High Pressure and Pasteurization Effects on Dairy Cream †
Abstract
1. Introduction
2. Materials and Methods
2.1. Cream Samples
2.2. Preparation of Cream Samples and Inoculation
2.3. HPP Treatment of Samples
2.4. Storage Conditions
2.5. Microbial Analyses
2.6. pH and Color
2.7. Apparent Viscosity Measurements
2.8. Fatty Acid Determination
2.9. Volatile Profile
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microbial Analysis
3.2. pH and Color
3.3. Viscosity
3.4. Fatty Acid Analysis
3.5. Volatile Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deosarkar, S.S.; Khedkar, C.D.; Kalyankar, S.D.; Sarode, A.R. Cream: Types of cream. In Encyclopedia of Food and Health; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 331–337. ISBN 9780123849533. [Google Scholar]
- Wang, T.; Lin, L.U.; Ou, J.I.E.; Chen, M.I.N.; Yan, W. The inhibitory effects of varying water activity, pH, and nisin content on Staphylococcus aureus growth and enterotoxin A production in whipping cream. J. Food Saf. 2016, 37, e12280. [Google Scholar] [CrossRef]
- O’Sullivan, M. The Technology of Dairy Products, 2nd ed.; Early, R., Ed.; Springer: New York, NY, USA, 1993; Volume 19. [Google Scholar]
- Hogan, E.; Kelly, A.L.; Sun, D.W. High Pressure Processing of Foods. An Overview. In Emerging Technologies for Food Processing; Academic Press: Cambridge, MA, USA, 2005; Volume 1, ISBN 9780126767575. [Google Scholar]
- Elamin, W.M.; Endan, J.B.; Yosuf, Y.A.; Shamsudin, R.; Anvarjon, A. High pressure processing technology and equipment evolution: A review. J. Eng. Sci. Technol. Rev. 8 2015, 8, 75–83. [Google Scholar] [CrossRef]
- Pinto, C.A.; Moreira, S.A.; Fidalgo, L.G.; Inácio, R.S.; Barba, F.J.; Saraiva, J.A. Effects of high-pressure processing on fungi spores: Factors affecting spore germination and inactivation and impact on ultrastructure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Ravash, N.; Peighambardoust, S.H.; Soltanzadeh, M.; Lorenzo, J.M. Impact of high-pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Dumay, E.; Lambert, C.; Funtenberger, S.; Cheftel, J.C. Effects of high pressure on the physico-chemical characteristics of dairy creams and model oil/water emulsions. LWT Food Sci. Technol. 1996, 29, 606–625. [Google Scholar] [CrossRef]
- Raffalli, J.; Rosec, J.C.; Carlez, A.; Dumay, E.; Richard, N.; Cheftel, J.C. High pressure stress and inactivation of Listeria innocua in inoculated dairy cream. Sci. Aliments 1994, 14, 349–358. [Google Scholar]
- Gervilla, R.; Ferragut, V.; Guamis, B. High pressure inactivation of microorganisms inoculated into ovine milk of different fat contents. J. Dairy Sci. 2000, 83, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Neoκleous, I.; Tarapata, J.; Papademas, P. Non-thermal Processing Technologies for Dairy Products: Their Effect on Safety and Quality Characteristics. Front. Sustain. Food Syst. 2022, 6, 856199. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission; Food and Agriculture Organization: Rome, Italy, 2000; Volume 1, pp. 1–10.
