Effect of Three Different Preservatives on the Microbiota of Shalgam, a Traditional Lactic Acid Fermented Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Materials
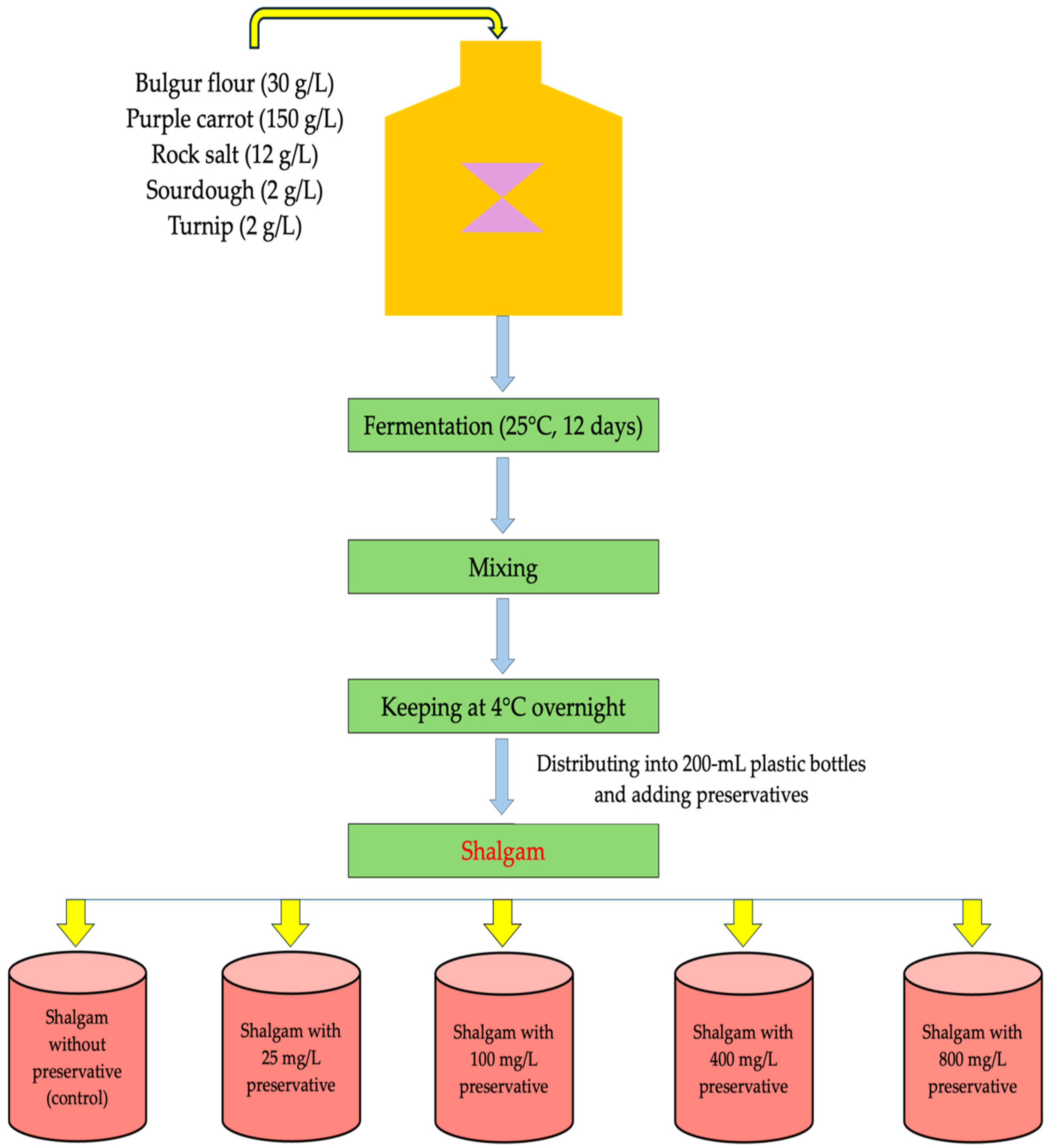
2.2. Shalgam Production
2.3. Determination of the Physicochemical Properties of Shalgam
2.4. Enumeration of LAB and Yeasts in Shalgam
2.5. Isolation of LAB
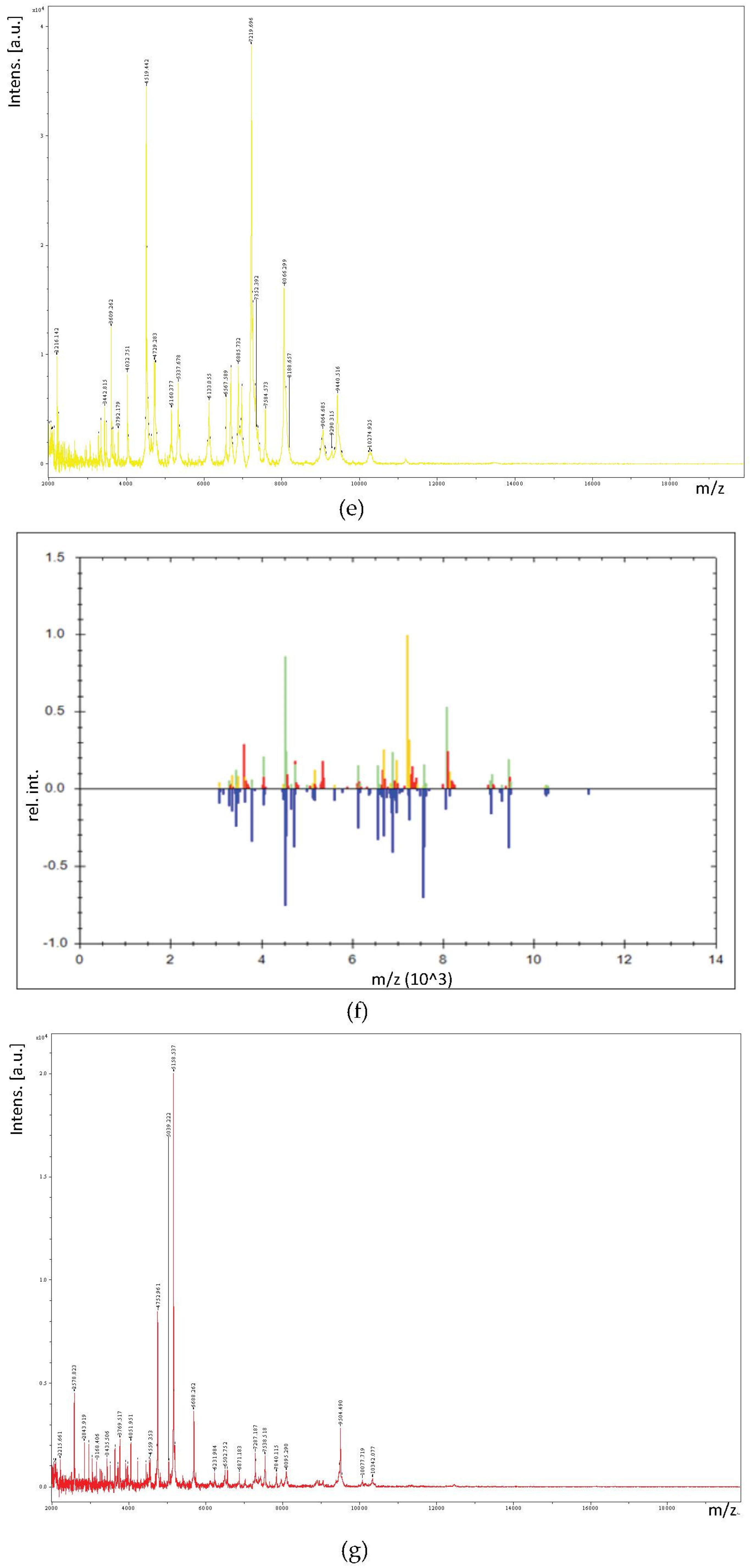
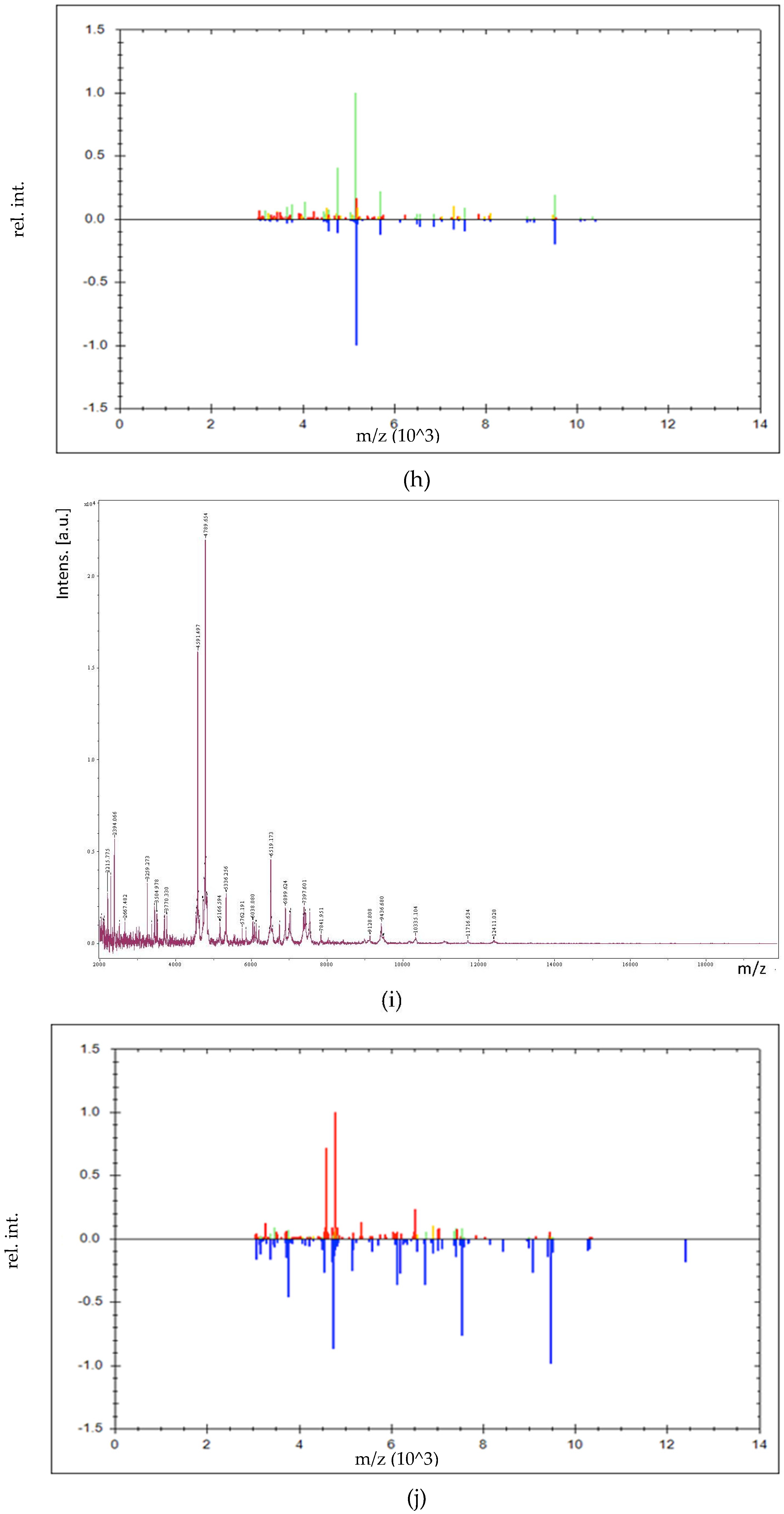
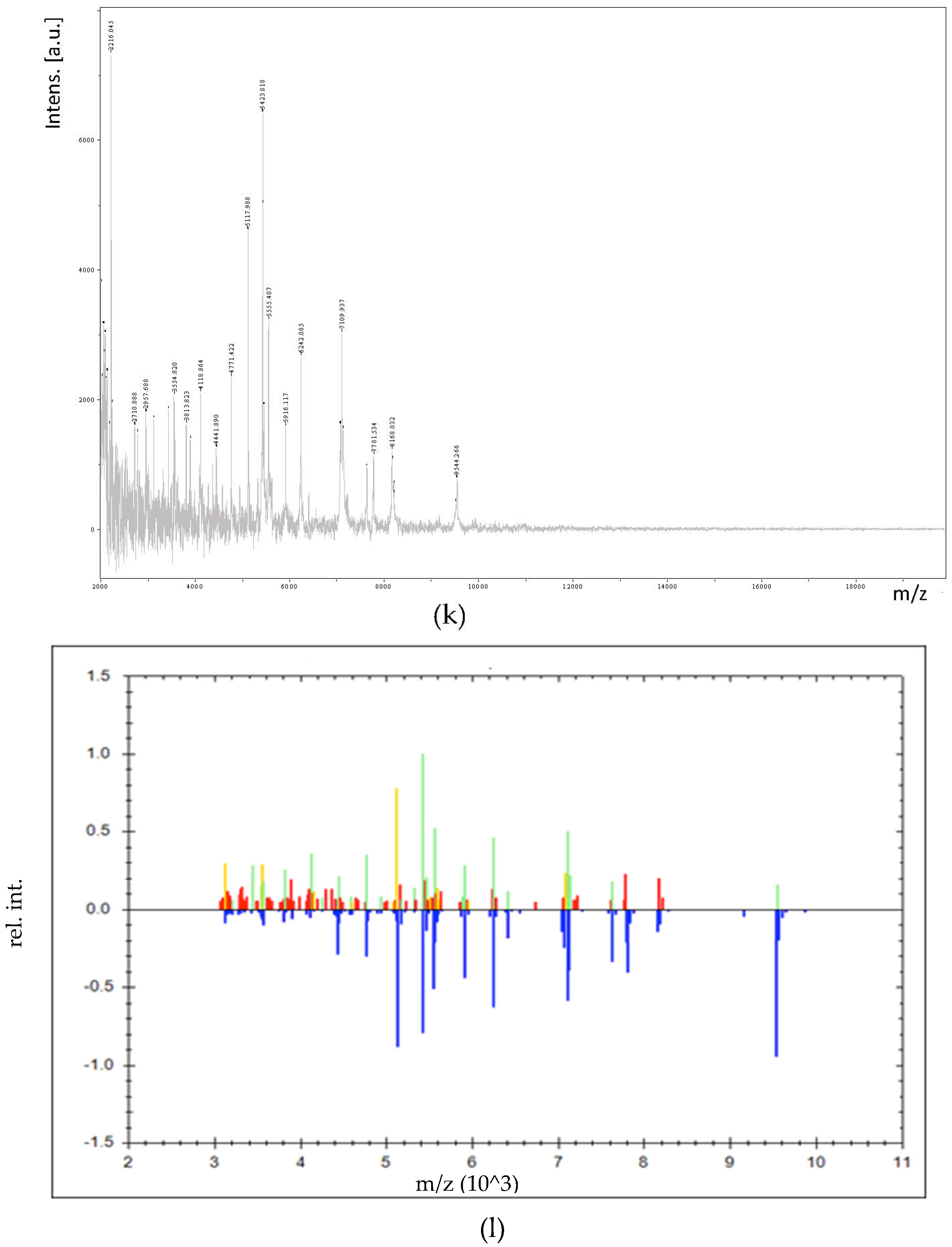
2.6. MALDI-TOF/TOF MS Profile Acquisition
2.7. Identification of LAB by MALDI-TOF/TOF MS
3. Results and Discussion
3.1. Physicochemical Properties of Shalgam
3.2. Enumeration of LAB and Yeast in Shalgam Samples
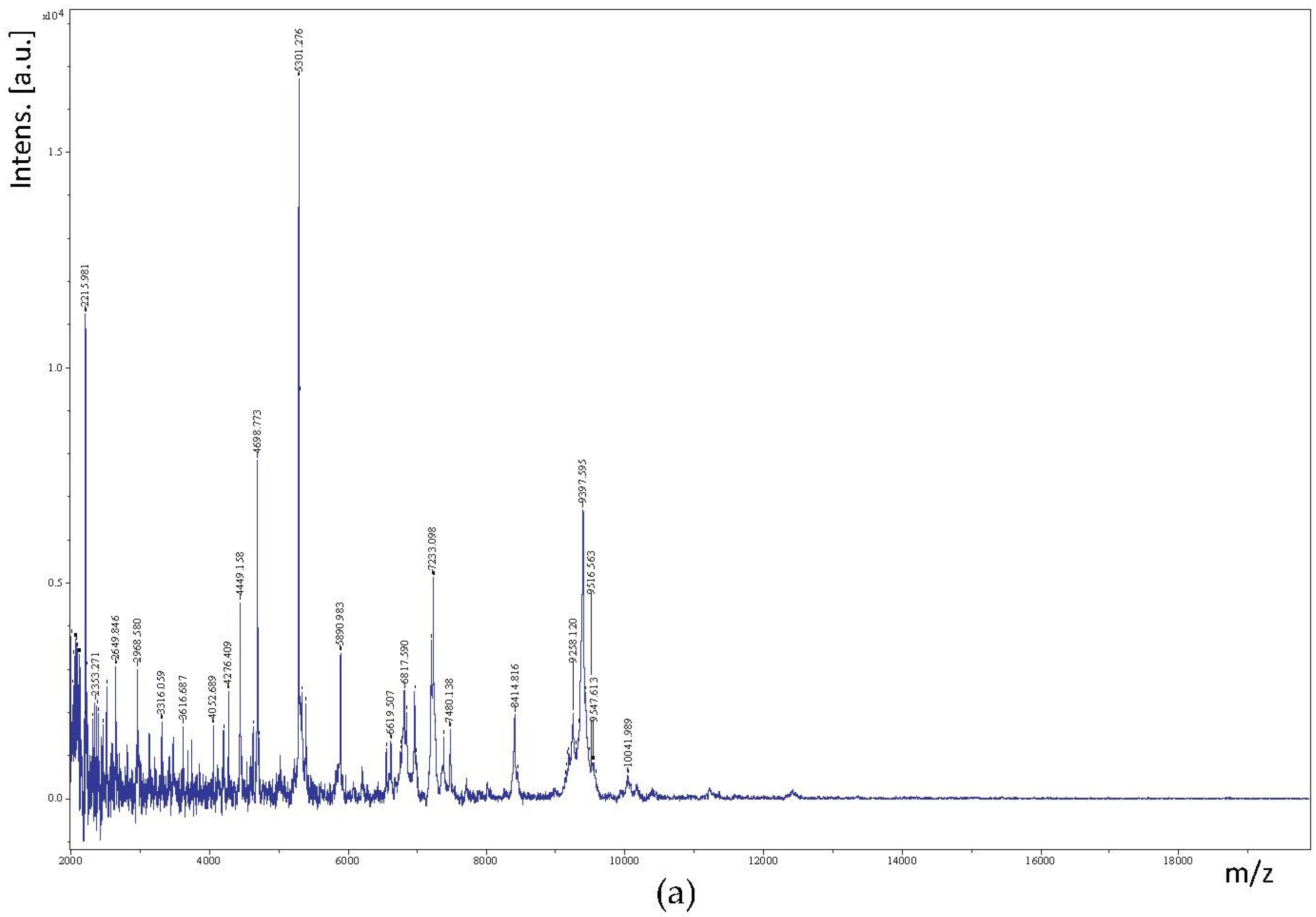
