Effect of High-Hydrostatic-Pressure Treatment on the Physicochemical Properties of Kafirin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Kafirin
2.3. HHP Treatment of Kafirin
2.4. Solubility
2.5. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Ultraviolet Spectrum
2.7. Endogenous Fluorescence Spectrum
2.8. Particle-Size Distribution
2.9. Particle Size and Zeta Potential
2.10. Fourier Transform Infrared (FTIR) Spectrum
2.11. Differential Scanning Calorimetry (DSC)
2.12. Surface and Total Free Sulfhydryl Group Content and Disulfide-Bond Content
2.13. Scanning Electron Microscope (SEM)
2.14. Statistical Analysis
3. Results and Discussion
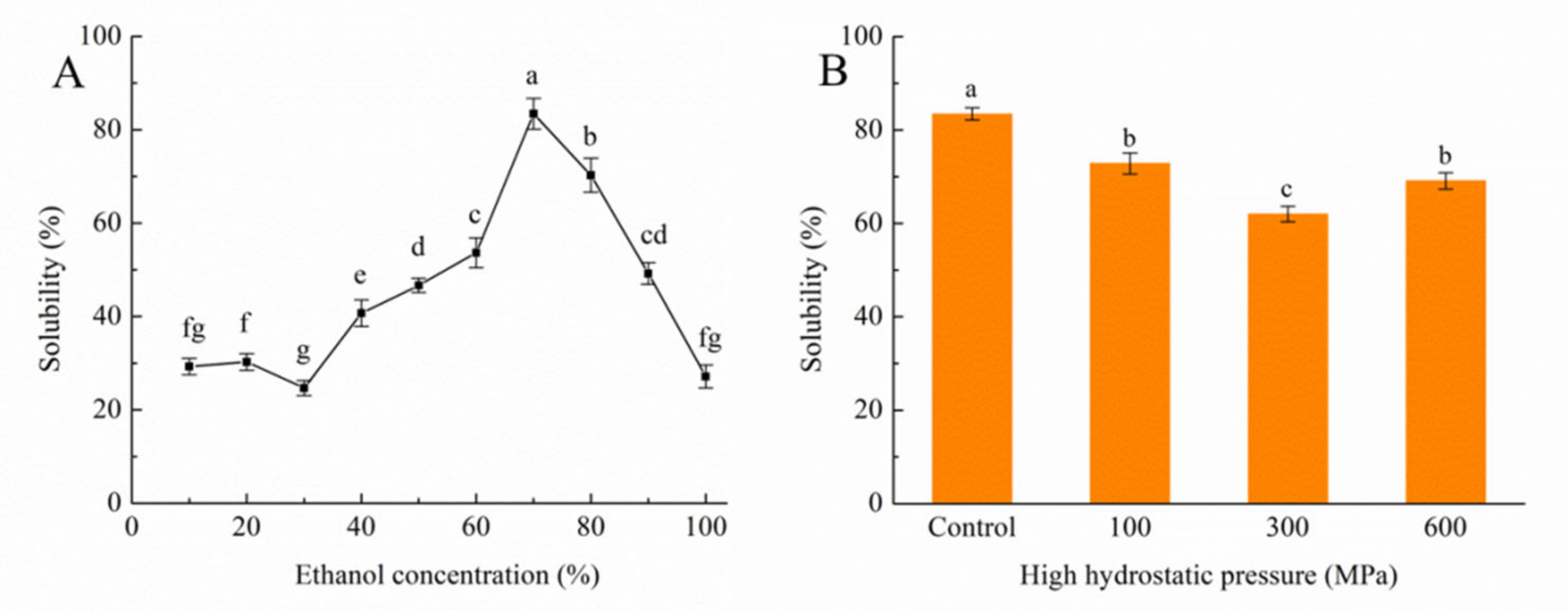
3.1. Impact of the HHP Treatment on Solubility
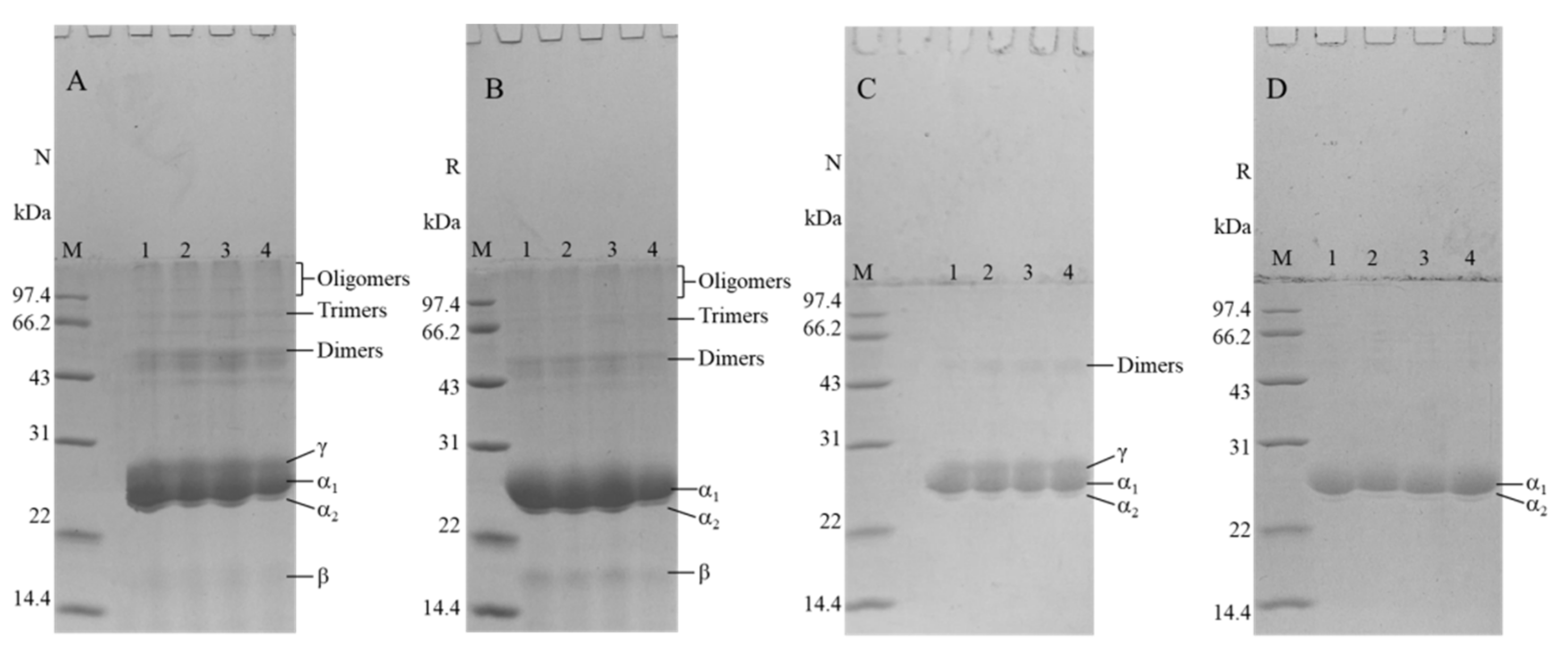
3.2. Impact of the HHP Treatment on SDS-PAGE
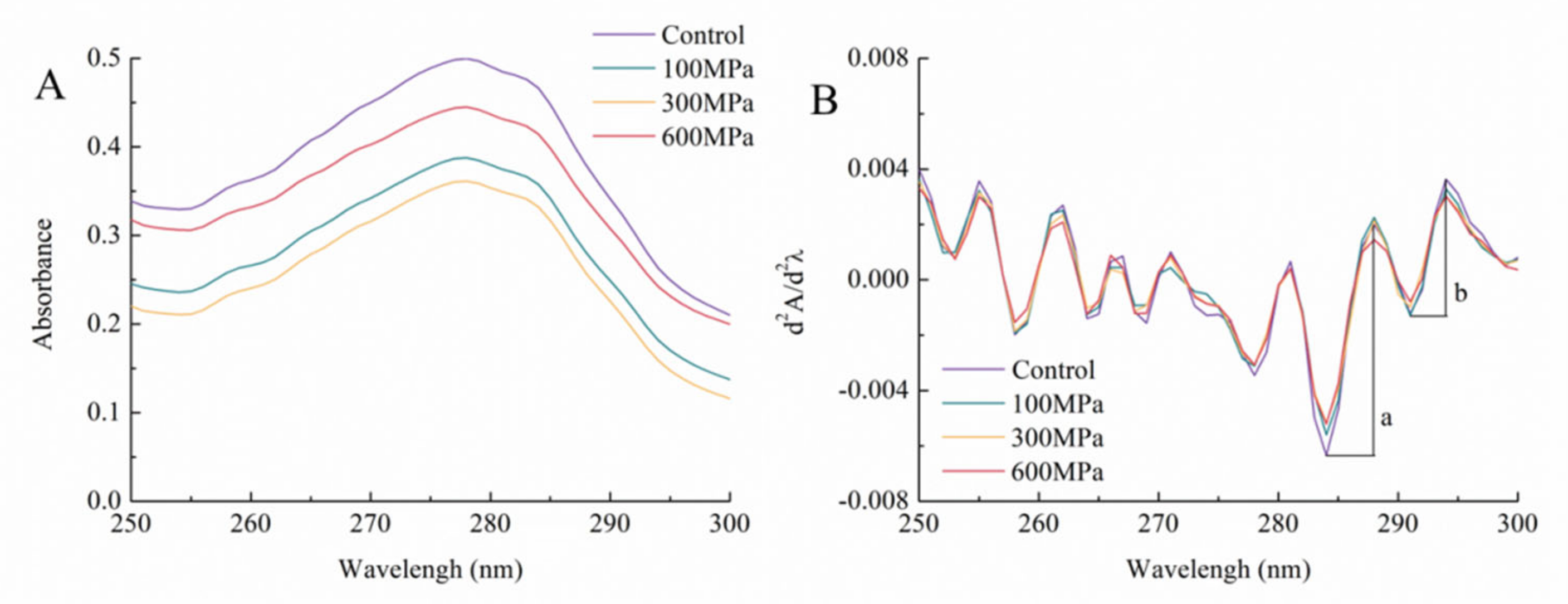
3.3. Impact of the HHP Treatment on the Ultraviolet Spectrum
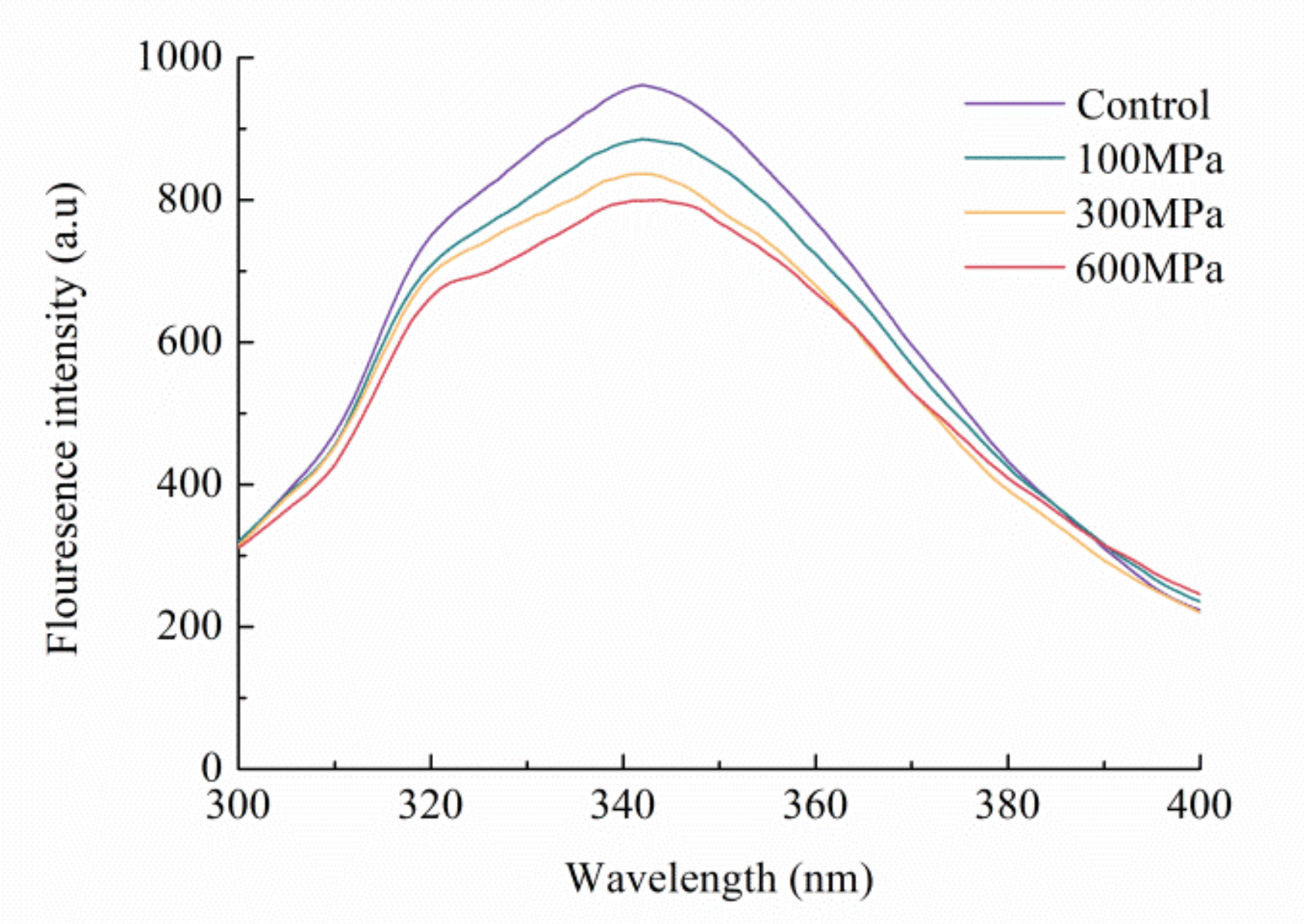
3.4. Impact of the HHP Treatment on the Endogenous Fluorescence Spectrum
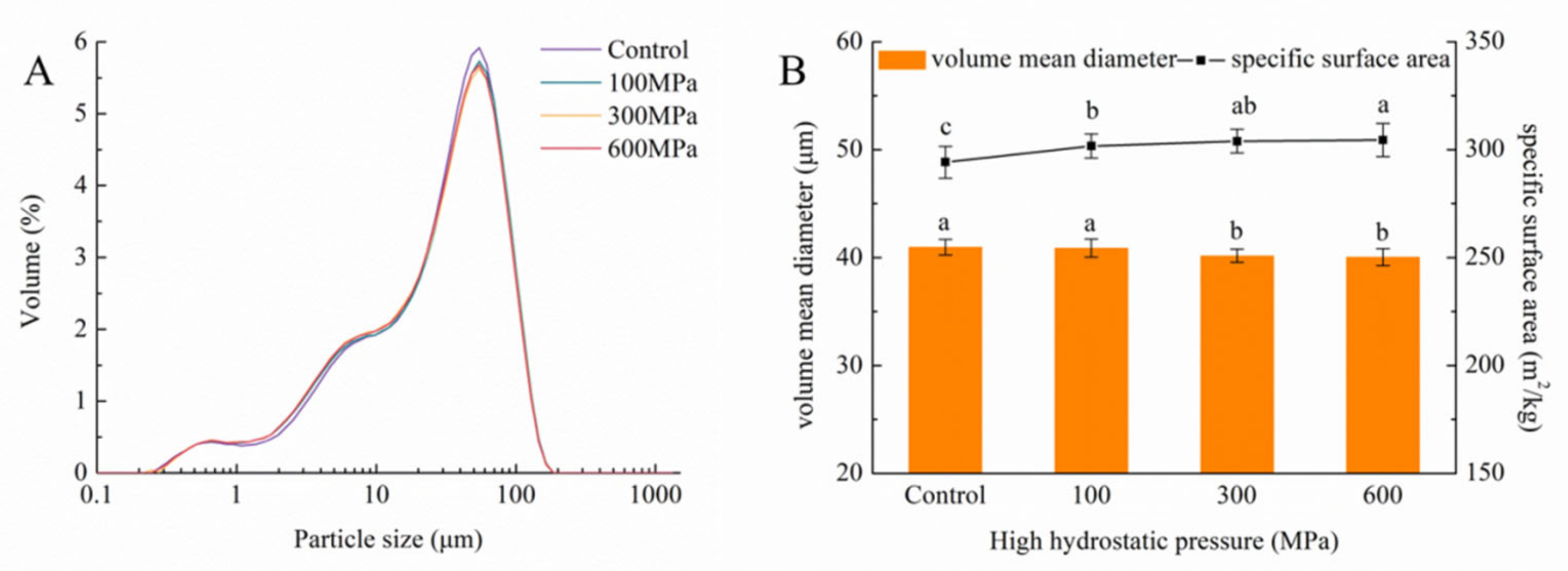
3.5. Impact of the HHP Treatment on Particle-Size Distribution
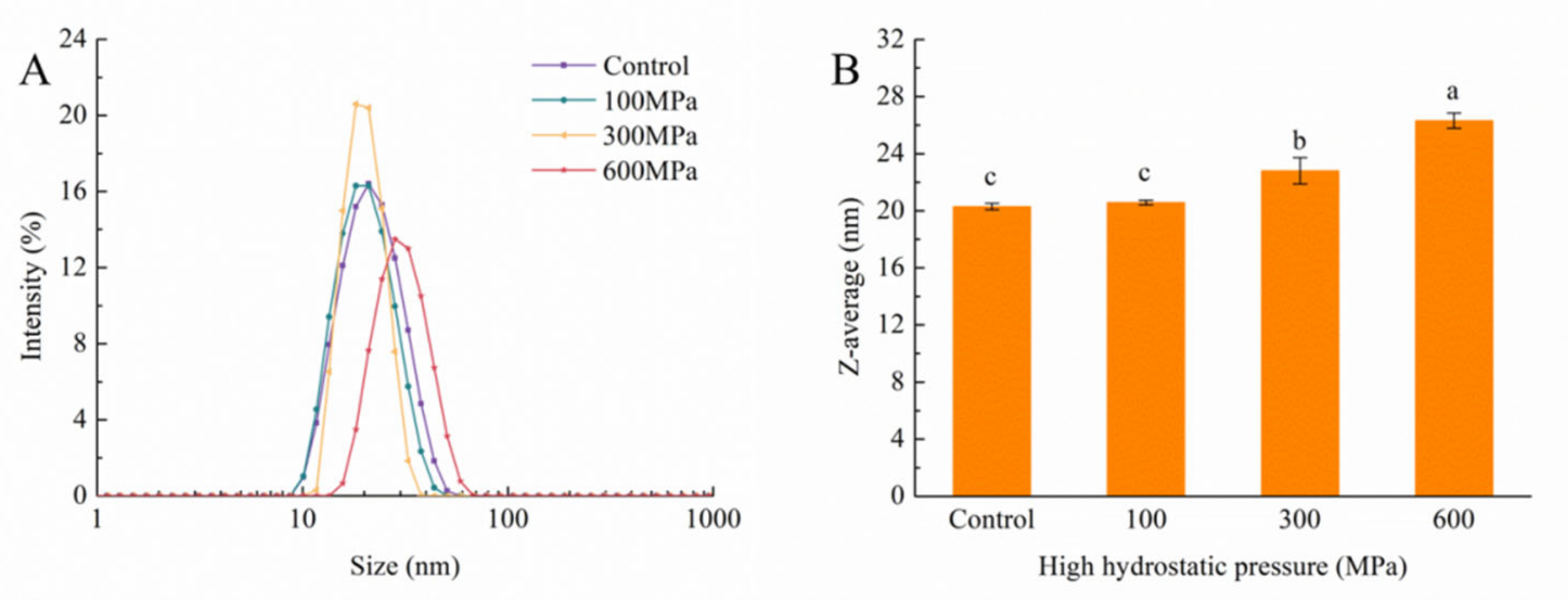
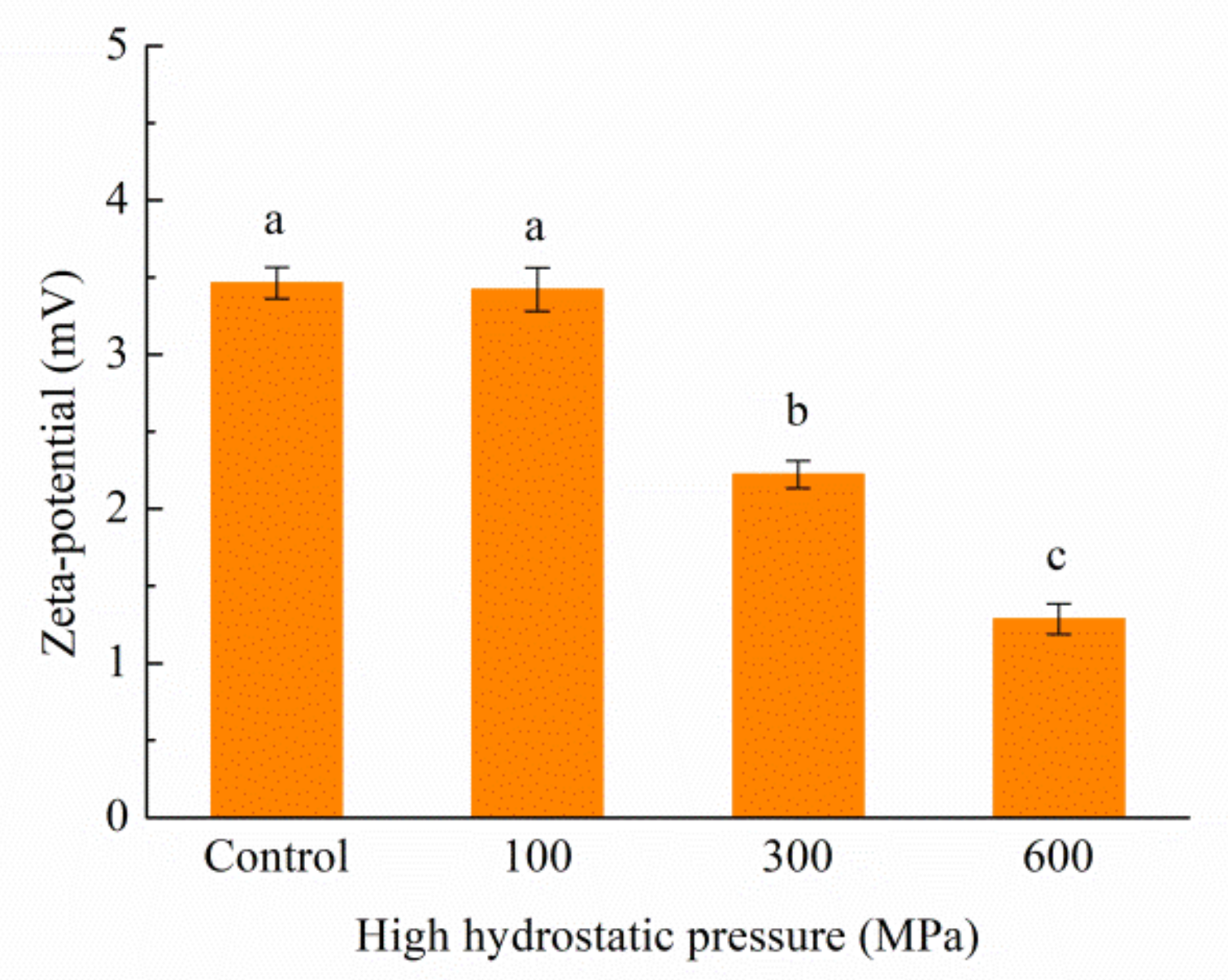
3.6. Impact of the HHP Treatment on Particle Size and Zeta Potential
3.7. Impact of the HHP Treatment on the FTIR Spectrum
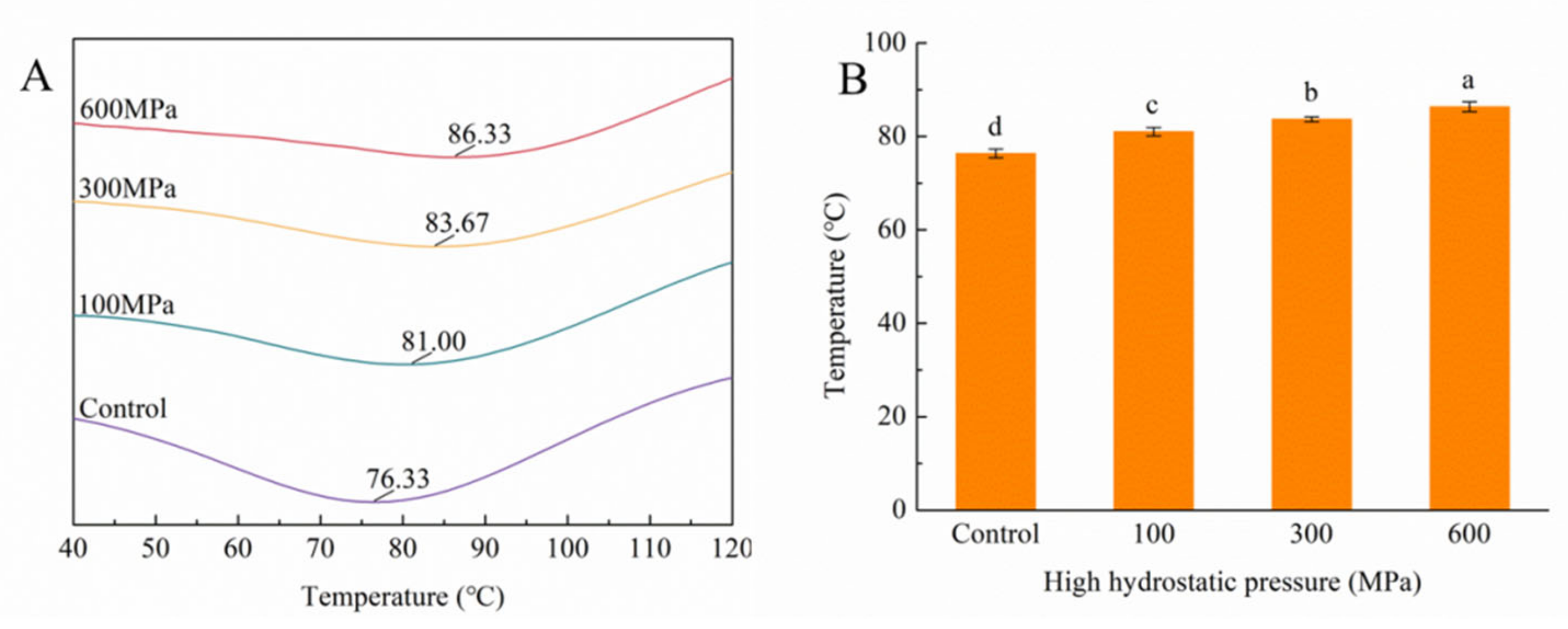
3.8. Impact of the HHP Treatment on DSC
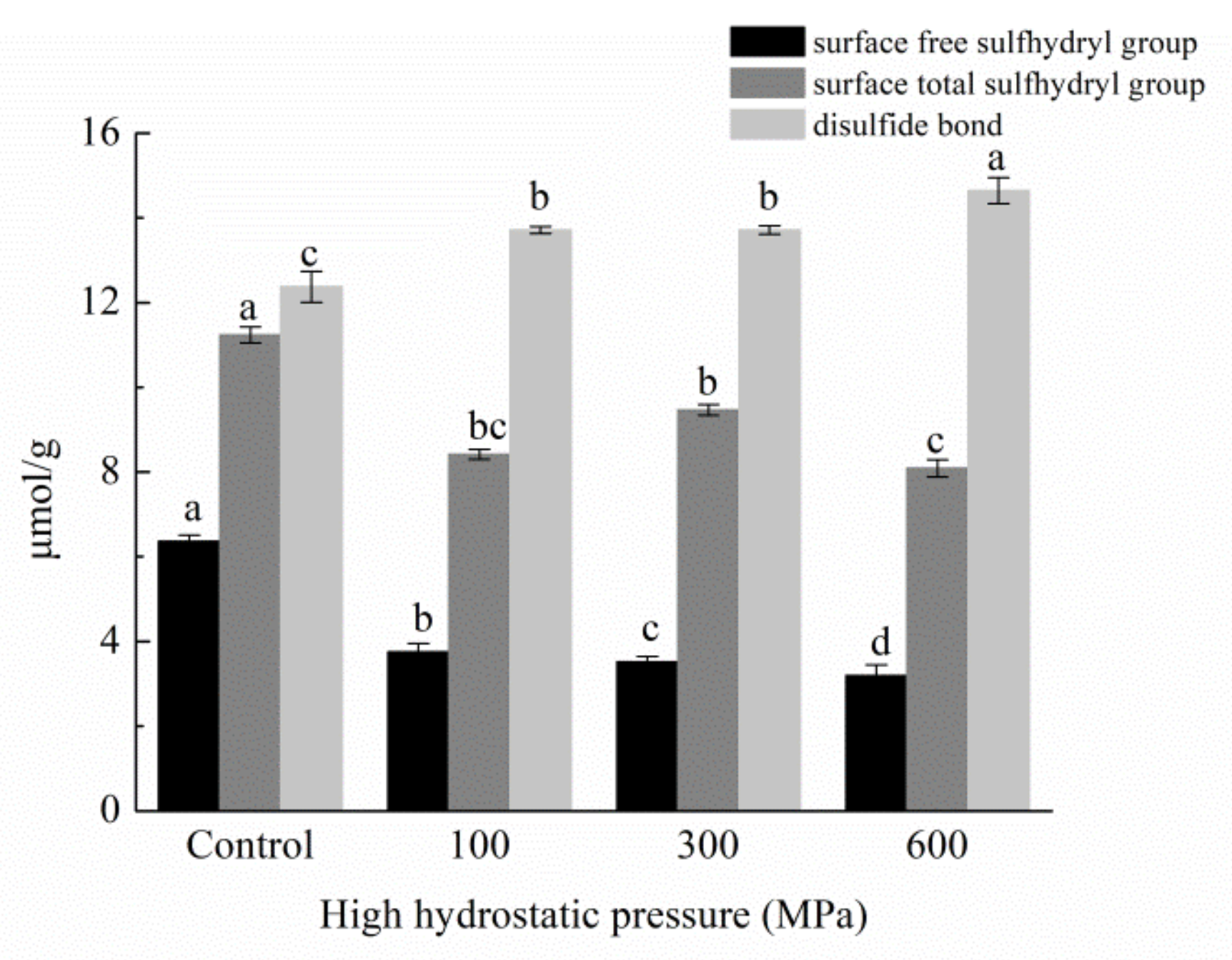
3.9. Impact of HHP Treatments on Surface Free Sulfhydryl Groups, Total Free Sulfhydryl Groups, and Disulfide-Bond Content
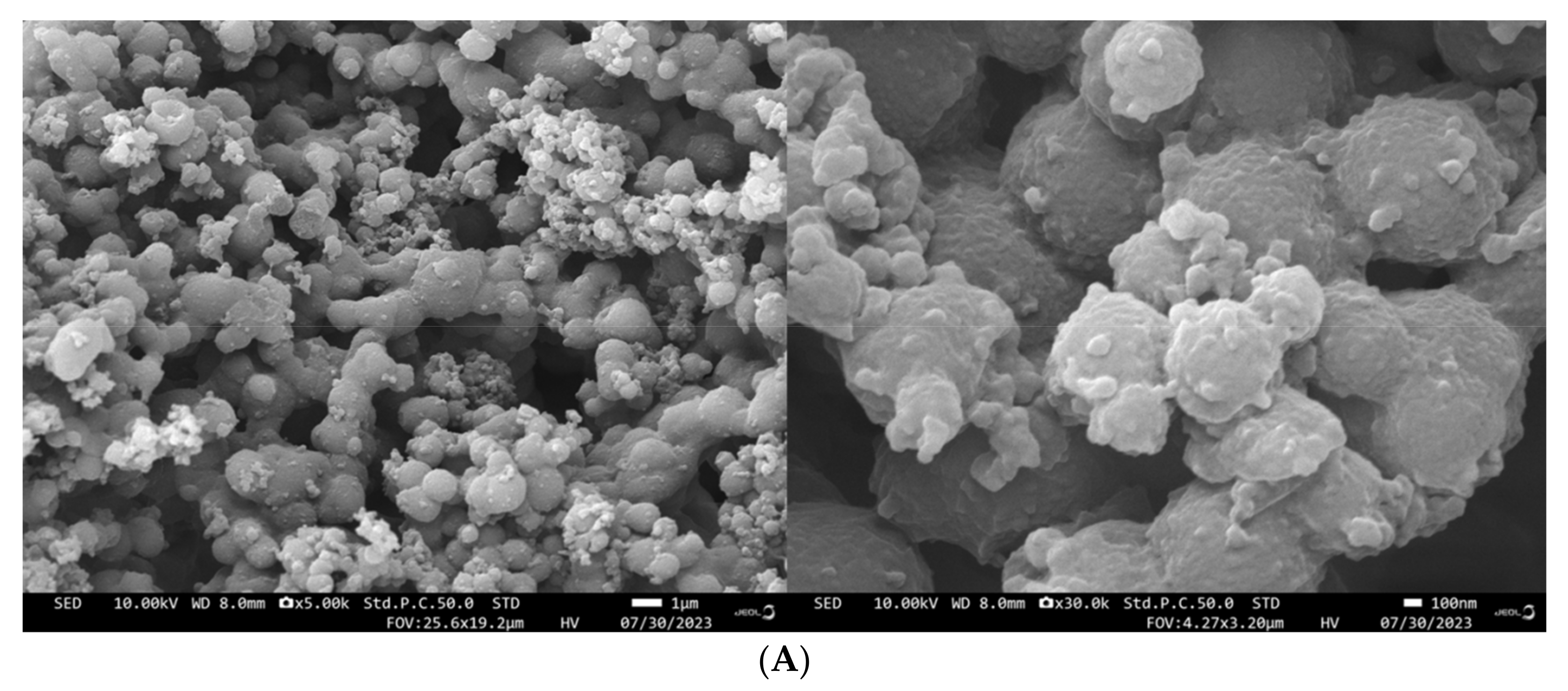
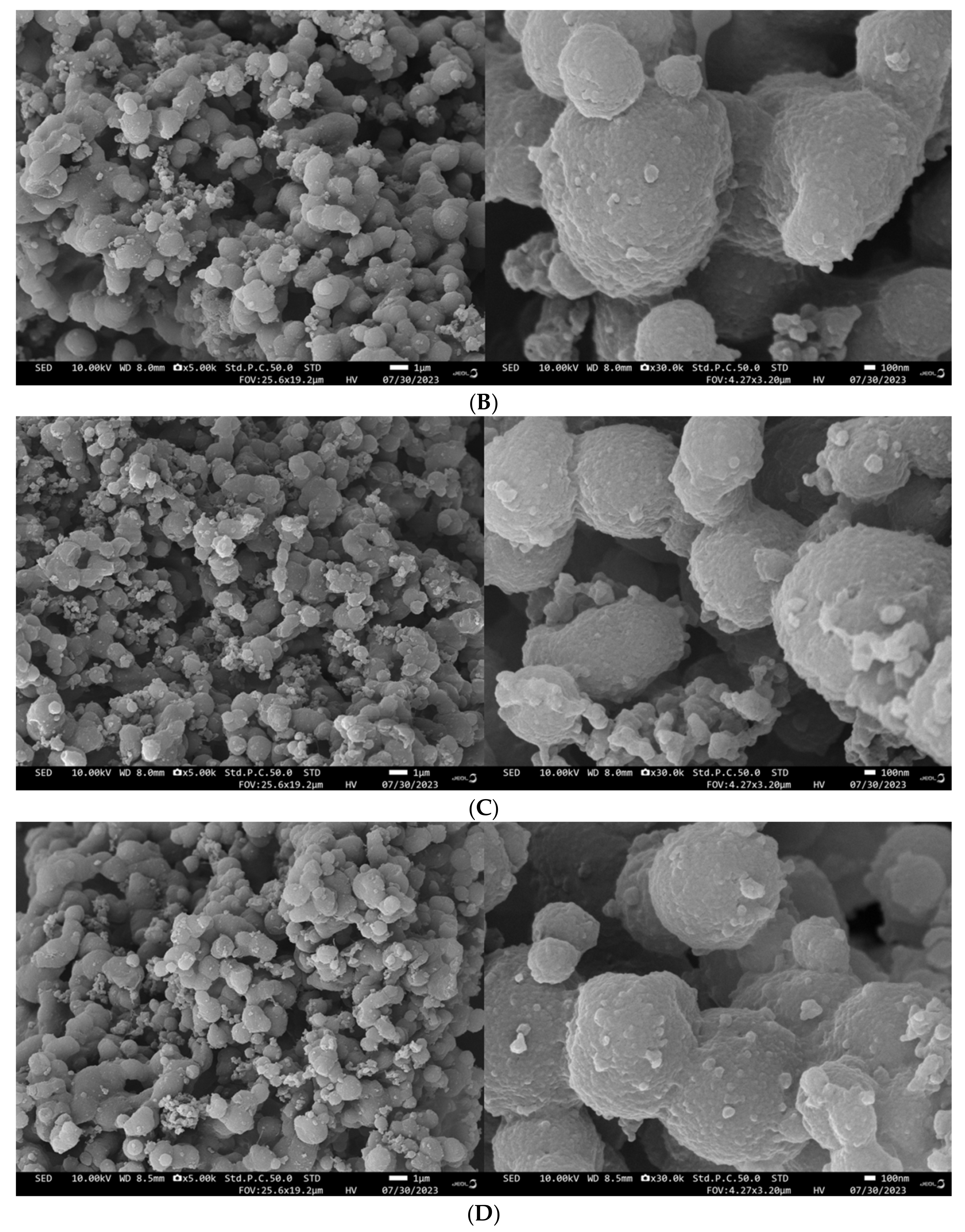
