Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Microalgae Isolation and Cultivation
2.3. Morphological and Molecular Identification
2.4. Centesimal Composition and Fatty Acids Profile
2.5. Preparation of the Microalgae Extract
2.6. Phenolic Content of ChA Extract
2.6.1. Bioactive Molecules Content
2.6.2. Phenolic Compounds Profiling by UHPLC-ESI-MS/MS
2.7. Antioxidant Activity Assays
2.8. Endothelial Cell Culture and Treatments
2.8.1. Immunoenzymatic Assays
2.8.2. RNA Extraction and Real-Time RT-PCR
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Molecular Identification
3.2. Centesimal and Fatty Acid Composition
3.3. Bioactive Compounds Quantitation and Antioxidant Activity of ChA Extract
3.4. Phenolic Compounds Profiling by UHPLC-ESI-MS/MS
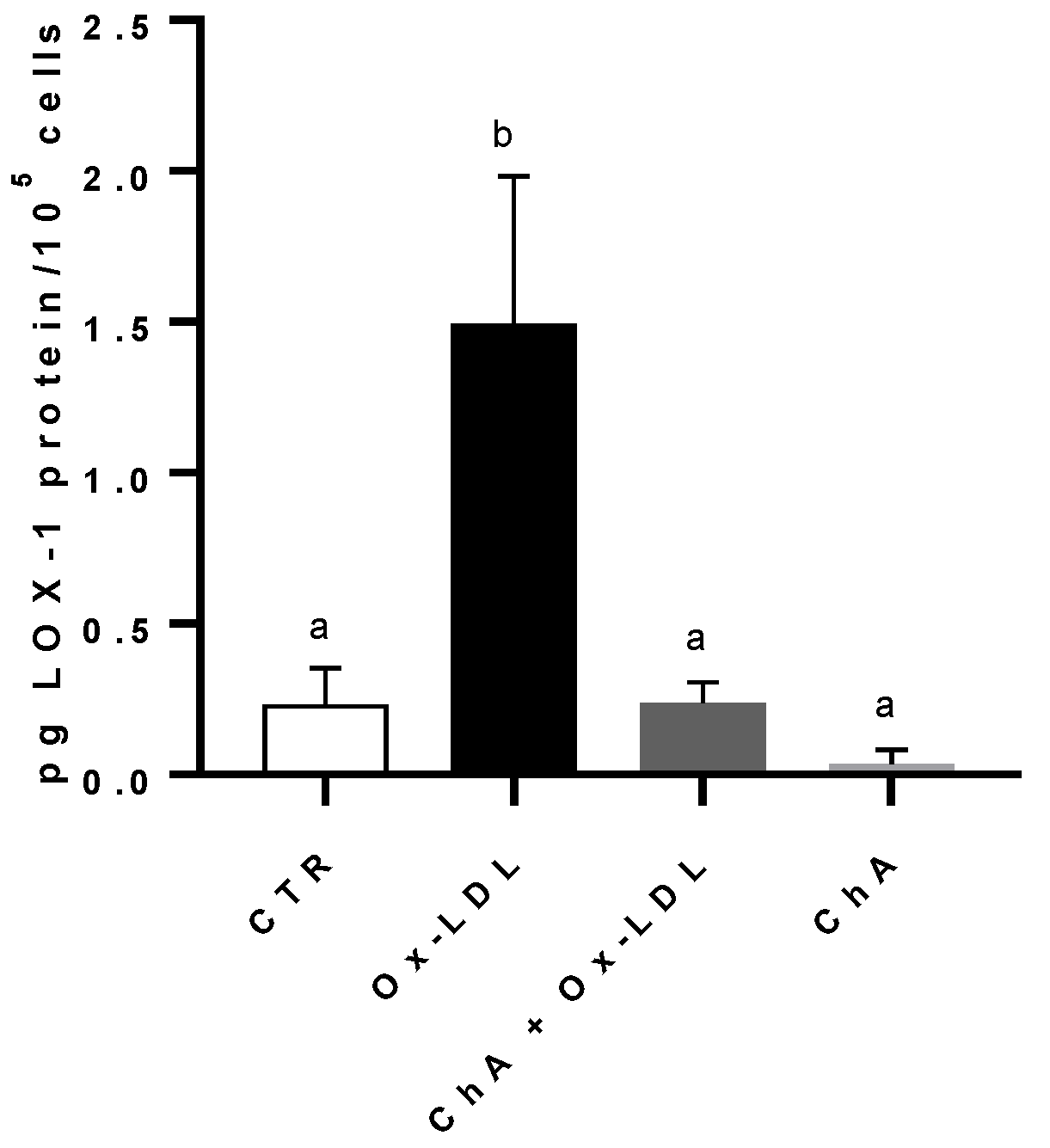
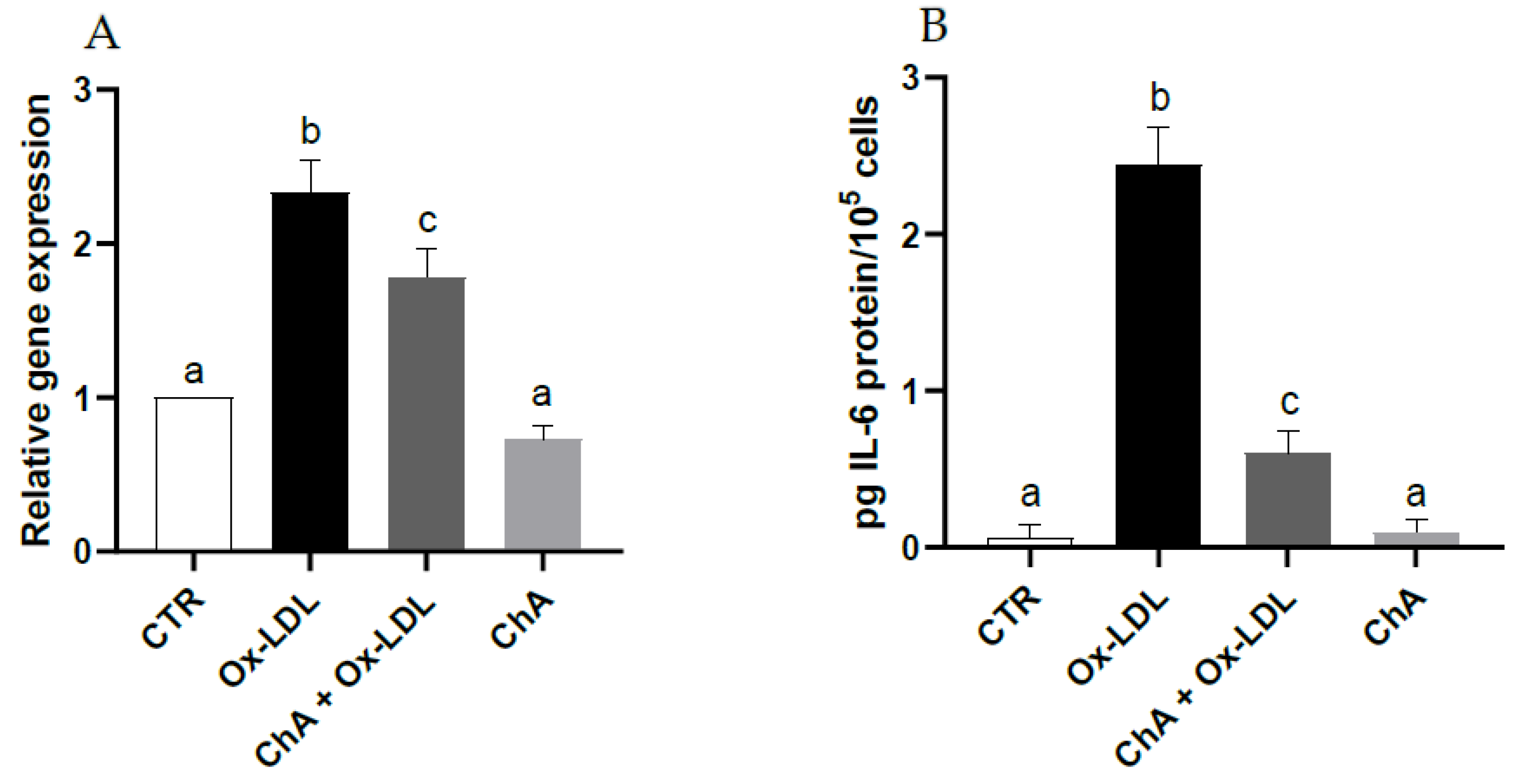
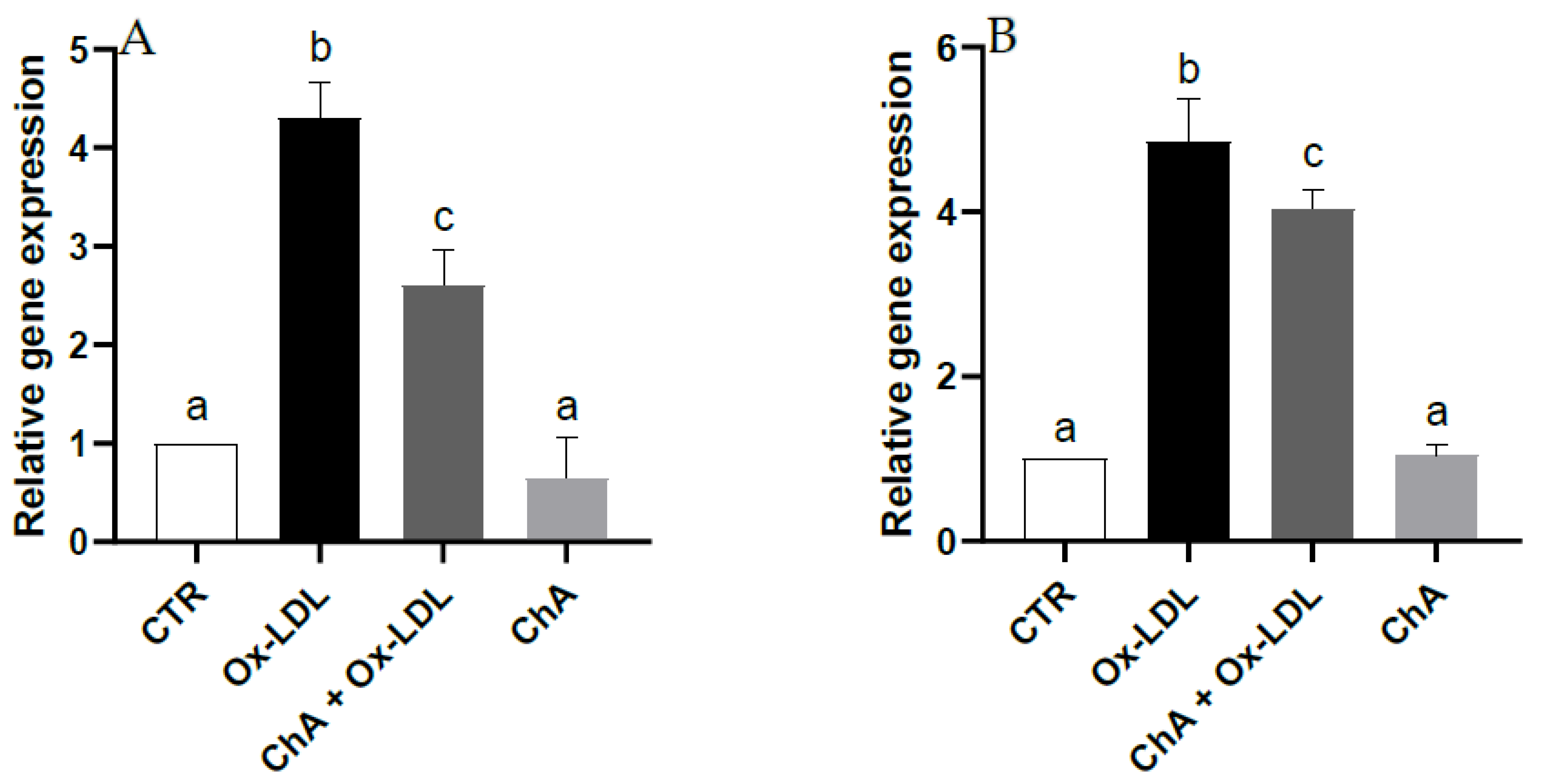
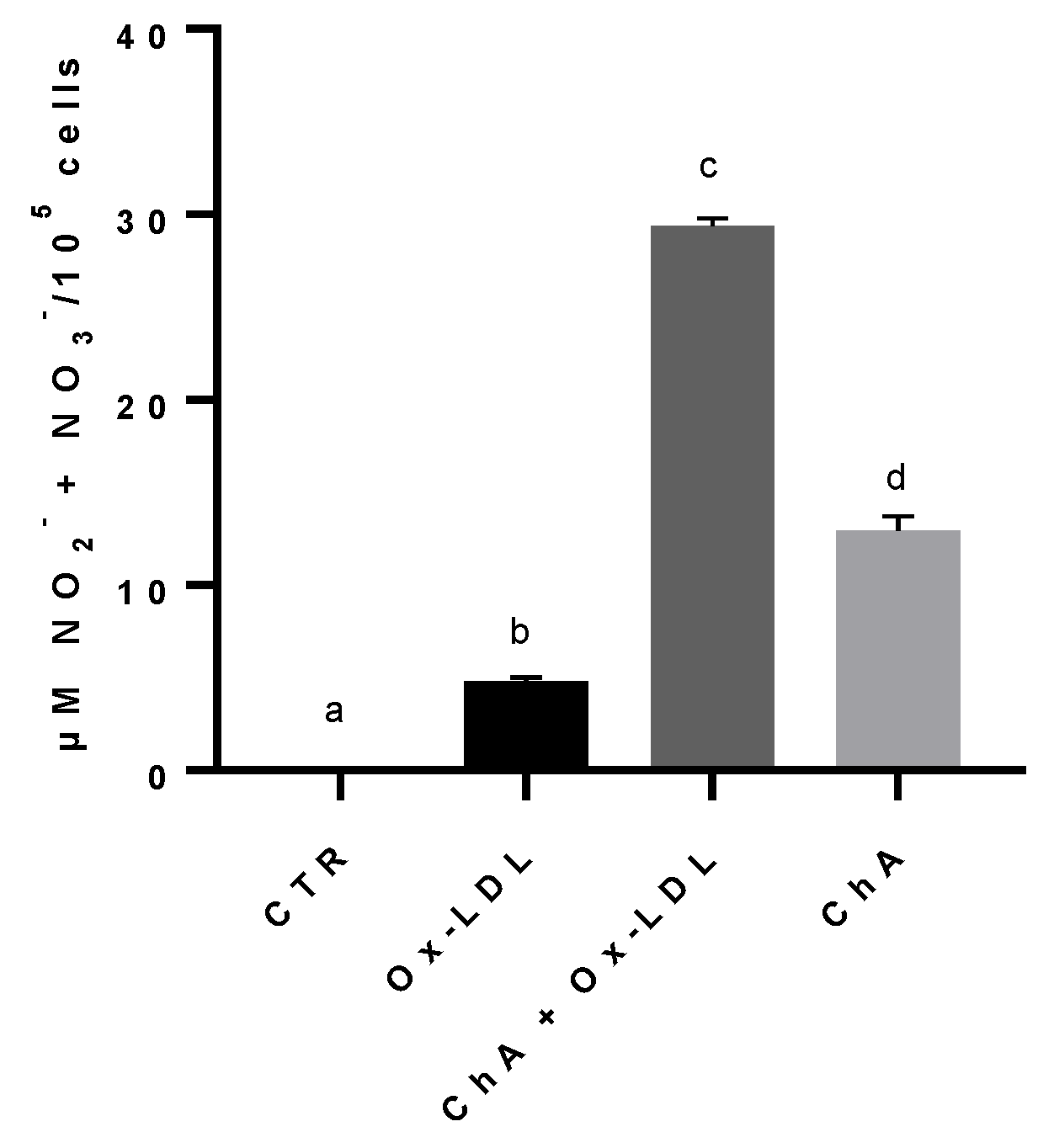
3.5. Effects on HMEC-1 Cells Treated with Ox-LDL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cezare-Gomes, E.A.; del Carmen Mejia-da-Silva, L.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications—A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Morales, E.; Luna, V.; Navarro, L.; Santana, V.; Gordillo, A.; Arévalo, A. Diversidad de microalgas y cianobacterias en muestras provenientes de diferentes provincias del Ecuador, destinadas a una colección de cultivos. Rev. Ecuat. Med. Cienc. Biol. 2013, 34, 129–149. [Google Scholar] [CrossRef]
- Kiran, B.R.; Venkata Mohan, S. Microalgal Cell Biofactory—Therapeutic, Nutraceutical and Functional Food Applications. Plants 2021, 10, 836. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health Applications of Bioactive Compounds from Marine Microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Promises and challenges of microalgal antioxidant production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Jatmiko, Y.D.; Nurdiani, R.; Miftachurrochmah, A.; Wakayama, M. Freshwater Microalgae as Promising Food Sources: Nutritional and Functional Properties. Open Microbiol. J. 2022, 16, e187428582206200. [Google Scholar] [CrossRef]
