Abstract
Tea is the most consumed drink worldwide due to its pleasant taste and various beneficial effects on human health. This paper assesses the physicochemical analysis of different varieties of tea (leaves, flowers, and instant) after prior drying and fine grinding. The thermal decomposition behavior of the tea components shows that the tea has three stages of decomposition, depending on temperature. The first stage was attributed to the volatilization of water, while the second stage involved the degradation of volatiles, polyphenols, and fatty acids. The degradation of cellulose, hemicellulose, and lignin content occurs at the highest temperature of 400 °C in the third stage. A total of 66 volatile compounds, divided into eight classes, were identified in the tea samples. The volatile compounds were classified into nine odor classes: floral, fruity, green, sweet, chemical, woody, citrus, roasted, and alcohol. In all flower and leaf tea samples, monounsaturated (MUFAs), polyunsaturated (PUFAs), and saturated fatty acids (SFAs) were identified. A high content of omega-6 was quantified in acacia, Saint John’s Wort, rose, and yarrow, while omega-3 was found in mint, Saint John’s Wort, green, blueberry, and lavender samples. The flower and leaf tea samples studied could be a good dietary source of polyphenolic compounds, essential elements. In instant tea samples, a low quantity of polyphenols and major elements were identified. The physicochemical analysis demonstrated that both flower and leaf teas have high-quality properties when compared to instant tea.
Keywords:
tea; thermal behavior; cellulose; lignin; volatile organic compounds; fatty acids; minerals 1. Introduction
Tea is the world’s most commonly consumed non-alcoholic beverage next to water because of its flavor and pleasant taste and perceived health benefits such as antioxidant, antimicrobial, immunostimulatory, and antimutagenic potential, as well as its capacity to reduce cardiovascular diseases and cholesterol levels [1,2]. Tea is globally grown in as many as 58 countries on all five continents. The total land area employed for tea is estimated to be 4.12 million ha, with a production of 5.36 million tons [3]. Tea can be divided into six types or five categories, namely non-fermented tea (green), lightly fermented tea (white), semi-fermented tea (oolong), fully fermented tea (black), and post-fermented tea (yellow and dark varieties) [4]. Green, oolong, and black tea are the most widely used tea products, having different fermentation degrees and sensory profiles, with green varieties being non-fermented and black ones being fermented [5]. The tea industry is a labor-intensive sector that employs more than 13 million people worldwide [6]. The vast consumption of tea results in more than 90% of the tea left after the beverage industry [7,8]. The tea plant cultivar, terroir (climate, geography, and soil), and growing conditions are critical in establishing each tea’s aroma profile and uniqueness. Though it is possible to produce different types of tea using the same leaves, it is challenging to find a tea cultivar appropriate to obtain all six tea types of aromas and authentic profiles [6,8].
Nowadays, the instant tea market has netted a significant amount of the world market due to its ease of use and convenience in the food industry [8,9]. The production of instant tea includes oil removal, water extraction, leaf filtration, concentration, and drying. Recent studies reported that sweet instant tea has a higher content of polyphenols, flavonoids, antioxidant capacity, phlorizin, and trilobatin than initial sweet tea. As a result, instant sweet tea can be an excellent dietary source of antioxidants for consumers [9]. Extensive differences in the metabolic profiles of early and late spring tea leaves like flavonoids, flavonols, and amino acids are acknowledged, resulting in huge discrepancies in the taste and suitability of tea at different stages [10,11]. One study analyzing green teas harvested in early, middle, and late spring at low altitudes highlighted that the concentration of amino acids decreased in a gradual manner, while the concentration of carbohydrates, flavonols, and their glycosides significantly increased following late spring [10,11]. Hence, researching the accumulation of compounds in tea samples under different harvesting seasons is of great benefit for the in-depth usage of tea resources [10,11]. Tea quality is strongly correlated with catechins, caffeine, volatile compounds, and amino acids in harvestable tea shoots [12].
