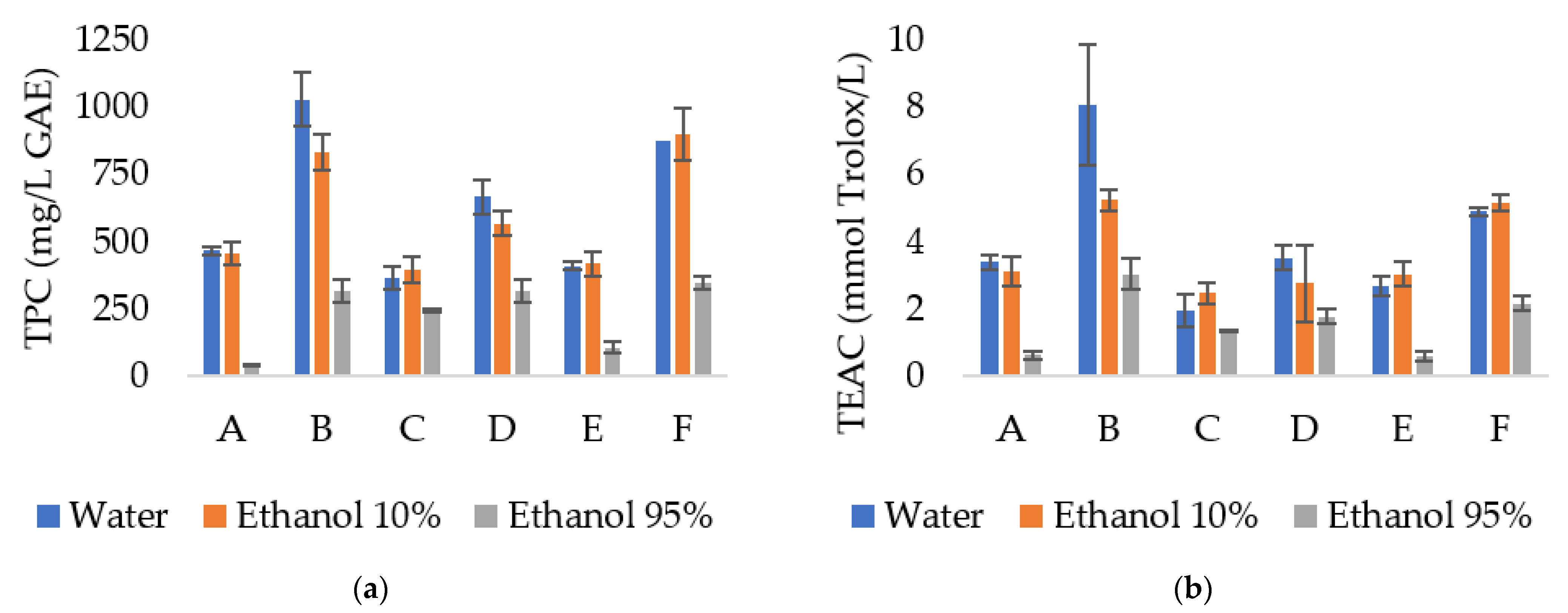
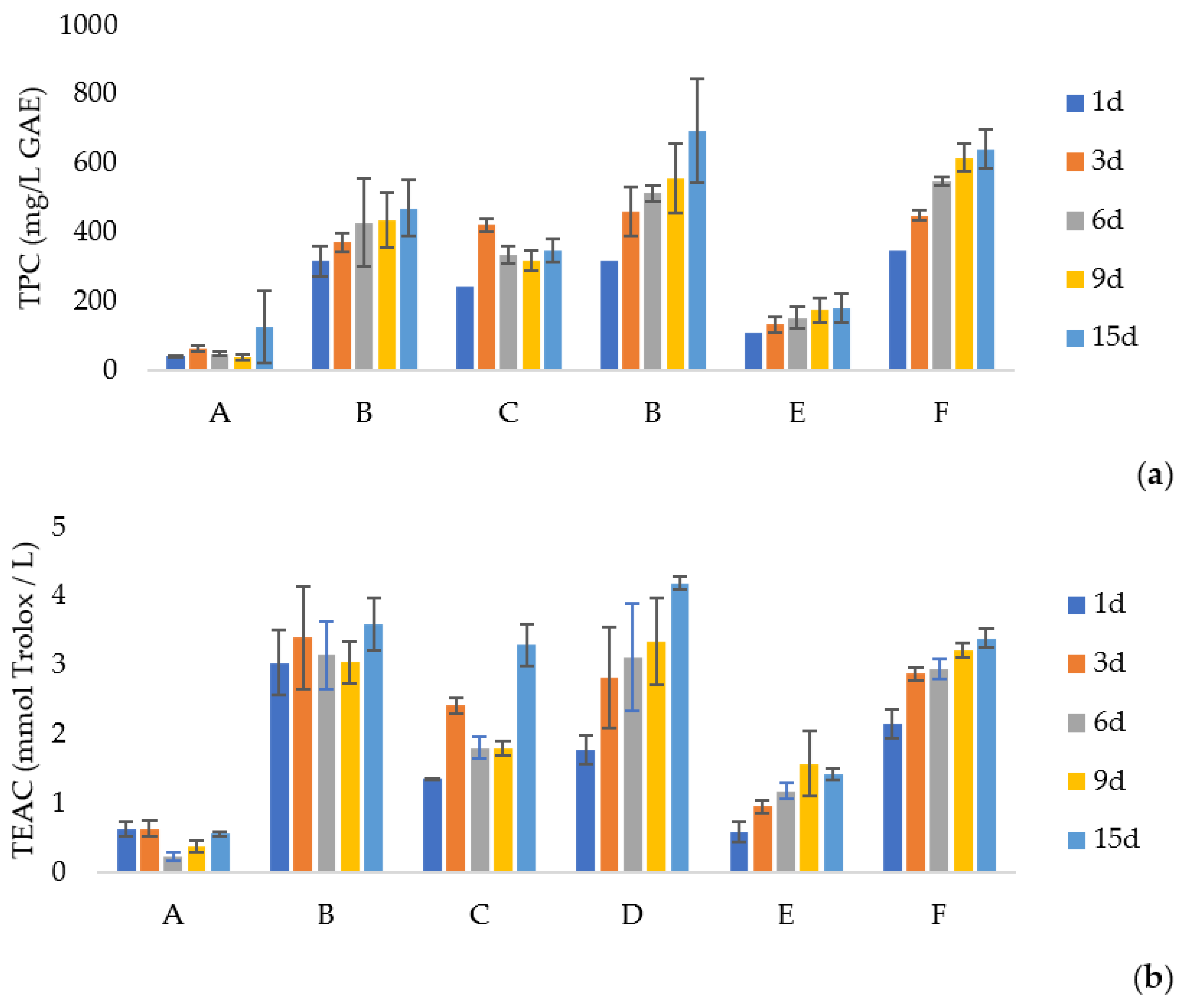
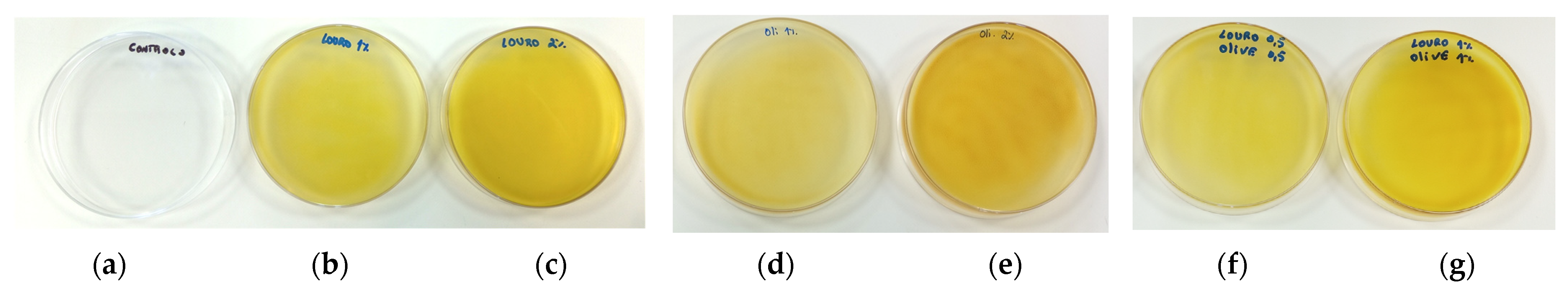
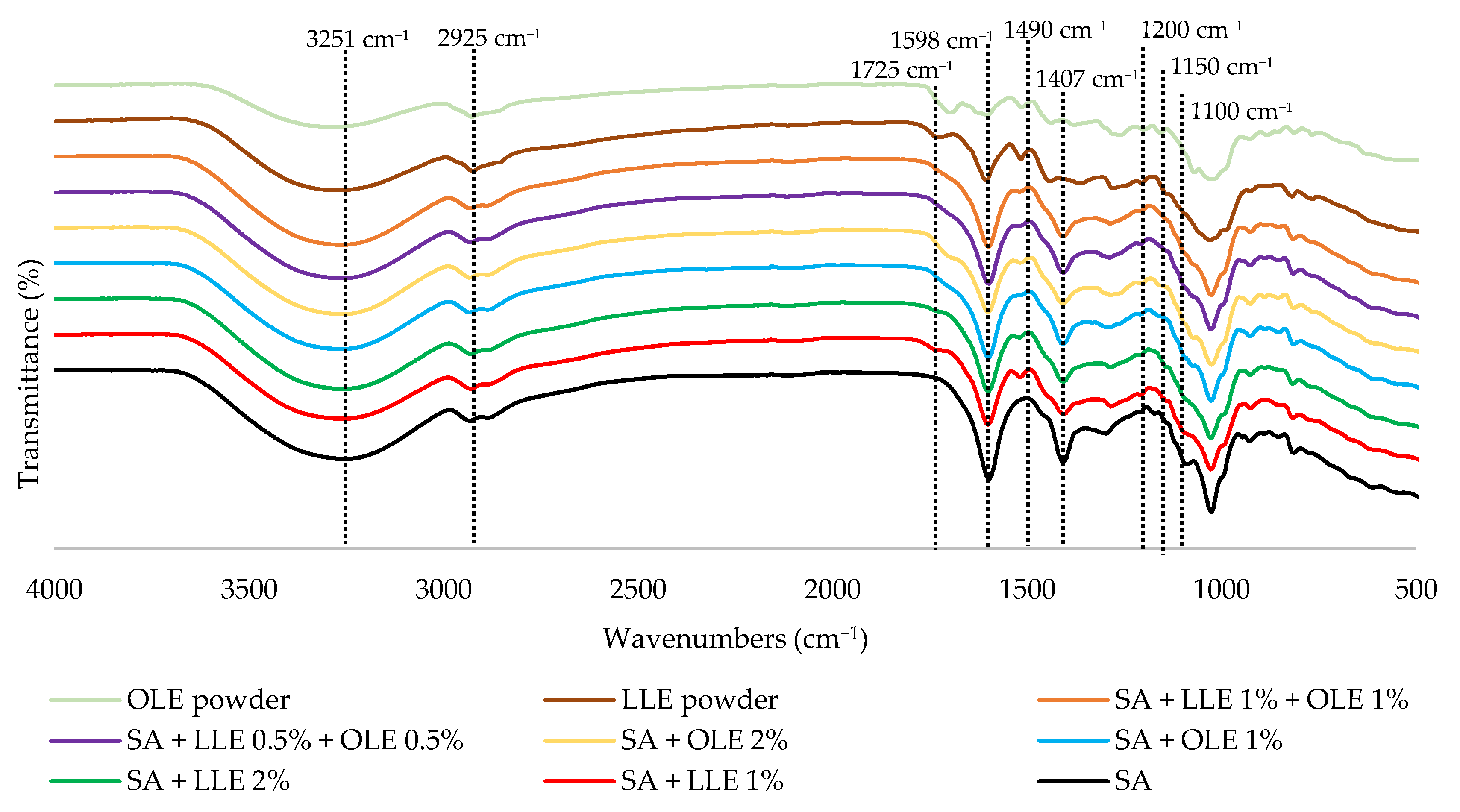
| Sample | TPC (mg GAE/g Extract) | TEAC (mmol TE/g Extract) | IC50 (mg/mL) |
|---|---|---|---|
| LLE | 195.75 ± 10.90 a | 2.07 ± 0.21 a | 0.74 ± 0.00 c |
| OLE | 147.04 ± 1.80 b | 0.78 ± 0.02 c | 1.77 ± 0.08 a |
| LLE + OLE | 179.13 ± 2.81 a | 1.13 ± 0.06 c | 0.98 ± 0.03 b |
| BHT | nd | 1.53 ± 0.16 b | 0.56 ± 0.04 d |
| p | <0.01 | <0.001 | <0.001 |
| Sample | S. aureus | L. monocytogenes | E. faecalis | S. Typhimurium | E. coli |
|---|---|---|---|---|---|
| BHI 0 h | 7.26 ± 0.06 c | 7.32 ± 0.03 c | 7.19 ± 0.05 d | 7.09 ± 0.04 c | 7.25 ± 0.08 f |
| BHI 24 h | 10.66 ± 0.24 a | 10.48 ± 0.19 a | 10.17 ± 0.09 a | 10.68 ± 0.05 a | 12.05 ± 0.02 a |
| SA | 10.70 ± 0.06 a | 9.93 ± 0.13 ab | 10.02 ± 0.14 ab | 10.77 ± 0.03 a | 12.05 ± 0.03 a |