- Ahn, D.U.; Mendonça, A.F.; Feng, X. The Storage and Preservation of Meat: II—Nonthermal Technologies. In Lawrie´s Meat Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–263. ISBN 9780081006948. [Google Scholar]
- Pinto, C.; Moreira, S.A.; Fidalgo, L.G.; Santos, M.D.; Vidal, M.; Delgadillo, I.; Saraiva, J.A. Impact of different hyperbaric storage conditions on microbial, physicochemical and enzymatic parameters of watermelon juice. Food Res. Int. 2017, 99, 123–132. [Google Scholar] [CrossRef]
- Institute of Medicine (US); National Research Council (US) Committee. International Microbiological Criteria for Dairy Products. In Scientific Criteria to Ensure Safe Food; National Academies Press: Washington, DC, USA, 2003; p. 424. ISBN 0309509203. [Google Scholar]
- Milovanovic, B.; Tomovic, V.; Djekic, I.; Miocinovic, J.; Solowiej, B.G.; Lorenzo, J.M.; Barba, F.J.; Tomasevic, I. Colour assessment of milk and milk products using computer vision system and colorimeter. Int. Dairy J. 2021, 120, 105084. [Google Scholar] [CrossRef]
- Duarte, R.V.; Casal, S.; Da Silva, J.A.L.; Gomes, A.; Delgadillo, I.; Saraiva, J.A. Nutritional, Physicochemical, and Endogenous Enzyme Assessment of Raw Milk Preserved under Hyperbaric Storage at Variable Room Temperature. ACS Food Sci. Technol. 2022, 2, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Shepard, L.; Miracle, R.E.; Leksrisompong, P.; Drake, M.A. Relating sensory and chemical properties of sour cream to consumer acceptance. J. Dairy Sci. 2013, 96, 5435–5454. [Google Scholar] [CrossRef] [PubMed]
- Permanyer, M.; Castellote, C.; Audí, C.; Castell, M. Maintenance of breast milk immunoglobulin A after high-pressure processing. J. Dairy Sci. 2010, 93, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Evert-Arriagada, K.; Hernández-Herrero, M.M.; Guamis, B.; Trujillo, A.J. Commercial application of high-pressure processing for increasing starter-free fresh cheese shelf-life. LWT Food Sci. Technol. 2014, 55, 498–505. [Google Scholar] [CrossRef]
- Arias, M.L.; Monge-Rojas, R.; Chaves, C.; Antillon, F. Effect of storage temperatures on growth and survival of Escherichia coli O157: H7 inoculated in foods from a neotropical environment. Rev. Biol. Trop. 2001, 49, 517–524. [Google Scholar] [PubMed]
- Trujillo, A.J. Applications of high-hydrostatic pressure on milk and dairy products. Int. J. High Press. Res. 2002, 22, 619–626. [Google Scholar] [CrossRef]
- Viazis, S.; Farkas, B.E.; Jaykus, L.A. Inactivation of bacterial pathogens in human milk by high-pressure processing. J. Food Prot. 2008, 71, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bozoglu, F.; Alpas, H. Injury recovery of foodborne pathogens in high hydrostatic pressure treated milk during storage. FEMS Immunol. Med. Microbiol. 2004, 40, 243–247. [Google Scholar] [CrossRef]