- Stepp, D.W.; Pollock, D.M.; Frisbee, J.C. Low-flow vascular remodeling in themetabolic syndrome X. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, 964–970. [Google Scholar] [CrossRef]
- Cardillo, C.; Campia, U.; Bryant, M.B.; Panza, J.A. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 2002, 106, 1783–1787. [Google Scholar] [CrossRef]
- Le Goff, M.; Delbrut, A.; Quinton, M.; Pradelles, R.; Bescher, M.; Burel, A.; Schoefs, B.; Sergent, O.; Lagadic-Gossmann, D.; Le Ferrec, E.; et al. Protective action of Ostreococcus tauri and Phaeodactylum tricornutum extracts towards benzo[a]pyrene-induced cytotoxicity in endothelial cells. Mar. Drugs 2019, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Kawachi, M. Traditional Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 83–100. ISBN 0-12-088426-7. [Google Scholar]
- Reyes, H.T.; Chiellini, C.; Barozzi, E.; Sandoval, C.; Echeverría, C.; Guglielminetti, L. Exploring the Physiological Multiplicity of Native Microalgae from the Ecuadorian Highland, Italian Lowland and Indoor Locations in Response to UV-B. Int. J. Mol. Sci. 2023, 24, 1346. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria 1. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, Y.T.; White, J.G. Species-specific PCR primers for Pythium developed from ribosomal ITS1 region. Lett. Appl. Microbiol. 2003, 37, 127–132. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Bianchi, L.; Mugnai, C. Effect of dietary α-linolenic acid and vitamin E on the fatty acid composition, storage stability and sensory traits of rabbit meat. Meat Sci. 2004, 66, 407–413. [Google Scholar] [CrossRef]