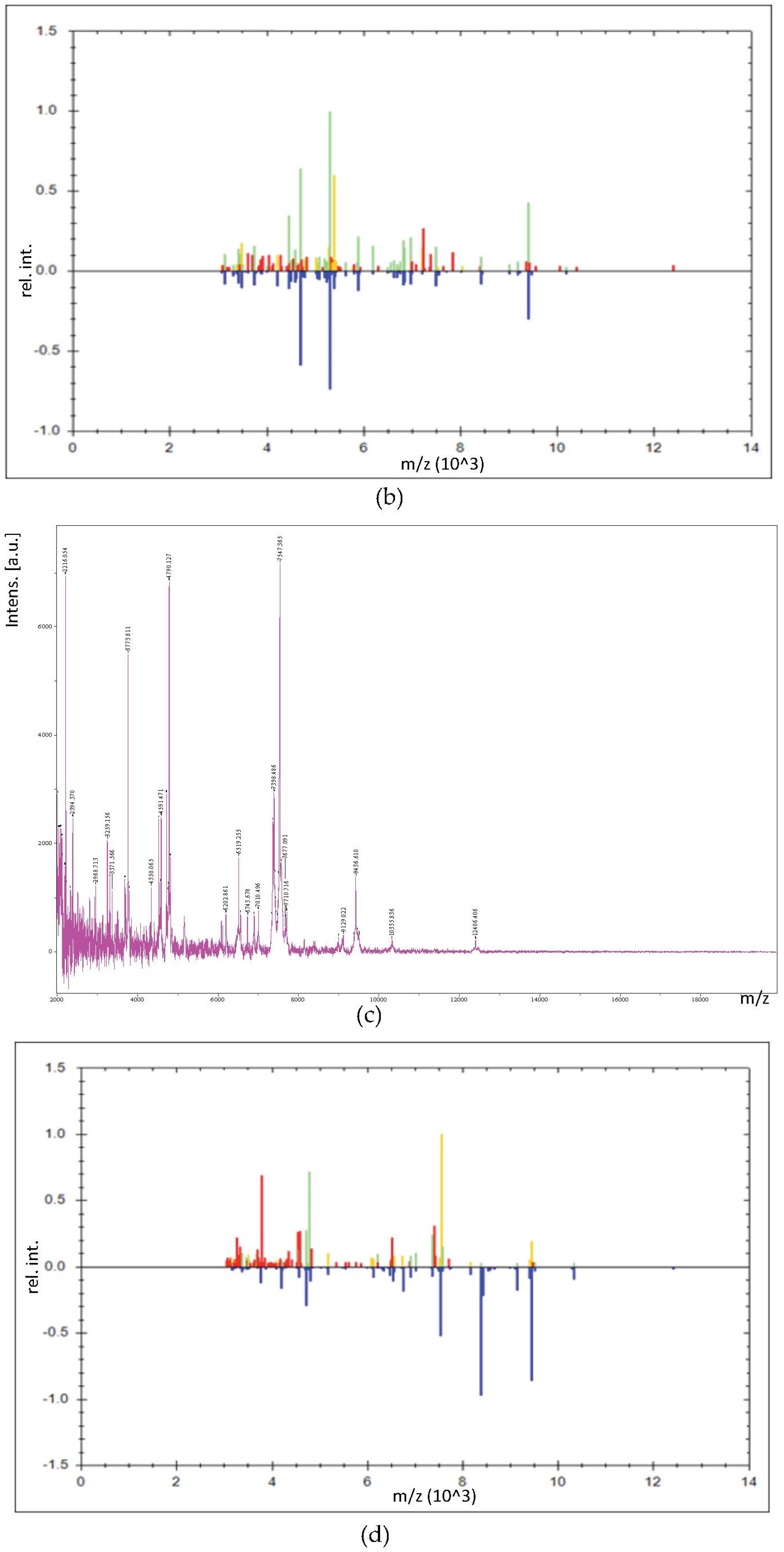
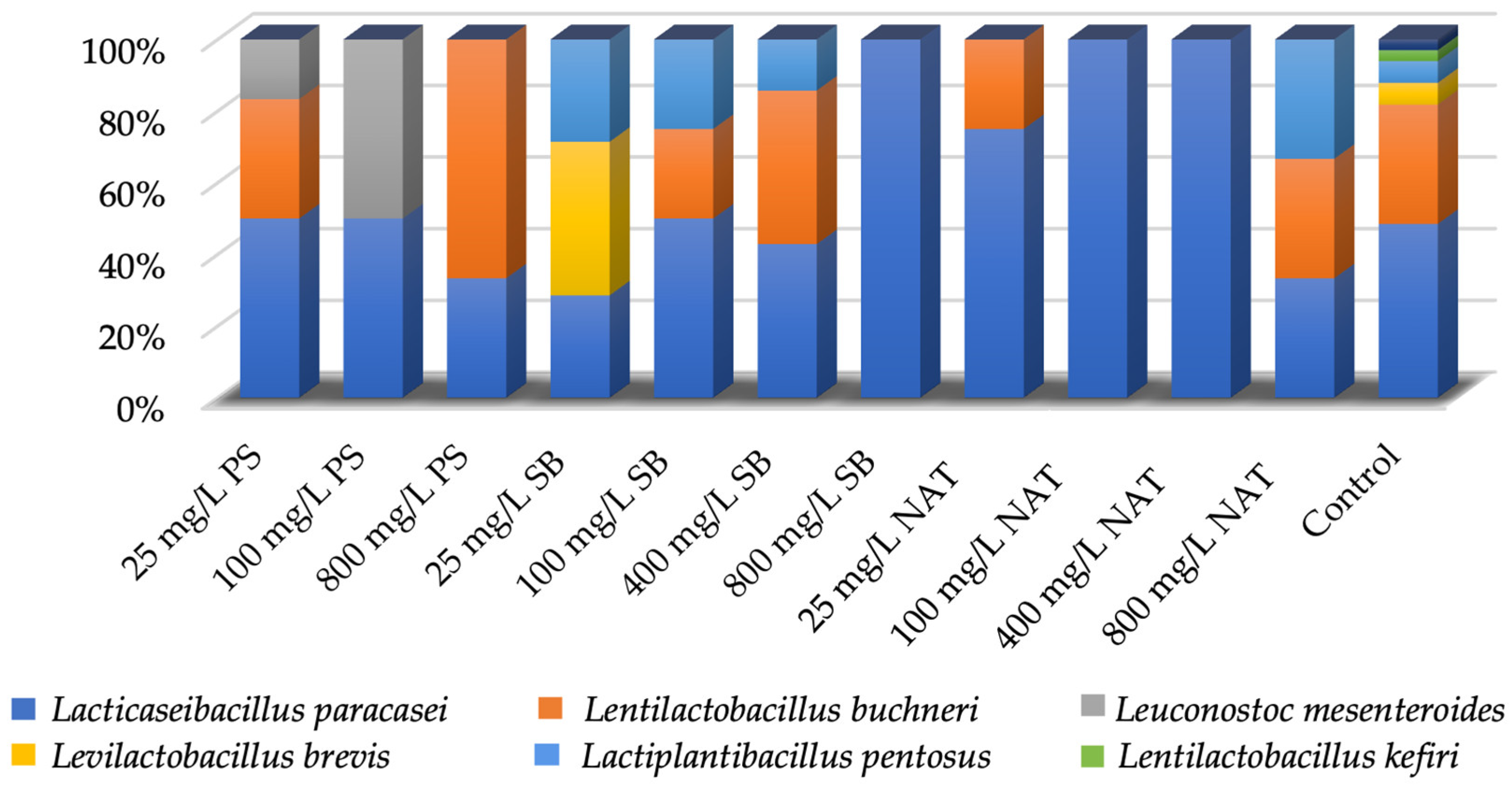
3.3. Identification of LAB by MALDI-TOF/TOF MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.D.L.; Baños-Lara, M.D.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional fermented foods and beverages from around the world and their health benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Tanguler, H.; Erten, H. Occurrence and growth of lactic acid bacteria species during the fermentation of shalgam (salgam), a traditional Turkish fermented beverage. LWT-Food Sci. Technol. 2012, 46, 36–41. [Google Scholar] [CrossRef]
- Gok, I. Functional potential of several Turkish fermented traditional foods: Biotic properties, bioactive compounds, and health benefits. Food Rev. Int. 2023, 39, 2568–2593. [Google Scholar] [CrossRef]