- Liepa, M.; Baltrukova, S.; Safety, F.; Health, A.; Bior, E.; Zagorska, J.; Galoburda, R. Survival of pathogens in high pressure processed milk. Food Sci. 2018, 1, 215–221. [Google Scholar] [CrossRef]
- Fortin, N.D. Codex alimentarius commission. Handb. Transnatl. Econ. Gov. Regimes 2009, 1, 645–653. [Google Scholar] [CrossRef]
- Gassi, J.; Famelart, M.; Lopez, C. Heat treatment of cream affects the physicochemical properties of sweet buttermilk. Dairy Sci. Technol. 2008, 88, 369–385. [Google Scholar] [CrossRef][Green Version]
- Decimo, M.; Ordónez, J.A.; Brasca, M.; Cabeza, M.C. Fatty acids released from cream by psychrotrophs isolated from bovine raw milk. Int. J. Dairy Technol. 2006, 70, 339–344. [Google Scholar] [CrossRef]
- Donsì, G.; Ferrari, G.; Maresca, P. Rheological Properties of High Pressure Milk Cream. Procedia Food Sci. 2011, 1, 862–868. [Google Scholar] [CrossRef]
- Rutkowska, J.; Bialek, M.; Adamska, A.; Zbikowska, A. Differentiation of geographical origin of cream products in Poland according to their fatty acid profile. Food Chem. 2015, 178, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Moltó-puigmartí, C.; Permanyer, M.; Isabel, A.; López-sabater, M.C. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- Juan, B.; Barron, L.J.R.; Ferragut, V.; Trujillo, A.J. Effects of high pressure treatment on volatile profile during ripening of ewe milk cheese. J. Dairy Sci. 2010, 90, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Duizer, L.; Griffiths, M.; Walkling-Ribeiro, M.; Corredig, M.; Khanal, D. Change in color and volatile composition of skim milk processed with pulsed electric field and microfiltration treatments or heat pasteurization. Foods 2014, 3, 250–268. [Google Scholar] [CrossRef]
- Garrido, M.; Contador, R.; García-Parra, J.; Delgado, F.J.; Delgado-Adámez, J.; Ramírez, R. Volatile profile of human milk subjected to high-pressure thermal processing. Food Res. Int. 2015, 78, 186–194. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Effect of high-pressure-moderate-temperature processing on the volatile profile of milk. J. Agric. Food Chem. 2006, 54, 9184–9192. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Van Der Plancken, I.; Verbeyst, L.; De Vleeschouwer, K.; Grauwet, T.; Heiniö, R.L.; Husband, F.A.; Lille, M.; Mackie, A.R.; Van Loey, A.; Viljanen, K.; et al. (Bio)chemical reactions during high pressure/high temperature processing affect safety and quality of plant-based foods. Trends Food Sci. Technol. 2012, 23, 28–38. [Google Scholar] [CrossRef]
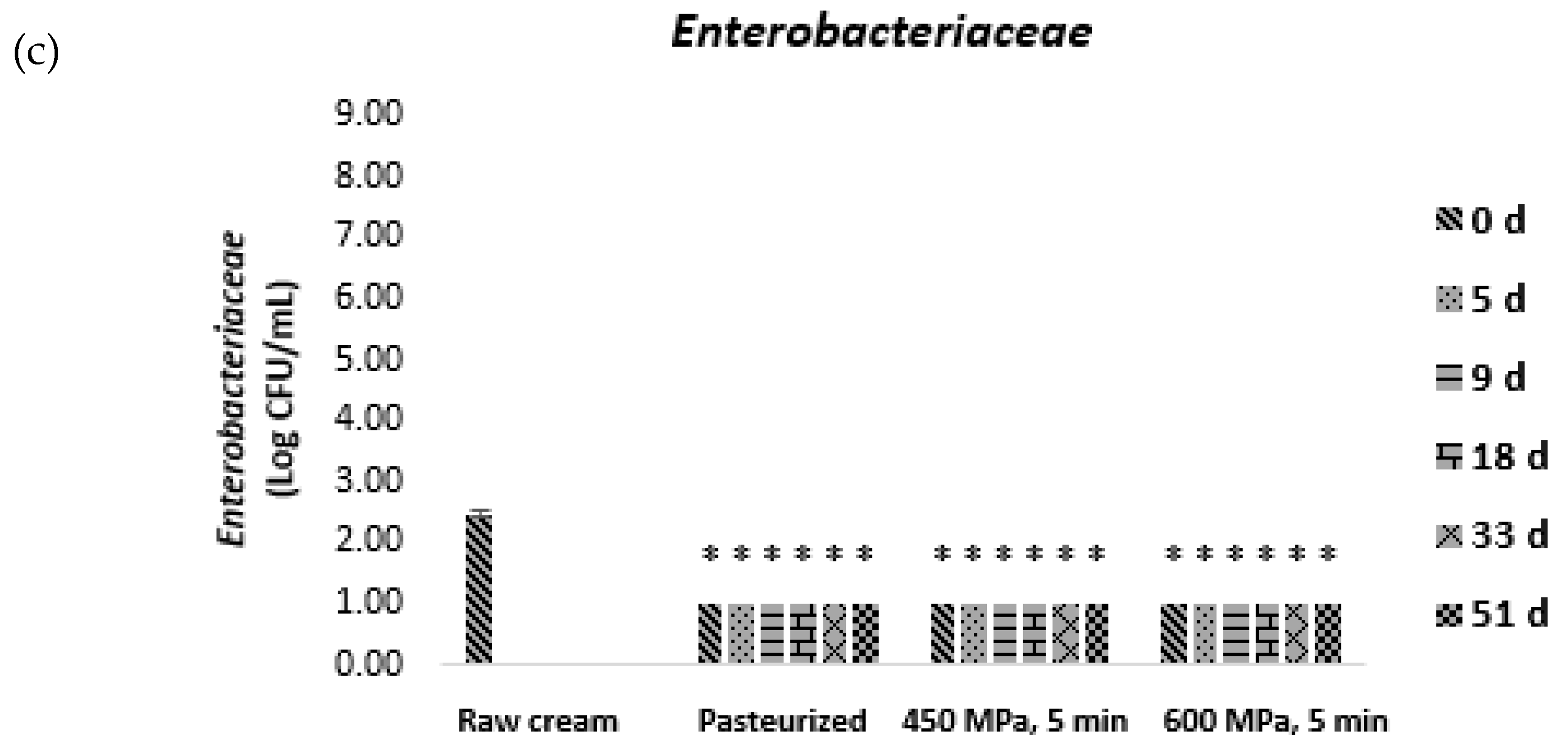
 ) and after 5 (
) and after 5 ( ), 9 (
), 9 ( ), 18 (
), 18 ( ), 33 (
), 33 ( ), and 51 (
), and 51 ( ) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a,b).
) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a,b).
 ) and after 5 (
) and after 5 ( ), 9 (
), 9 ( ), 18 (
), 18 ( ), 33 (
), 33 ( ), and 51 (
), and 51 ( ) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a,b).
) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a,b).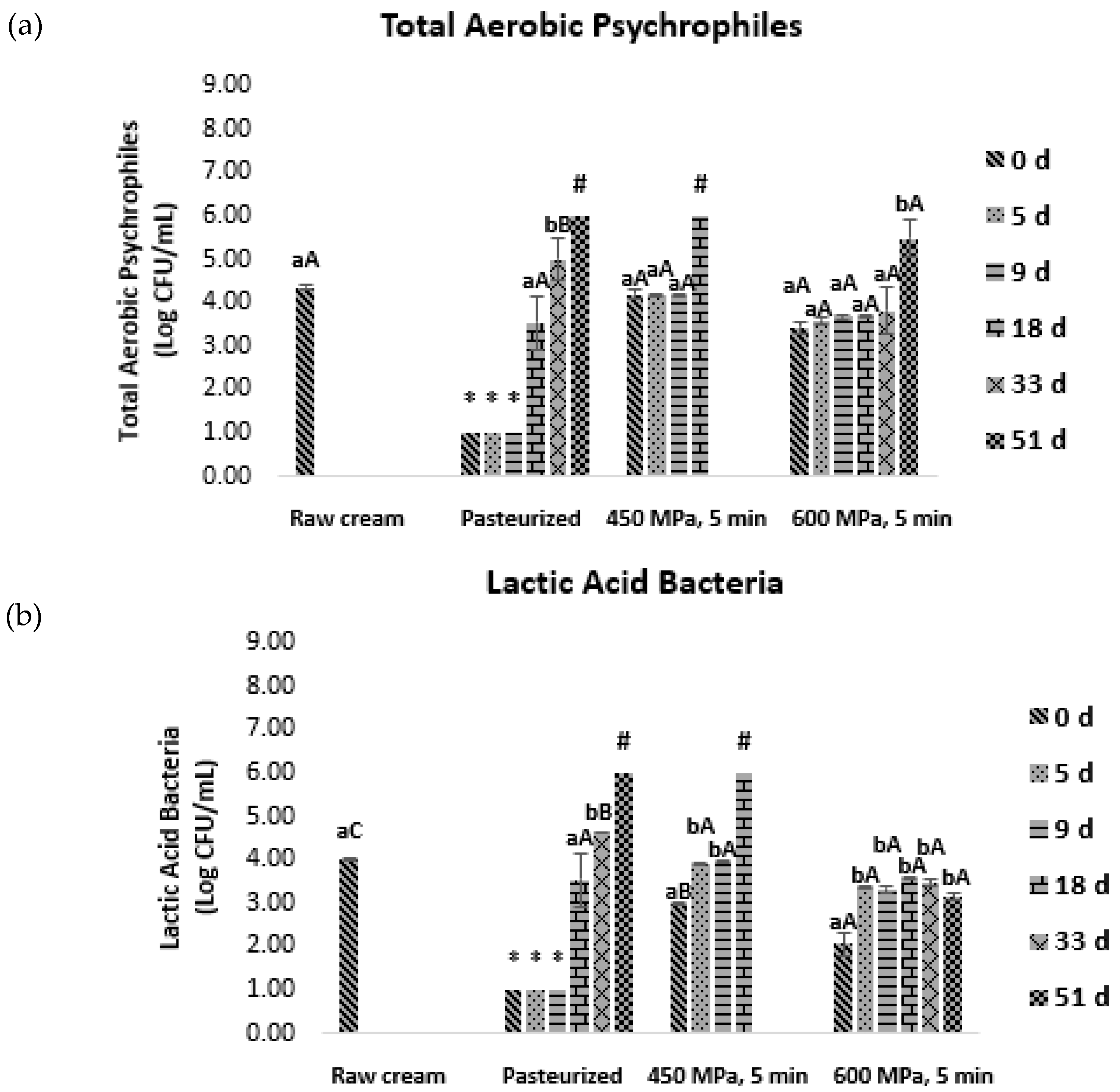
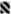 ) and after 3 (
) and after 3 ( ), 10 (
), 10 ( ), 28 (
), 28 ( ), and 52 (
), and 52 ( ) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a–c).
) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a–c).
 ) and after 3 (
) and after 3 ( ), 10 (
), 10 ( ), 28 (
), 28 ( ), and 52 (
), and 52 ( ) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a–c).
) days of storage at 4 °C. Bars with * and # represent microbial loads below the detection limit (lower than 1.00 log CFU/mL) and above 6.00 log CFU/mL, respectively. Different letters denote significant differences (p ≤ 0.05) between storage days for each condition (A,B) and treatment conditions for each storage day (a–c).

| HPP Conditions | Storage Period (Days) | Nomenclature | To Study the Effect of HPP after Processing and during Each Storage Period On: | ||
|---|---|---|---|---|---|
| Pressure (MPa) | Duration (min) | ||||
| First batch | - | - | - | Raw |
|
| - | - | 0, 5, 9, 18, 33, 51 | Pasteurized | ||
| 450 | 5 | 450/5 | |||
| 600 | 5 | 600/5 | |||
| Second batch | - | - | - | Raw |
|
| - | - | 0, 3, 10, 28, 52 | Pasteurized | ||
| 600 | 5 | 600/5 | |||
| 600 | 15 | 600/15 | |||
| Storage Time (Days) | Conditions | Shear Rate (1/s) | Viscosity (Pa·s) |
|---|---|---|---|
| 0 | Initial | 33.19 | 0.015 ± 0.001 aA |
| Heat treated | 0.017 ± 0.001 aA | ||
| 600 MPa/5 min | 0.016 ± 0.001 aA | ||
| 600 MPa/15 min | 0.015 ± 0.001 aA | ||
| 3 | Heat treated | 0.018 ± 0.002 aA | |
| 600 MPa/5 min | 0.031 ± 0.003 bB | ||
| 600 MPa/15 min | 0.026 ± 0.002 bB | ||
| 10 | Heat treated | 0.017 ± 0.002 aA | |
| 600 MPa/5 min | 0.027 ± 0.003 bB | ||
| 600 MPa/15 min | 0.026 ± 0.002 bB | ||
| 28 | Heat treated | 0.016 ± 0.002 aA | |
| 600 MPa/5 min | 0.028 ± 0.003 bB | ||
| 600 MPa/15 min | 0.030 ± 0.003 bBC | ||
| 52 | Heat treated | – | |
| 600 MPa/5 min | 0.030 ± 0.003 aB | ||
| 600 MPa/15 min | 0.034 ± 0.003 aC |
| Storage Time (Days) | Conditions | Alcohols | Acids | Aldehydes/Ketones | Aliphatic Hydrocarbons | Lactones | Total Volatiles |
|---|---|---|---|---|---|---|---|
| 0 | Initial | Nd | 16.4 ± 2.5 aA | 19.5 ± 1.3 aA | 51.6 ± 1.9 aAB | 0.2 ± 0.1 aA | 315.1 ± 26.9 aAB |
| Heat treated | 3.2 ± 0.4 aAB | 33.5 ± 4.7 aAB | 36.9 ± 1.0 aAB | 80.5 ± 0.8 aB | 1.1 ± 0.1 aB | 492.8 ± 36.7 aB | |
| 600 MPa/5 min | 4.1 ± 0.6 aAB | 54.8 ± 3.3 aB | 26.2 ± 4.6 aAB | 41.6 ± 0.4 aA | 0.4 ± 0.1 aAB | 291.1 ± 11.4 aA | |
| 600 MPa/15 min | 6.6 ± 0.6 aB | 52.6 ± 6.4 aB | 47.5 ± 1.0 bB | 6.9 ± 0.2 aA | 0.4 ± 0.1 aAB | 314.1 ± 10.7 aAB | |