| SA + LLE 1% | 7.50 ± 0.25 c | 6.39 ± 0.52 cd | 9.28 ± 0.37 bc | 9.76 ± 0.14 b | 11.25 ± 0.15 d |
| SA + LLE 2% | 6.31 ± 0.23 d | 5.58 ± 0.66 d | 7.41 ± 0.31 d | 9.22 ± 0.46 b | 11.03 ± 0.10 e |
| SA + OLE 1% | 9.20 ± 0.14 b | 9.96 ± 0.17 ab | 9.85 ± 0.02 abc | 9.86 ± 0.01 b | 11.59 ± 0.17 b |
| SA + OLE 2% | 9.35 ± 0.09 b | 10.02 ± 0.06 ab | 9.52 ± 0.24 abc | 9.69 ± 0.20 b | 11.37 ± 0.05 cd |
| SA + LLE 0.5% + OLE 0.5% | 6.00 ± 0.26 de | 9.55 ± 0.27 ab | 9.65 ± 0.02 abc | 9.51 ± 0.06 b | 11.45 ± 0.11 bc |
| SA + LLE 1% + OLE 1% | 5.31 ± 0.05 e | 8.84 ± 0.19 b | 9.03 ± 0.33 c | 9.35 ± 0.04 b | 11.20 ± 0.05 de |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Film | Moisture Content (%) | WVP × 10−11 (g m−1 s−1 Pa−1) | L* | a* | b* | ΔE | Opacity (%) |
|---|---|---|---|---|---|---|---|
| PLA | nd | 87.7 ± 0.10 a | 96.77 ± 0.07 a | 0.19 ± 0.02 c | 2.01 ± 0.07 e | 0.87 ± 0.09 f | 9.28 ± 0.10 e |
| SA | 31.55 ± 3.82 a | 22.08 ± 1.13 a | 96.50 ± 0.38 a | 0.16 ± 0.06 c | 3.20 ± 0.37 e | 1.84 ± 0.47 f | 4.33 ± 0.93 f |