- Kırlangıç, O.; Ilgaz, C.; Kadiroğlu, P. Influence of pasteurization and storage conditions on microbiological quality and aroma profiles of shalgam. Food Biosci. 2021, 44, 101350. [Google Scholar] [CrossRef]
- Karaoglan, H.A.; Keklik, N.M.; Develi Işikli, N. Modeling inactivation of Candida inconspicua isolated from turnip juice using pulsed UV light. J. Food Process. Eng. 2017, 40, e12418. [Google Scholar] [CrossRef]
- Coskun, F. A traditional Turkish fermented non-alcoholic beverage, “Shalgam”. Beverages 2017, 3, 49. [Google Scholar] [CrossRef]
- Yanardağ Karabulut, Ş. Glutensiz ve Katkısız Şalgam Suyu Üretimi ve Yüksek Hidrostatik Basınç ile raf Ömrünün Uzatılması. Master’s Thesis, Trakya University, Edirne, Turkey, 2020. [Google Scholar]
- Ulucan, E.; Çoklar, H.; Akbulut, M. Application of ultrasound to extend the shelf life of the shalgam juice: Changes in various physicochemical, nutritional, and microbiological properties. J. Food Process. Preserv. 2022, 46, e16501. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. Food preservatives—An overview on applications and side effects. Emir. J. Food Agric. 2016, 28, 366–373. [Google Scholar] [CrossRef]
- Hoca, G. Bursa Ilinde Tüketime Sunulan nar Ekşisi ve Nar Ekşili Soslarda Sorbik asit ve Benzoik Asit Miktarlarının Belirlenmesi. Master’s Thesis, Bursa Uludag University, Bursa, Turkey, 2019. [Google Scholar]
- Piper, J.D.; Piper, P.W. Benzoate and sorbate salts: A systematic review of the potential hazards of these invaluable preservatives and the expanding spectrum of clinical uses for sodium benzoate. Comr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Piper, P.W. Potential safety issues surrounding the use of benzoate preservatives. Beverages 2018, 4, 33. [Google Scholar] [CrossRef]
- Stark, J. Preservatives/permitted preservatives-natamycin. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1776–1781. [Google Scholar]
- Lule, V.K.; Garg, S.; Gosewade, S.C.; Khedkar, C.D. Natamycin. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 56–62. [Google Scholar]
- Turkish Standard (TS) 11149/T3; Turnip Juice. Turkish Standard Institute: Ankara, Turkey, 2016.