3.10. Impact of the HHP Treatment on SEMs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef] [PubMed]
- Ananda, G.K.S.; Myrans, H.; Norton, S.L.; Gleadow, R.; Furtado, A.; Henry, R.J. Wild Sorghum as a Promising Resource for Crop Improvement. Front. Plant Sci. 2020, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Yones, H.A.; Karim, N.; Taha, E.M.; Chen, W. Potential Processing Technologies for Developing Sorghum-Based Food Products: An Update and Comprehensive Review. Trends Food Sci. Technol. 2021, 110, 168–182. [Google Scholar] [CrossRef]
- Castro-Jácome, T.P.; Alcántara-Quintana, L.E.; Tovar-Pérez, E.G. Optimization of Sorghum Kafirin Extraction Conditions and Identification of Potential Bioactive Peptides. BioRes. Open Access 2020, 9, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Li, J.; Gonzalez, A.P.; Xia, Q.; Huang, Q. Structure, Morphology, and Assembly Behavior of Kafirin. J. Agric. Food Chem. 2015, 63, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Duressa, D.; Weerasoriya, D.; Bean, S.R.; Tilley, M.; Tesso, T. Genetic Basis of Protein Digestibility in Grain Sorghum. Crop Sci. 2018, 58, 2183–2199. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors Affecting Sorghum Protein Digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Duodu, K.G.; Nunes, A.; Delgadillo, I.; Parker, M.L.; Mills, E.N.C.; Belton, P.S.; Taylor, J.R.N. Effect of Grain Structure and Cooking on Sorghum and Maize in Vitro Protein Digestibility. J. Cereal Sci. 2002, 35, 161–174. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Huang, H.-W.; Hsu, C.