The elderflower flowers of Sambucus nigra from the Caprofoliaceae family are a good source of volatile oils, anthocyanins, vitamins, and phenolic compounds such as flavonoids, tannins, and coumarins. Elderflowers have a long-standing tradition in herbal medicine, displaying diuretic, laxative, antirheumatic, antiviral, and anti-inflammatory actions, leading to a strengthening of the body’s resistance and vitality [13]. Linden tea (Tilia cordata) is part of the Tilinaceae family and a highly popular herbal plant due to its beneficial properties for the central nervous system. The linden extract possesses numerous health benefits, such as being sedative, anti-inflammatory, alleviating high blood pressure, reducing anxiety, soothing digestion, and helping respiratory tract infections and insomnia [14]. Matricaria chamomilla is one of the most popular single-ingredient herbal teas due to its numerous health benefits, some of which include treating infections, respiratory, neuropsychiatric, gastrointestinal, and liver disorders. It is also largely used as an antispasmodic, sedative, antiemetic, and antiseptic. The main constituents of the flowers are divided into two classes: hydrophobic (terpenoids and azulenes) and hydrophilic compounds (polyphenols) [15,16]. St. John’s wort (Hypericaceae family) tea is the most commonly used for the treatment of water retention, insomnia, anxiety, depression, and gastritis. Its phenolic compounds include phenol carboxylic and cinnamic acids as well as flavonoids (i.e., flavones, flavanols, flavanones, flavans, catechins, bioflavonoids, and anthocyanidins). In addition to these, hypericin, pseudohypericin, prenylated phloroglucinols, and their derivatives have also been identified [17]. Mentha piperita and Levandula officinalis are two popular plants whose oils and teas are widely employed due to their antibacterial, antioxidant, and antidiabetic activities. Additionally, many studies support the use of Lavandula tea as a sedative, anxiolytic, and mood modulator, and Mentha tea in treating irritation and inflammation [13,18].
The volatile compounds (alcohols, aldehydes, aromatic hydrocarbons, esters, ketones, and terpenes) are principally formed during tea processing [19]. Moreover, as the main aroma substances and functional components, phenolic compounds, purine alkaloids, and free amino acids are key indicators for identifying and distinguishing the category and origin of teas [4]. Volatile compounds are easily liberated or degraded during tea processing, which can significantly affect the sensory characteristics of tea samples and the quality of the final tea product [2]. In this regard, the specific processing strongly impacts the aroma characteristics of tea, with the floral and fruity odors being mainly produced by withering or shaking and the nitrogen-containing volatiles by the combination of drying [2]. Notably, the prolonged withering for the quality formation of white tea significantly impacts the presence of volatile and non-volatile compounds, e.g., phenolic components and free amino acids, through hydrolyzation, condensation, and polymerization catalyzed by endogenous enzymes [20]. Therefore, identifying the key odorants will help understand the tea aroma chemistry and sensory markers and provide a valuable scientific basis for the different tea classifications based on the aroma quality [6,21].
Fatty acids (FAs) are essential constituents of each plant [22,23,24] and various analytical methods for the determination of fatty acids from oil, biological fluids, microalgae, etc. are reported [25]. Lipids are regarded as one of the essential nutrients for humans. Based on the presence of double bonds, lipids are classified as unsaturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs with one double bond), and polyunsaturated fatty acids (PUFAs with two or up to six double bonds). Based on the number of carbon atoms, there are FAs with 14–20 carbon atoms and polyunsaturated fatty acids (PUFAs) with more than 20 carbon atoms in structure (like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)). A high PUFA content is essential for food nutrition [26,27,28,29]. The important PUFAs are α-linolenic acid (C18:3, (n3)) and linoleic acid (C18:2, (n6)). Omega-3 FA was recommended for consumption due to its high properties to help immunity, the brain, and human health [25]. Omega-6 and omega-3 are considered essential FAs because they cannot be synthesized by humans and must be supplemented by food. Moreover, a lack of omega-3 FAs can cause stroke, age-related cognitive decline, and Alzheimer’s disease [25]. Fatty acid types give the aroma of tea and differ depending on the type, maturity, harvest time, and storage time [30]. The efficiency of lipid extraction is dependent on the utilized method, solvent, and type. Usually, classical extraction with solvents is used. Different studies showed that using the pre-treatment method for lipid extraction substantially improved the oil yield. The pre-treatment methods, such as ultrasonication, microwave irradiation, autoclaving, etc., are reported in the literature [31]. A high content of linoleic (C18:2) and palmitic (C16:0) acids and a low content of oleic acid (C18:1) were reported in tea seed oil from Camellia species from the China region [32], and a high content of PUFA (42.79%), followed by SFA (29.18%) and MUFA (12.57%), related to the fatty acid content, were reported for the lavender extract [33]. Moreover, 10% palmitic acid, 2.6% stearic acid, 17% oleic acid, 42% linoleic acid, and 26% linolenic acid were also reported by Dai et al. (2021) for blueberry fruit [34].