- Topić Popović, N.; Kazazić, S.P.; Bojanić, K.; Strunjak-Perović, I.; Čož-Rakovac, R. Sample preparation and culture condition effects on MALDI-TOF MS identification of bacteria: A review. Mass Spectrom. Rev. 2023, 42, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Karasu-Yalcin, S.; Soylemez-Milli, N.; Eren, O.; Eryasar-Orer, K. Reducing time in detection of Listeria monocytogenes from food by MALDI-TOF mass spectrometry. J. Food Sci. Technol. 2021, 58, 4102–4109. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R. Scales for the measurements of color difference. In The Measurements of the Appearance; Hunter, R., Harold, R.V., Eds.; John Wiley and Sons: New York, NY, USA, 1975; pp. 133–140. [Google Scholar]
- Tamang, J.P.; Tamang, B.; Schillinger, U.; Franz, C.M.A.P.; Gores, M.; Holzapfel, W.H. Identification of predominant lactic acid bacteria isolated from traditionally fermented vegetable products of the Eastern Himalayas. Int. J. Food Microbiol. 2005, 105, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, D.; Nuraida, L.; Dewanti-Hariyadi, R.; Hunaefi, D. Lactic acid bacteria and yeasts from Indonesian kefir grains and their growth interaction. Asian J. Microbiol. Biotechnol. Environ. Sci. 2020, 22, 44–49. [Google Scholar]
- Ateş, C. Effectiveness of Ultrasonication and High Pressure Processing Pasteurization on Quality Characteristics and Shelf Life of Fermented Shalgam Drink. Master’s Thesis, Abant Izzet Baysal University, Bolu, Turkey, 2019. [Google Scholar]
- Tangüler, H. Identification of Predominant Lactic acid Bacteria Isolated from Shalgam Beverage and Improvement of Its Production Technique. Ph.D. Thesis, Çukurova University, Adana, Turkey, 2010. [Google Scholar]
- Ulucan, E. Effects of Some Physicochemical and Microbiological Properties of Ultrasonic Application on Shalgam Juice after Fermentation. Master’s Thesis, Selcuk University, Konya, Turkey, 2019. [Google Scholar]
- Altay, F.; Karbancioglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef]
- Şanlibaba, P.; Tezel, B.U. Traditional fermented foods in Anatolia. Acta Sci. Pol. Technol. Aliment. 2023, 22, 193–215. [Google Scholar] [CrossRef]
- Mete, A.; Coşansu, S.; Demirkol, O.; Ayhan, K. Amino acid decarboxylase activities and biogenic amine formation abilities of lactic acid bacteria isolated from shalgam. Int. J. Food Prop. 2017, 20, 171–178. [Google Scholar] [CrossRef]
- Tanguler, H.; Saris, P.E.J.; Erten, H. Microbial, chemical and sensory properties of shalgams made using different production methods. J. Sci. Food Agric. 2015, 95, 1008–1015. [Google Scholar] [CrossRef]
- Kafkasgiray, E.S. Determination of the Microbial Profile of Shalgam Beverage Fermentation Process by Molecular Methods. Master’s Thesis, Istanbul Sabahattin Zaim University, Istanbul, Turkey, 2020. [Google Scholar]
- Erginkaya, Z.; Ünal Turhan, E. Enumeration and identification of dominant microflora during the fermentation of shalgam. Akademik Gıda 2016, 14, 92–97. [Google Scholar]
- Bu, Y.; Liu, Y.; Liu, Y.; Wang, S.; Liu, Q.; Hao, H.; Yi, H. Screening and probiotic potential evaluation of bacteriocin-producing Lactiplantibacillus plantarum in vitro. Foods 2022, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
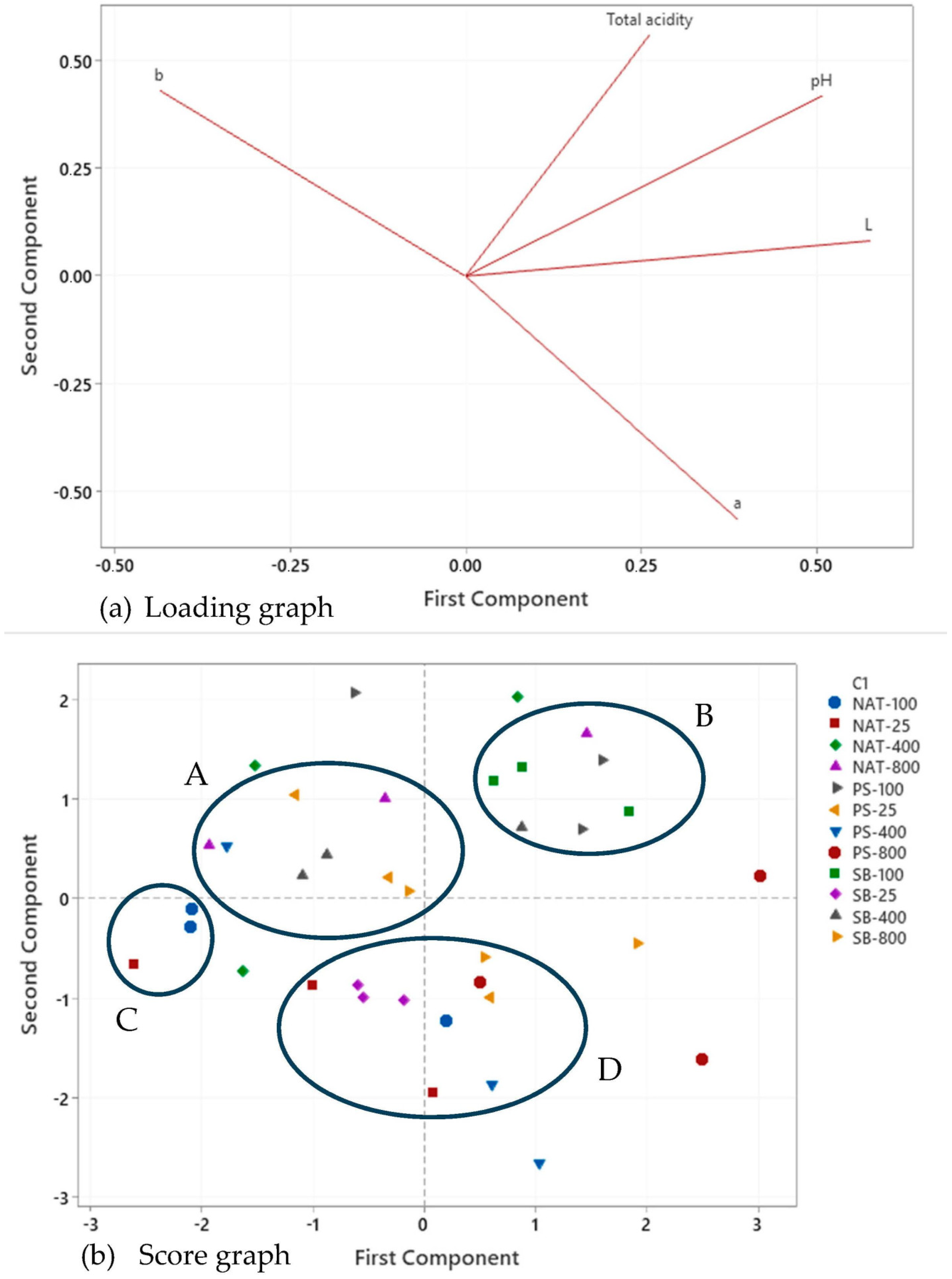
| Samples | pH | Total Acididy (as Lactic Acid g/L) | Instrumental Color Parameters | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Control | 3.43 ± 0.01 | 4.41 ± 0.13 e | 23.97 ± 1.67 | −2.467 ± 0.70 b | −4.387 ± 1.22 |
| SB-25 | 3.46 ± 0.01 | 5.13 ± 0.04 bcd | 24.88 ± 0.46 | 0.525 ± 0.01 a | −4.433 ± 0.22 |
| SB-100 | 3.52 ± 0.03 | 5.61 ± 0.13 a | 26.05 ± 1.44 | 0.448 ± 0.09 a | −4.436 ± 0.22 |
| SB-400 | 3.45 ± 0.04 | 5.37 ± 0.04 abcd | 26.97 ± 2.40 | 0.368 ± 0.09 a | −3.979 ± 0.22 |
| SB-800 | 3.45 ± 0.01 | 5.46 ± 0.08 abc | 27.20 ± 1.87 | 0.608 ± 0.09 a | −4.502 ± 0.24 |
| NAT-25 | 3.44 ± 0.03 | 4.95 ± 0.13 d | 24.90 ± 2.36 | 0.441 ± 0.09 a | −4.213 ± 0.48 |
| NAT-100 | 3.45 ± 0.01 | 5.04 ± 0.17 cd | 24.35 ± 2.83 | 0.304 ± 0.12 a | −4.220 ± 0.61 |
| NAT-400 | 3.46 ± 0.09 | 5.31 ± 0.13 abcd | 26.29 ± 2.86 | 0.167 ± 0.17 a | −3.988 ± 0.29 |
| NAT-800 | 3.49 ± 0.11 | 5.31 ± 0.13 abcd | 26.44 ± 2.69 | 0.166 ± 0.10 a | −4.098 ± 0.32 |
| PS-25 | 3.47 ± 0.01 | 5.27 ± 0.02 abcd | 26.09 ± 1.37 | 0.559 ± 0.32 a | −3.827 ± 0.50 |