-P.; Yang, B.B. Recent Advances in Food Processing Using High Hydrostatic Pressure Technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy Expectations of High Hydrostatic Pressure Treatment in Food Processing Industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing Enzymatic Hydrolysis of Food Proteins and Production of Bioactive Peptides Using High Hydrostatic Pressure Technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; Da Silva, J.A.L. Tailoring Structure and Technological Properties of Plant Proteins Using High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, Z.; Chen, Z.; Jing, X.; Wang, X. Effect of High Hydrostatic Pressure Treatment on the Structure and Physicochemical Properties of Millet Gliadin. LWT 2022, 154, 112755. [Google Scholar] [CrossRef]
- Kieffer, R.; Schurer, F.; Köhler, P.; Wieser, H. Effect of Hydrostatic Pressure and Temperature on the Chemical and Functional Properties of Wheat Gluten: Studies on Gluten, Gliadin and Glutenin. J. Cereal Sci. 2007, 45, 285–292. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Sun, X.S.; Wang, D. Overview of Sorghum Industrial Utilization. In Agronomy Monographs; Ciampitti, I.A., Vara Prasad, P.V., Eds.; Soil Science Society of America: Madison, WI, USA, 2019; pp. 463–476. ISBN 978-0-89118-628-1. [Google Scholar]
- Taylor, J.; Taylor, J.R.N.; Dutton, M.F.; De Kock, S. Identification of Kafirin Film Casting Solvents. Food Chem. 2005, 90, 401–408. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant Activity of Zein Hydrolysates in a Liposome System and the Possible Mode of Action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Zheng, W.; Pan, Q.; Zhu, Y. Effect of Multi-Mode Sweep Frequency Ultrasound Pretreatment on Properties of the Zeins and ACE Inhibitory Peptides Activity of the Hydrolysates. Food Chem. 2023, 407, 135126. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Li, X.-Y.; Liu, B.-H.; Yin, Y.-Q.; Zhang, H.-L.; Zhang, Y.-H. Comprehensive Application Possibility: Construction Hydrophilic, Amphiphilic and Hydrophobic System of Modified Zein by Enzymatic or Cysteine Modification. Food Hydrocoll. 2023, 135, 108159. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.-T.; Zhang, B.; Li, X.-L.; Liu, W.-J.; Tao, H.; Li, P.-J. Zein/Carboxymethyl Dextrin Nanoparticles Stabilized Pickering Emulsions as Delivery Vehicles: Effect of Interfacial Composition on Lipid Oxidation and in Vitro Digestion. Food Hydrocoll. 2020, 108, 106020. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, H.; Ling, J.; Ouyang, X.; Song, X. Enhancing the Stability of Zein/Fucoidan Composite Nanoparticles with Calcium Ions for Quercetin Delivery. Int. J. Biol. Macromol. 2021, 193, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and Characterization of Zein Edible Films Incorporated with Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, D.; Tao, S.; Hu, X.; Wang, C.; Sun, Y.; Zhao, B.; Li, Y. Facile Encapsulation of Thymol within Deamidated Zein Nanoparticles for Enhanced Stability and Antibacterial Properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 126940. [Google Scholar] [CrossRef]
- Yang, Y.; He, S.; Zhang, Y.; Li, X.; Liu, H.; Li, Q.; Cao, X.; Ye, Y.; Sun, H. Comparison of Crude Prolamins from Seven Kidney Beans (Phaseolus Vulgaris L.) Based on Composition, Structure and Functionality. Food Chem. 2021, 357, 129748. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.