| SA + OLE 1% | 23.69 ± 2.23 b | 6.49 ± 0.27 b | 77.14 ± 2.92 d | 4.24 ± 1.71 b | 46.20 ± 1.73 d | 49.22 ± 1.92 e | 11.74 ± 3.12 d |
| SA + OLE 2% | 19.36 ± 2.43 cd | 4.29 ± 0.16 cd | 68.38 ± 4.01 f | 9.89 ± 2.01 a | 58.12 ± 3.58 b | 64.43 ± 2.70 c | 13.37 ± 2.17 bcd |
| SA + LLE 1% | 15.59 ± 2.05 d | 5.20 ± 0.11 bc | 85.92 ± 1.28 b | −5.24 ± 0.31 e | 52.60 ± 4.45 c | 52.46 ± 4.56 d | 11.93 ± 1.45 cd |
| SA + LLE 2% | 15.07 ± 1.97 d | 3.53 ± 0.09 cd | 73.82 ± 2.10 e | 5.00 ± 1.02 b | 71.47 ± 1.50 a | 73.87 ± 1.41 a | 16.43 ± 1.53 a |
| SA + OLE 0.5% + LLE 0.5% | 20.35 ± 3.23 bc | 6.08 ± 0.32 b | 81.53 ± 2.10 c | 1.34 ± 1.56 c | 52.80 ± 4.46 c | 53.58 ± 4.67 d | 14.00 ± 2.17 bc |
| SA + OLE 1% + LLE 1% | 17.47 ± 3.45 cd | 3.49 ± 0.11 d | 77.87 ± 2.94 d | −2.72 ± 1.24 d | 68.92 ± 2.18 a | 70.15 ± 2.19 b | 14.14 ± 1.65 b |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Film | Thickness (µm) | Tensile Strength (MPa) | Elongation at Break (%) | Modulus of Elasticity (Mpa) |