- Uma, R.; Sivasubramanian, V.; Niranjali, D.S. Preliminary phytochemical analysis and in vitro antibacterial screening of green microalgae Desmococcus olivaceous, Chlorococcum humicola and Chlorella vulgaris. J. Algal Biomass Util. 2011, 2, 82–93. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct. Plant Biol. 2016, 43, 607–619. [Google Scholar]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The effect of sourdough fermentation on Triticum dicoccum from Garfagnana: 1H NMR characterization and analysis of the antioxidant activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef] [PubMed]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic. Res. 2014, 48, 841–848. [Google Scholar] [CrossRef]
- Pombert, J.F.; Lemieux, C.; Turmel, M. The complete chloroplast DNA sequence of the green alga Oltmannsiellopsis viridis reveals a distinctive quadripartite architecture in the chloroplast genome of early diverging ulvophytes. BMC Biol. 2006, 4, 3. [Google Scholar] [CrossRef]
- Vornoli, A.; Grande, T.; Lubrano, V.; Vizzarri, F.; Gorelli, C.; Raffaelli, A.; Della Croce, C.M.; Zarate Baca, S.; Sandoval, C.; Longo, V.; et al. In vitro characterization of antioxidant, antibacterial and antimutagenic activities of the green microalga Ettlia pseudoalveolaris. Antioxidants 2023, 12, 1308. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L. Annals of Internal Medicine Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Caroppo, C.; Pagliara, P. Microalgae: A Promising Future. Microorganisms 2022, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.M.; Farooq, Z.; Hafeez, A.; Sajid, M.W.; Tariq, M.R.; Ali, S.W.; Ali, S.; Shafiq, M.; Iftikhar, M.; Safdar, W.; et al. Supplementation of PUFA extracted from microalgae for the development of chicken patties. Peer J. 2023, 11, e15355. [Google Scholar]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.T.; da Costa, D.C.; Vieira, A.A.H.; Antoniosi Filho, N.R. Analysis of Major Carotenoids and Fatty Acid Composition of Freshwater Microalgae. Heliyon 2019, 5, e01529. [Google Scholar] [CrossRef]