-H.; Redl, A.; Guilbert, S. Mechanism of Heat and Shear Mediated Aggregation of Wheat Gluten Protein upon Mixing. Biomacromolecules 2002, 3, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, J.-Y. Microspheres of Corn Protein, Zein, for an Ivermectin Drug Delivery System. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current Insights into Protein Solubility: A Review of Its Importance for Alternative Proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Kim, S.; Xu, J. Aggregate Formation of Zein and Its Structural Inversion in Aqueous Ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Qin, Z.; Guo, X.; Lin, Y.; Chen, J.; Liao, X.; Hu, X.; Wu, J. Effects of High Hydrostatic Pressure on Physicochemical and Functional Properties of Walnut (Juglans Regia L.) Protein Isolate: Effects of HHP on Walnut Protein Isolate. J. Sci. Food Agric. 2013, 93, 1105–1111. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Zhang, M.; Goffin, D. Effect of High Hydrostatic Pressure on the Structure, Physicochemical and Functional Properties of Protein Isolates from Cumin (Cuminum Cyminum) Seeds. Int. J. Food Sci. Tech. 2019, 54, 752–761. [Google Scholar] [CrossRef]
- Da Silva, L.S.; Taylor, J.; Taylor, J.R.N. Transgenic Sorghum with Altered Kafirin Synthesis: Kafirin Solubility, Polymerization, and Protein Digestion. J. Agric. Food Chem. 2011, 59, 9265–9270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R.; Cao, F. Effects of High Hydrostatic Pressure Treatment on Structural, Allergenicity, and Functional Properties of Proteins from Ginkgo Seeds. Innov. Food Sci. Emerg. Technol. 2016, 34, 187–195. [Google Scholar] [CrossRef]