Tea comprises mainly polyphenols, polysaccharides, multivitamins, beta-carotene, caffeine, flavonoids, pyrroloquinoline quinone, protein, and amino acids [35]. Tea polyphenols play an important role in fixing metals inside plant cells, protecting the cell constituents against oxidative damage, and consequently limiting the risk of several degenerative diseases related to oxidative stress [36,37]. Moreover, the tea leaves are rich in mineral elements such as Co, Mn, Fe, Zn, Mg, Ca, Na, and K, and they have an important role in the development of tea trees but are also a key expression of the nutritional value of tea since minerals are present in all body tissues and are involved in many life processes [38]. Consequently, frequent consumption of tea may contribute significantly to the recommended daily intake. Ca2+ and Fe3+ notably exert an influence on the content of alcohols and aldehydes in volatile compounds [39]. Some mineral substances, such as Fe, Zn, Cu, and Se, add value to the human body. Some biological functions they have an effect on include antioxidation, immunomodulation, inhibition of cancer, and playing an important role in energy metabolism and gene expression [39]. On the contrary, toxic mineral substances such as As, Cd, Cr, Ni, and Pb can cause neurotoxicity, nephrotoxicity, and a variety of other adverse effects on health [39]. Mineral-rich water not only deteriorates the flavor profiles but also reduces the contents of catechins and the antioxidant capacity of tea infusions [5]. The factors responsible for the mineral content in plant samples refer to the geographic origin, soil type, use of fertilizers and pesticides, climatic factors, harvest time, type of processing, mining activities, and vehicular emissions [1]. Green tea leaves are rich in bioactive compounds, particularly phenolic compounds exhibiting antioxidant activity, probably due to the low oxidation degree of the young leaves. Polyphenols, which account for 20–30% of the dry weight of tea leaves, are the most abundant class of soluble components that influence the color, taste, and aroma of tea leaves and are the most important substance to exert their health benefits. Tea polyphenolic compounds play an important role in metal binding within plant cells and protect cells from oxidative stress due to their antioxidant properties [35].
Many studies reported the chemical analysis of single or different tea plants, but a complete chemical analysis and correlation between all components still require attention. In this regard, concerning their benefits for human health, the present study aims to compare three tea types (flowers, leaves, and instant) in terms of physicochemical analysis and their thermal behavior.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents were of analytical grade and were used as received from Merck, except for sodium chlorite purchased from Alfa Aesar. Ultrapure water from a Buckinghamshire Purelab Flex 3 system was used to prepare the standard solutions and dilute the samples.
2.2. Tea Samples
Lavender (T1), chamomile (T2), blueberry (T3), Breackland thyme (T4), Yarrow (T5), mint (T6), black (T7), acacia (T8), elderflower (T9), Saint John’s Wort (T10), linden (T11), rose (T12), green (T13), lemon (T14), and pomegranate (T15) were used for this study. Eleven tea samples as mature flowers (T1, T2, T4, T5, T8, T9, T10, T11, and T12) and two mature leaves (T3 and T6) were wild-harvested from an unpolluted area located in Northern Romania. The collected samples were washed, air dried, and ground to a fine powder. Alongside, black leaves (T7), green leaves (T13), instant lemon (T14), and instant pomegranate (T15) tea samples were purchased from a local market and covered different countries of origin. All samples were stored at −20 °C until further processing.
2.3. Thermal Analysis
The thermal behavior of the grounded tea sample was investigated by thermogravimetry (TG) and differential thermal analysis (DTA) using a SDTQ600 instrument (TA Instruments, New Castle, DE, USA) in air and argon atmospheres up to 1000 °C at a 10 °C/min heating rate using alumina standards.
2.4. Oil Extraction from Teas
The method used to extract oil from tea samples was realized according to a previously published method, with improvements according to Wang et al. (2021) [40]. Approximately 0.5 g were extracted from each sample with 25 mL of chloroform:methanol (2:1, v/v) in an ultrasonic bath for one hour at room temperature. The organic phase was separated by filtration. Ten milliliters of KCl (0.74%) were added to the organic phase and separated again into a 250 mL separating funnel. The water was removed by filtration using Na2SO4. A rotary evaporator, Laborota 4010 (Heidolph, Schwabach, Germany), was used to separate the solvent. The obtained oil was weighted and analyzed for fatty acid constituents. The fatty acids were transformed into FAMEs by transesterification with alcohol. The samples (30 mg) were dissolved in 4 mL of isooctane and reacted with 200 µL of methanol potassium hydroxide (CH5KO2) (2 mol/L) under stirring. One gram of sodium hydrogen sulfate (NaHSO4·H2O) was added to the final solution. The FAME standard mixture (CRM47885) was acquired from Sigma-Aldrich. The FAMEs content was determined by GC-FID (Agilent Technologies, Santa Clara, CA, USA, 6890N) equipped with a ZB-WAX capillary column (30 m × 0.25 mm × 0.25 µm). Helium was used as the gas carrier at a constant flow rate of 1 mL min−1. The furnace temperature started at 60 °C (held for 1 min) and was increased to 200 °C (held for a 2 min plateau) with a rate of 10 °C min−1, and, finally, increased to 220 °C (held for a 20 min plateau) with a rate of 5 °C min−1. The injector and temperature detector were established at 250 °C. The standard mixture calibration curve was used to quantify all FAs.