- Maheshwari, N.; Krishna, P.K.; Thakur, I.S.; Srivastava, S. Biological fixation of carbon dioxide and biodiesel production using microalgae isolated from sewage waste water. Environ. Sci. Pollut. Res. 2020, 27, 27319–27329. [Google Scholar] [CrossRef]
- Maurício, T.; Couto, D.; Lopes, D.; Conde, T.; Pais, R.; Batista, J.; Melo, T.; Pinho, M.; Moreira, A.S.P.; Trovão, M.; et al. Differences and similarities in lipid composition, nutritional value, and bioactive potential of four edible Chlorella vulgaris strains. Foods 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Rugnini, L.; Rossi, C.; Antonaroli, S.; Rakaj, A.; Bruno, L. The influence of light and nutrient starvation on morphology, biomass and lipid content in seven strains of green microalgae as a source of biodiesel. Microorganisms 2020, 8, 1254. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; Suárez de Tangil, M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Faraloni, C.; Di Lorenzo, T.; Bonetti, A. Impact of light stress on the synthesis of both antioxidants polyphenols and carotenoids, as fast photoprotective response in Chlamydomonas reinhardtii: New prospective for biotechnological potential of this microalga. Symmetry 2021, 13, 2220. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 1999, 11, 559–566. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K. Induced accumulation of polyphenolics and flavonoids in cyanobacteria under salt stress protects organisms through enhanced antioxidant activity. Am. J. Plant Sci. 2014, 5, 43916. [Google Scholar] [CrossRef]
- Orr, V.; Rehmann, L. Improvement of the Nile Red fluorescence assay for determination of total lipid content in microalgae independent of chlorophyll content. J. Appl. Phycol. 2015, 27, 2181–2189. [Google Scholar] [CrossRef]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic Compounds, Carotenoids, and Antioxidant Capacities of a Thermo-Tolerant Scenedesmus sp. (Chlorophyta) Extracted with Different Solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef]