- Lange, R.; Balny, C. UV-Visible Derivative Spectroscopy under High Pressure. Biochim. Et Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2002, 1595, 80–93. [Google Scholar] [CrossRef]
- Wang, J.-M.; Yang, X.-Q.; Yin, S.-W.; Zhang, Y.; Tang, C.-H.; Li, B.-S.; Yuan, D.-B.; Guo, J. Structural Rearrangement of Ethanol-Denatured Soy Proteins by High Hydrostatic Pressure Treatment. J. Agric. Food Chem. 2011, 59, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Yun, S.; Liao, X.; Zhao, G.; Leng, X. Effect of High Hydrostatic Pressure (HHP) on Structure and Activity of Phytoferritin. Food Chem. 2012, 130, 273–278. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Cao, A.; Zhang, Y.; Lv, Y.; Li, J. Denaturation Kinetics and Aggregation Mechanism of the Sarcoplasmic and Myofibril Proteins from Grass Carp During Microwave Processing. Food Bioprocess Technol. 2018, 11, 417–426. [Google Scholar] [CrossRef]
- Li, N.; Yu, J.; Jin, N.; Chen, Y.; Li, S.; Chen, Y. Modification of the Physicochemical and Structural Characteristics of Zein Suspension by Dielectric Barrier Discharge Cold Plasma Treatment. J. Food Sci. 2020, 85, 2452–2460. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; De Oliveira Brisola Maciel, M.V.; De Carvalho, S.M.; De Melo, A.P.Z.; Jummes, B.; Da Silva, T.; Martelli, S.M.; Villetti, M.A.; Bertoldi, F.C.; Barreto, P.L.M. Characterization and Evaluation of Physicochemical and Antimicrobial Properties of Zein Nanoparticles Loaded with Phenolics Monoterpenes. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 337–344. [Google Scholar] [CrossRef]