|---|---|---|---|---|
| PLA | 50.00 ± 1.09 e | |||
| SA | 63.67 ± 9.36 de | 14.27 ± 5.38 a | 40.89 ± 7.37 b | 17.65 ± 1.31 c |
| SA + OLE 1% | 97.06 ± 8.76 bc | 11.75 ± 2.04 a | 48.65 ± 3.78 ab | 11.44 ± 0.74 d |
| SA + OLE 2% | 137.37 ± 15.55 a | 9.63 ± 1.39 ab | 54.39 ± 3.94 a | 8.00 ± 0.65 e |
| SA + LLE 1% | 83.77 ± 10.96 cd | 10.27 ± 2.86 a | 29.40 ± 6.23 c | 27.32 ± 2.84 b |
| SA + LLE 2% | 119.03 ± 12.43 ab | 4.91 ± 1.18 c | 25.59 ± 6.04 cd | 15.18 ± 1.42 c |
| SA + OLE 0.5% + LLE 0.5% | 90.47 ± 11.55 c | 5.46 ± 0.96 bc | 16.83 ± 3.65 d | 32.97 ± 3.27 a |
| SA + OLE 1% + LLE 1% | 119.37 ± 18.87 ab | 4.90 ± 1.37 c | 28.86 ± 5.32 c | 11.74 ± 1.17 d |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura-Alves, M.; Souza, V.G.L.; Silva, J.A.; Esteves, A.; Pastrana, L.M.; Saraiva, C.; Cerqueira, M.A. Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology. Foods 2023, 12, 4076. https://doi.org/10.3390/foods12224076
Moura-Alves M, Souza VGL, Silva JA, Esteves A, Pastrana LM, Saraiva C, Cerqueira MA. Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology. Foods. 2023; 12(22):4076. https://doi.org/10.3390/foods12224076
Chicago/Turabian StyleMoura-Alves, Márcio, Victor Gomes Lauriano Souza, Jose A. Silva, Alexandra Esteves, Lorenzo M. Pastrana, Cristina Saraiva, and Miguel A. Cerqueira. 2023. "Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology" Foods 12, no. 22: 4076. https://doi.org/10.3390/foods12224076
APA StyleMoura-Alves, M., Souza, V. G. L., Silva, J. A., Esteves, A., Pastrana, L. M., Saraiva, C., & Cerqueira, M. A. (2023). Characterization of Sodium Alginate-Based Films Blended with Olive Leaf and Laurel Leaf Extracts Obtained by Ultrasound-Assisted Technology. Foods, 12(22), 4076. https://doi.org/10.3390/foods12224076