- Shetty, V.; Sibi, G. Relationship between total phenolics content and antioxidant activities of microalgae under autotrophic, heterotrophic and mixotrophic growth. J. Food Resour. Sci. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Molnár, Z.; Stirk, W.A.; Ördög, V.; Van Staden, J. Changes in Phytochemical Content and Pharmacological Activities of Three Chlorella Strains Grown in Different Nitrogen Conditions. J. Appl. Phycol. 2016, 28, 149–159. [Google Scholar] [CrossRef]
- Dinev, T.; Tzanova, M.; Velichkova, K.; Dermendzhieva, D.; Beev, G. Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L., Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Appl. Sci. 2021, 11, 4745. [Google Scholar] [CrossRef]
- Tiong, I.K.R.; Nagappan, T.; Wahid, M.E.A.; Muhammad, T.S.T.; Tatsuki, T.; Satyantini, W.H.; Mahasri, G.; Sorgeloos, P.; Sung, Y.Y. Antioxidant capacity of five microalgae species and their effect on heat shock protein 70 expression in the brine shrimp Artemia. Aquac. Rep. 2020, 18, 100433. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Mfotie Njoya, E. Chapter 31: Medicinal plants, antioxidant potential, and cancer. In Cancer, 2nd ed.; Academic Press: San Diego, CA, USA, 2021; pp. 349–357. [Google Scholar]
- Avila, J.G.; de Liverant, J.G.; Martınez, A.; Martınez, G.; Munoz, J.L.; Arciniegas, A.; de Vivar, A.R. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J. Ethnopharmacol. 