- Perreault, V.; Hénaux, L.; Bazinet, L.; Doyen, A. Pretreatment of Flaxseed Protein Isolate by High Hydrostatic Pressure: Impacts on Protein Structure, Enzymatic Hydrolysis and Final Hydrolysate Antioxidant Capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef]
- Manassero, C.A.; David-Briand, E.; Vaudagna, S.R.; Anton, M.; Speroni, F. Calcium Addition, pH, and High Hydrostatic Pressure Effects on Soybean Protein Isolates—Part 1: Colloidal Stability Improvement. Food Bioprocess Technol. 2018, 11, 1125–1138. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Xu, M.; Zhang, X.; Cao, S.; Hu, Y.; Luan, G. Physicochemical Properties and Protein Structure of Extruded Corn Gluten Meal: Implication of Temperature. Food Chem. 2023, 399, 133985. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, L.; Nikoo, M.; Ocen, D.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of Frozen Storage on the Conformational, Thermal and Microscopic Properties of Gluten: Comparative Studies on Gluten-, Glutenin- and Gliadin-Rich Fractions. Food Hydrocoll. 2014, 35, 238–246. [Google Scholar] [CrossRef]
- Sun, M.; Mu, T.; Sun, H.; Zhang, M. Digestibility and Structural Properties of Thermal and High Hydrostatic Pressure Treated Sweet Potato (Ipomoea Batatas L.) Protein. Plant Foods Hum. Nutr. 2014, 69, 270–275. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Liu, H.-Z.; Liu, L.; Zhao, G.-L.; Wang, Q.; Chen, Q.-L. Effects of High Pressure on the Physicochemical and Functional Properties of Peanut Protein Isolates. Food Hydrocoll. 2014, 36, 123–129. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Wu, W. Effects of High Pressure Treatments (Ultra-High Hydrostatic Pressure and High Pressure Homogenization) on Bighead Carp (Aristichthys Nobilis) Myofibrillar Protein Native State and Its Hydrolysate. Food Bioprocess Technol. 2022, 15, 2252–2266. [Google Scholar] [CrossRef]