| 3 | Heat treated | 3.6 ± 0.3 aA | 34.3 ± 2.8 aA | 49.6 ± 10.2 abB | 94.4 ± 2.8 abA | 0.7 ± 0.1 aA | 427.2 ± 64.5 aA |
| 600 MPa/5 min | 5.2 ± 0.3 aA | 114.2 ± 13.7 cB | 42.0 ± 0.7 abAB | 152.5 ± 3.8 bB | 0.9 ± 0.1 aAB | 493.9 ± 21.7 bA | |
| 600 MPa/15 min | 4.4 ± 0.7 aA | 53.1 ± 0.7 aA | 23.0 ± 0.8 aA | 79.4 ± 2.9 bA | 1.6 ± 0.1 bB | 479.9 ± 58.2 aA | |
| 10 | Heat treated | 4.1 ± 0.2 aA | 26.1 ± 3.7 aA | 60.5 ± 1.7 bAB | 121.6 ± 3.2 bA | 1.5 ± 0.3 aA | 373.5 ± 35.8 aA |
| 600 MPa/5 min | 6.6 ± 0.3 aA | 84.4 ± 4.8 bB | 70.8 ± 6.4 bB | 220.2 ± 13.4 cB | 2.4 ± 0.3 bB | 703.2 ± 66.1 cB | |
| 600 MPa/15 min | 8.3 ± 0.2 aA | 78.9 ± 4.12 bB | 43.4 ± 8.0 abA | 310.2 ± 6.8 dC | 2.3 ± 0.2 bAB | 782.4 ± 32.4 bB | |
| 28 | Heat treated | 20.3 ± 5.0 bB | 62.8 ± 9.8 bA | 74.5 ± 11.8 bA | 69.2 ± 5.7 aA | 2.6 ± 0.2 bA | 687.9 ± 37.8 bA |
| 600 MPa/5 min | 5.9 ± 1.2 aA | 152.5 ± 3.8 dB | 73.9 ± 2.6 bA | 346.5 ± 36.4 dB | 4.9 ± 0.3 cB | 1007.3 ± 49.9 dC | |
| 600 MPa/15 min | 4.6 ± 0.4 aA | 220.7 ± 6.7 cC | 71.4 ± 6.4 cA | 83.3 ± 2.2 bA | 5.6 ± 0.1 cB | 797.6 ± 28.6 bB | |
| 52 | Heat treated | 50.5 ± 3.7 cB | 126.1 ± 8.9 cA | 61.9 ± 12.7 bB | 64.2 ± 4.2 aA | 2.6 ± 0.2 bA | 751.2 ± 56.4 bA |
| 600 MPa/5 min | 4.1 ± 0.3 aA | 167.1 ± 6.4 dB | 52.2 ± 2.2 bAB | 119.9 ± 8.1 bB | 5.3 ± 0.5 cB | 783.3 ± 30.2 cA | |
| 600 MPa/15 min | 6.1 ± 0.3 aA | 307.0 ± 3.3 dC | 36.2 ± 3.4 abA | 224.2 ± 1.2 cC | 5.7 ± 0.2 cB | 881.1 ± 112.7 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, F.; Duarte, R.V.; Pinto, C.A.; Casal, S.; Lopes-da-Silva, J.A.; Saraiva, J.A. High Pressure and Pasteurization Effects on Dairy Cream. Foods 2023, 12, 3640. https://doi.org/10.3390/foods12193640
Machado F, Duarte RV, Pinto CA, Casal S, Lopes-da-Silva JA, Saraiva JA. High Pressure and Pasteurization Effects on Dairy Cream. Foods. 2023; 12(19):3640. https://doi.org/10.3390/foods12193640
Chicago/Turabian StyleMachado, Fernanda, Ricardo V. Duarte, Carlos A. Pinto, Susana Casal, José A. Lopes-da-Silva, and Jorge A. Saraiva. 2023. "High Pressure and Pasteurization Effects on Dairy Cream" Foods 12, no. 19: 3640. https://doi.org/10.3390/foods12193640
APA StyleMachado, F., Duarte, R. V., Pinto, C. A., Casal, S., Lopes-da-Silva, J. A., & Saraiva, J. A. (2023). High Pressure and Pasteurization Effects on Dairy Cream. Foods, 12(19), 3640. https://doi.org/10.3390/foods12193640