| PS-100 | 3.51 ± 0.01 | 5.58 ± 0.08 ab | 26.86 ± 1.48 | 0.445 ± 0.23 a | −4.028 ± 0.38 |
| PS-400 | 3.44 ± 0.01 | 5.04 ± 0.17 cd | 27.29 ± 1.57 | 0.669 ± 0.45 a | −4.182 ± 0.67 |
| PS-800 | 3.50 ± 0.12 | 5.34 ± 0.17 abcd | 28.12 ± 1.33 | 0.747 ± 0.05 a | −4.818 ± 0.64 |
| Treatment | LAB (log CFU/mL) | Yeast (log CFU/mL) |
|---|---|---|
| Control | 6.58 ± 0.1 a | 6.39 ± 0.3 a |
| SB-25 | 4.60 ± 0.4 ab | 4.01 ± 0.3 b |
| SB-100 | 3.03 ± 0.4 b | 3.12 ± 0.3 bcd |
| SB-400 | 2.65 ± 0.5 b | 2.42 ± 0.3 def |
| SB-800 | 2.48 ± 1.3 b | 1.31 ± 0.7 f |
| NAT-25 | 4.55 ± 0.6 ab | 3.12 ± 0.2 bcd |
| NAT-100 | 3.95 ± 0.9 b | 2.92 ± 0.1 bcd |
| NAT-400 | 4.03 ± 0.7 b | 2.96 ± 0.4 bcd |
| NAT-800 | 3.58 ± 0.5 b | 1.57 ± 0.2 ef |
| PS-25 | 3.93 ± 0.3 b | 3.63 ± 0.2 bc |
| PS-100 | 3.83 ± 0.1 b | 3.39 ± 0.2 bcd |
| PS-400 | 3.05 ± 0.3 b | 3.19 ± 0.3 bcd |
| PS-800 | 2.78 ± 0.8 b | 2.52 ± 0.3 cde |
| Isolate No | Isolate Code | Isolation Source | Identification Result |
|---|---|---|---|
| 1 | C-1 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 2 | C-2 | Non-treated shalgam | Levilactobacillus brevis |
| 3 | C-3 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 4 | C-4 | Non-treated shalgam | Lentilactobacillus buchneri |
| 5 | C-5 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 6 | C-6 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 7 | C-7 | Non-treated shalgam | Lentilactobacillus buchneri |
| 8 | C-8 | Non-treated shalgam | Lactiplantibacillus pentosus |
| 9 | C-9 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 10 | C-10 | Non-treated shalgam | Lentilactobacillus buchneri |
| 11 | C-11 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 12 | C-12 | Non-treated shalgam | Lentilactobacillus buchneri |
| 13 | C-13 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 14 | C-14 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 15 | C-15 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 16 | C-16 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 17 | C-17 | Non-treated shalgam | Lentilactobacillus buchneri |
| 18 | C-18 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 19 | C-19 | Non-treated shalgam | Lentilactobacillus buchneri |
| 20 | C-20 | Non-treated shalgam | Lentilactobacillus kefiri |
| 21 | C-21 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 22 | C-22 | Non-treated shalgam | Lactiplantibacillus pentosus |
| 23 | C-23 | Non-treated shalgam | Lentilactobacillus buchneri |
| 24 | C-24 | Non-treated shalgam | Lentilactobacillus buchneri |
| 25 | C-25 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 26 | C-26 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 27 | C-27 | Non-treated shalgam | Lentilactobacillus buchneri |
| 28 | C-28 | Non-treated shalgam | Levilactobacillus brevis |
| 29 | C-29 | Non-treated shalgam | Lacticaseibacillus paracasei subsp. paracasei |
| 30 | C-30 | Non-treated shalgam | Lentilactobacillus buchneri |
| 31 | C-31 | Non-treated shalgam | Lentilactobacillus buchneri |
| 32 | C-32 | Non-treated shalgam | Lentilactobacillus kefiri |
| 33 | PS-1 | Shalgam containing 25 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 34 | PS-2 | Shalgam containing 25 mg/L PS | Lentilactobacillus buchneri |
| 35 | PS-3 | Shalgam containing 25 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 36 | PS-4 | Shalgam containing 25 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 37 | PS-5 | Shalgam containing 25 mg/L PS | Lentilactobacillus buchneri |
| 38 | PS-6 | Shalgam containing 25 mg/L PS | Leuconostoc mesenteroides |
| 39 | PS-7 | Shalgam containing 100 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 40 | PS-8 | Shalgam containing 100 mg/L PS | Leuconostoc mesenteroides |
| 41 | PS-9 | Shalgam containing 100 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 42 | PS-10 | Shalgam containing 100 mg/L PS | Leuconostoc mesenteroides |
| 43 | PS-11 | Shalgam containing 800 mg/L PS | Lentilactobacillus buchneri |
| 44 | PS-12 | Shalgam containing 800 mg/L PS | Lacticaseibacillus paracasei subsp. paracasei |
| 45 | PS-13 | Shalgam containing 800 mg/L PS | Lentilactobacillus buchneri |
| 46 | SB-1 | Shalgam containing 25 mg/L SB | Levilactobacillus brevis |
| 47 | SB-2 | Shalgam containing 25 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 48 | SB-3 | Shalgam containing 25 mg/L SB | Lactiplantibacillus pentosus |