| High Hydrostatic Pressure | Comparative Content/% | ||||
|---|---|---|---|---|---|
| Intermolecular β-Sheet | Intramolecular β-Sheet | Random Coil | α-Helix | β-Turn | |
| Control | 19.97 ± 0.17 b | 41.06 ± 0.05 a | 12.66 ± 0.01 a | 13.52 ± 0.07 ab | 12.79 ± 0.05 ab |
| 100 MPa | 19.95 ± 0.33 b | 40.76 ± 0.13 ab | 12.51 ± 0.11 a | 13.78 ± 0.13 a | 13.00 ± 0.06 a |
| 300 MPa | 20.55 ± 0.36 ab | 40.58 ± 0.09 b | 12.45 ± 0.09 a | 13.55 ± 0.16 ab | 12.87 ± 0.16 ab |
| 600 MPa | 21.06 ± 0.91 a | 40.59 ± 0.36 b | 12.29 ± 0.37 a | 13.39 ± 0.29 b | 12.67 ± 0.27 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, Q.; Liu, Q.; Wang, X.; Bai, W.; Chen, Z. Effect of High-Hydrostatic-Pressure Treatment on the Physicochemical Properties of Kafirin. Foods 2023, 12, 4077. https://doi.org/10.3390/foods12224077
Yang Y, Chen Q, Liu Q, Wang X, Bai W, Chen Z. Effect of High-Hydrostatic-Pressure Treatment on the Physicochemical Properties of Kafirin. Foods. 2023; 12(22):4077. https://doi.org/10.3390/foods12224077
Chicago/Turabian StyleYang, Yajing, Qiongling Chen, Qingshan Liu, Xiaowen Wang, Wenbin Bai, and Zhenjia Chen. 2023. "Effect of High-Hydrostatic-Pressure Treatment on the Physicochemical Properties of Kafirin" Foods 12, no. 22: 4077. https://doi.org/10.3390/foods12224077
APA StyleYang, Y., Chen, Q., Liu, Q., Wang, X., Bai, W., & Chen, Z. (2023). Effect of High-Hydrostatic-Pressure Treatment on the Physicochemical Properties of Kafirin. Foods, 12(22), 4077. https://doi.org/10.3390/foods12224077