1999, 66, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.E.; De Cooman, L. Detection of Flavonoids in Microalgae from Different Evolutionary Lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside protects pancreatic β-cells against ER-stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Suzuki, T.; Kohno, H.; Hasegawa, A.; Toshima, S.; Amaki, T.; Kurabayashi, M.; Nagai, R.; Clinical Trial of Oxidized LDL (CTOL) investigators. Diagnostic implications of circulating oxidized low density lipoprotein levels as a biochemical risk marker of coronary artery disease. Clin. Biochem. 2002, 35, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.W.; Freestone, K.; Homer-Vanniasinkam, S.; Ponnambalam, S. The LOX-1 scavenger receptor and its implications in the treatment of vascular disease. Cardiol. Res. Pract. 2012, 2012, 632408. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Mitra, S.; Khaidakov, M.; Wang, X.; Singla, S.; Ding, Z.; Liu, S.; Mehta, J.L. Current concepts of the role of oxidized LDL receptors in atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 150–159. [Google Scholar] [CrossRef]
- Cominacini, L.; Fratta Pasini, A.; Garbin, U.; Pastorino, A.; Rigoni, A.; Nava, C.; Davoli, A.; Lo Cascio, V.; Sawamura, T. The platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells. J. Am. Coll. Cardiol. 2003, 41, 499–507. [Google Scholar] [CrossRef]
- Kita, T.; Kume, N.; Minami, M.; Hayashida, K.; Murayama, T.; Sano, H.; Moriwaki, H.; Kataoka, H.; Yokode, M. Role of oxidized LDL in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 199–206. [Google Scholar] [CrossRef]
- Lubrano, V.; Gabriele, M.; Puntoni, M.R.; Longo, V.; Pucci, L. Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell. Mol. Biol. Lett. 2015, 20, 310–322. [Google Scholar] [CrossRef]
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Silva, H. The role of food supplementation in microcirculation—A comprehensive review. Biology 2021, 10, 616. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Silva, H. The vascular effects of isolated isoflavones—A focus on the determinants of blood pressure regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Oxidative stress, nitric oxide synthase, and superoxide dismutase: A matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension 2008, 51, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Vassalle, C.; Blandizzi, C.; Del Tacca, M.; Palombo, C.; L’Abbate, A.; Baldi, S.; Natali, A. The effect of lipoproteins on endothelial nitric oxide synthase is modulated by lipoperoxides. Eur. J. Clin. Investig. 2003, 33, 117–125. [Google Scholar] [CrossRef]
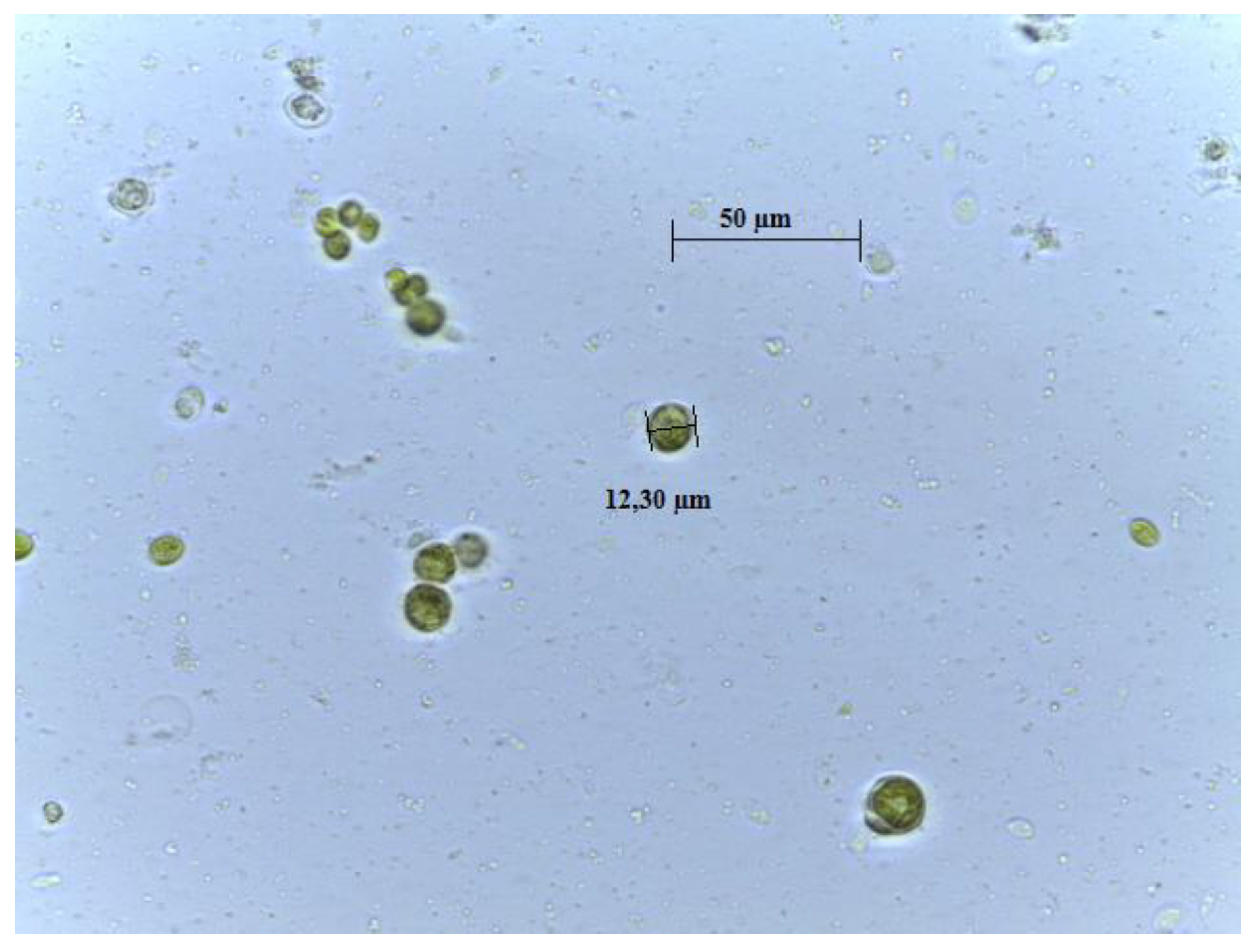
| Sequency | Taxonomy | Identity (%) | Sequency GenBank (NCBI) | References |
|---|---|---|---|---|
| Chla | Chlamydomonas agloeformis | 99.5 | L43351.1 | [30] |
| Reference Compounds | ChA |
|---|---|
| Moisture | 50.25 ± 0.03 |
| Ashes | 28.15 ± 0.04 |
| Lipids | 1.21 ± 0.11 |
| Protein | 13.46 ± 0.12 |
| Crude Fiber | 6.93 ± 0.01 |
| SFAs | |
| C14:0 | 1.45 ± 0.03 |
| C15:0 | 0.51 ± 0.01 |
| C16:0 | 39.46 ± 0.25 |
| C18:0 | 1.74 ± 0.02 |
| C20:0 | 0.74 ± 0.00 |
| MUFAs | |
| C14:1 | 0.38 ± 0.01 |
| C16:1-n7 | 2.85 ± 0.03 |
| C18:1-n9 | 1.53 ± 0.02 |
| PUFAs | |
| C18:2-n6 (LA) | 17.07 ± 0.09 |
| C18:3-n3 (ALA) | 32.49 ± 0.19 |
| C20:4 (AA) | 0.95 ± 0.03 |
| C22:5-n3 (DPA) | 0.83 ± 0.01 |
| ChA | ||
|---|---|---|
| Bioactive compounds | Total phenols (mg GAE/100 g dw) | 177.55 ± 25.94 |
| Flavonoids (mg CE/100 g dw) | 440.15 ± 152.38 | |
| Flavonols (mg QE/100 g dw) | 203.80 ± 97.02 | |
| Antioxidant activity | ORAC (µmol TE/100 g dw) | 4827.66 ±1.33 |
| FRAP (µmol TE/100 g dw) | 45.53 ± 5.53 | |
| DPPH (µmol TE/100 g dw) | 375.51 ± 3.6 |
| Phenolic Compound | Concentration (µg/g dw) |
|---|---|
| Gallic Acid | 1.81 ± 0.01 |
| Cyanidin 3,5-O-diglucoside | 0.06 ± 0.00 |
| 3-O-Caffeoylquinic acid | 1.64 ± 0.05 |
| Caffeic Acid | 1.82 ± 0.02 |
| Vanillic Acid | 1.33 ± 0.10 |
| 4-Coumaric Acid | 0.49 ± 0.01 |
| trans-Ferulic Acid | 0.12 ± 0.04 |
| Rosmarinic Acid | 0.43 ± 0.18 |
| ∑ Phenolic acids * | 7.69 |
| Quercetin | 1.99 ± 0.33 |