| 49 | SB-4 | Shalgam containing 25 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 50 | SB-5 | Shalgam containing 25 mg/L SB | Levilactobacillus brevis |
| 51 | SB-6 | Shalgam containing 25 mg/L SB | Levilactobacillus brevis |
| 52 | SB-7 | Shalgam containing 25 mg/L SB | Lactiplantibacillus pentosus |
| 53 | SB-8 | Shalgam containing 25 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 54 | SB-9 | Shalgam containing 100 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 55 | SB-10 | Shalgam containing 100 mg/L SB | Lactiplantibacillus pentosus |
| 56 | SB-11 | Shalgam containing 100 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 57 | SB-12 | Shalgam containing 100 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 58 | SB-13 | Shalgam containing 100 mg/L SB | Lentilactobacillus buchneri |
| 59 | SB-14 | Shalgam containing 100 mg/L SB | Lactiplantibacillus pentosus |
| 60 | SB-15 | Shalgam containing 100 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 61 | SB-16 | Shalgam containing 100 mg/L SB | Lentilactobacillus buchneri |
| 62 | SB-17 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 63 | SB-18 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 64 | SB-19 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 65 | SB-20 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 66 | SB-21 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 67 | SB-22 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 68 | SB-23 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 69 | SB-24 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 70 | SB-25 | Shalgam containing 400 mg/L SB | Lactiplantibacillus pentosus |
| 71 | SB-26 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 72 | SB-27 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 73 | SB-28 | Shalgam containing 400 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 74 | SB-29 | Shalgam containing 400 mg/L SB | Lactiplantibacillus pentosus |
| 75 | SB-30 | Shalgam containing 400 mg/L SB | Lentilactobacillus buchneri |
| 76 | SB-31 | Shalgam containing 800 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 77 | SB-32 | Shalgam containing 800 mg/L SB | Lentilactobacillus buchneri |
| 78 | SB-33 | Shalgam containing 800 mg/L SB | Lacticaseibacillus paracasei subsp. paracasei |
| 79 | NAT-1 | Shalgam containing 25 mg/L NAT | Lentilactobacillus buchneri |
| 80 | NAT-2 | Shalgam containing 25 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 81 | NAT-3 | Shalgam containing 25 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 82 | NAT-4 | Shalgam containing 25 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 83 | NAT-5 | Shalgam containing 100 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 84 | NAT-6 | Shalgam containing 100 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 85 | NAT-7 | Shalgam containing 100 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 86 | NAT-8 | Shalgam containing 400 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 87 | NAT-9 | Shalgam containing 800 mg/L NAT | Lacticaseibacillus paracasei subsp. paracasei |
| 88 | NAT-10 | Shalgam containing 800 mg/L NAT | Lentilactobacillus buchneri |
| 89 | NAT-11 | Shalgam containing 800 mg/L NAT | Lactiplantibacillus pentosus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujdeci, G.N.; Tanguler, H.; Macit, H.; Kabak, B. Effect of Three Different Preservatives on the Microbiota of Shalgam, a Traditional Lactic Acid Fermented Beverage. Foods 2023, 12, 4075. https://doi.org/10.3390/foods12224075
Mujdeci GN, Tanguler H, Macit H, Kabak B. Effect of Three Different Preservatives on the Microbiota of Shalgam, a Traditional Lactic Acid Fermented Beverage. Foods. 2023; 12(22):4075. https://doi.org/10.3390/foods12224075
Chicago/Turabian StyleMujdeci, Gamze Nur, Hasan Tanguler, Hasan Macit, and Bulent Kabak. 2023. "Effect of Three Different Preservatives on the Microbiota of Shalgam, a Traditional Lactic Acid Fermented Beverage" Foods 12, no. 22: 4075. https://doi.org/10.3390/foods12224075
APA StyleMujdeci, G. N., Tanguler, H., Macit, H., & Kabak, B. (2023). Effect of Three Different Preservatives on the Microbiota of Shalgam, a Traditional Lactic Acid Fermented Beverage. Foods, 12(22), 4075. https://doi.org/10.3390/foods12224075