| Quercetin 3-O-glucoside | 46.14 ± 8.13 |
| Quercetin 3,4-O-diglucoside | 0.83 ± 0.03 |
| Quercetin 3-O-rutinoside | 1.65 ± 0.33 |
| Kaempferol 7-O-glucoside | 1.02 ± 0.12 |
| Kaempferol 3-O-glucoside | 0.31 ± 0.19 |
| Kaempferol 3-O-rutinoside | 0.38 ± 0.05 |
| ∑ Flavonols * | 52.32 |
| Luteolin | 0.34 ± 0.06 |
| ∑ Flavones * | 0.34 |
| Apigenin | 0.02 ± 0.01 |
| (+)-Catechin | 1.71 ± 0.09 |
| (−)-Epicatechin | 29.40 ± 2.51 |
| ∑ Flavan-3-ols * | 31.14 |
| Hydroxytyrosol | 0.34 ± 0.02 |
| Verbascoside | 158.17 ± 16.67 |
| Oleuropein | 3.08 ± 0.00 |
| Ligstroside | 0.40 ± 0.00 |
| Phloridzin | 0.02 ± 0.00 |
| Phloretin | 7.06 ± 0.10 |
| Resveratrol 3-O-glucoside | 0.03 ± 0.01 |
| Naringenin | 0.03 ± 0.02 |
| ∑ Others * | 169.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, T.; Vornoli, A.; Lubrano, V.; Vizzarri, F.; Raffaelli, A.; Gabriele, M.; Novoa, J.; Sandoval, C.; Longo, V.; Echeverria, M.C.; et al. Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity. Foods 2023, 12, 3147. https://doi.org/10.3390/foods12173147
Grande T, Vornoli A, Lubrano V, Vizzarri F, Raffaelli A, Gabriele M, Novoa J, Sandoval C, Longo V, Echeverria MC, et al. Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity. Foods. 2023; 12(17):3147. https://doi.org/10.3390/foods12173147
Chicago/Turabian StyleGrande, Teresa, Andrea Vornoli, Valter Lubrano, Francesco Vizzarri, Andrea Raffaelli, Morena Gabriele, Jeniffer Novoa, Carla Sandoval, Vincenzo Longo, Maria Cristina Echeverria, and et al. 2023. "Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity" Foods 12, no. 17: 3147. https://doi.org/10.3390/foods12173147
APA StyleGrande, T., Vornoli, A., Lubrano, V., Vizzarri, F., Raffaelli, A., Gabriele, M., Novoa, J., Sandoval, C., Longo, V., Echeverria, M. C., & Pozzo, L. (2023). Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity. Foods, 12(17), 3147. https://doi.org/10.3390/foods12173147