2.5. Volatile Composition
The volatile compositions of tea samples were determined by headspace-solid phase microextraction (HS-SPME) according to our previously published method [41].
2.6. Mineral Profile
The major and trace elements were determined using an inductively coupled plasma optical emission Perkin Elmer Optima 5300DV (ICP-OES) spectrometer after microwave-assisted digestion using a Berghof Xpert system. An amount of 500 mg of ground tea was digested using 4 mL of HNO3 at 65% and 6 mL of H2O2 at 30% in polytetrafluoroethylene digestion vessels using a four-step digestion program (145, 170, and 190 °C—heating; 50 °C—cooling) for a total digestion time of 40 min. Subsequently, the vessels were cooled down, and the volume was made up to the mark with ultrapure water. Blanks were prepared for each lot of samples. The calibration standards were prepared from Merck ICP multi-element standard solution IV, 1000 mg/L (Na, K, Ca, Mg, Fe, Cu, Mn, and Zn), and a mono-element standard solution, 1000 mg/L P. The accuracy in determining the major and trace element concentrations in tea samples was evaluated via NIST SRM 1515 Apple Leaves, achieving satisfactory recoveries (%) of K, Ca, Mg, Fe, Cu, Zn, and P (85.6–105.3%). The total nitrogen (N) was determined by combustion using a Flash EA 2000 CHNS/O analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Cellulose, Hemicelluloses, and Lignin Content
The amount of cellulose, hemicelluloses, and lignin was established from each tea sample according to our previously published method [41]. The content of holocelluloses (total polysaccharide fraction) was determined as a residue insoluble in sodium chlorite and acetic acid (1%) at 75 °C for 1 h (process repeated four times). The cellulose content was determined as a residue obtained after the reaction of holocellulose with the holocellulose residue insoluble in NaOH (17.5%). The difference between holocellulose and cellulose content was used to determine hemicellulose. The lignin content was established as the insoluble residue in 72% H2SO4 for 4 h at 20 °C. [41].
2.8. Polyphenol Content
The content of polyphenols was determined by the Folin-Ciocalteu colorimetric method using a Lambda 25 spectrophotometer (Perkin-Elmer, Waltham, MA, USA), according to [42].
2.9. Statistical Analysis
For all analyses, the mean and standard deviation (SD) were determined for each variable. The multivariate statistical method of hierarchical cluster analysis was employed to find the correlation of the variables and presented as a dendrogram realized with the Minitab Statistics Software (version 21.1. 0, Minitab Inc., PA, USA). The statistical differences of the analyzed parameters were evaluated through Tukey’s test (p = 0.05) with the Paired Comparison App (Two-Way ANOVA) by using Origin software (version 2020b, OriginLab, Northampton, MA, USA). The letters indicate a statistically significant difference at p < 0.05.
3. Results and Discussions
3.1. Thermal Behavior
The decomposition stages of the tea samples were investigated by TG-DTA up to 600 °C (Figure 1). On the DTA curve, the tea samples generally show three exothermic effects. However, some samples display six exothermic effects.
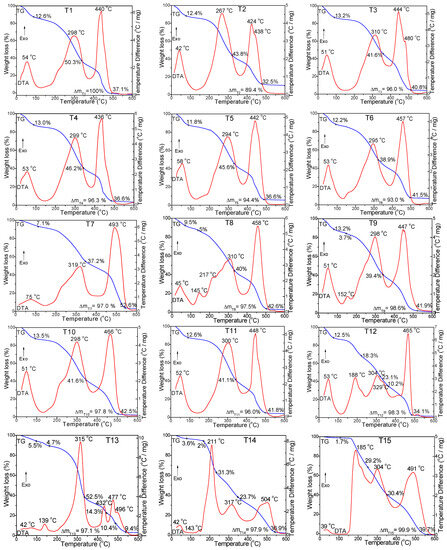
Figure 1.
TG (blue line) and DTA (red line) curves of the tea sample.
The first stage was set apart by an exothermic effect at around 39–75 °C, accompanied by a mass loss of 1.7–13.5%, which can be attributed to the evaporation of adsorbed water and solvent [7]. The second stage involved the decomposition of volatile compounds, such as polyphenols and fatty acids. It is characterized by (i) a single exothermic effect at 267–319 °C in the case of T1, T2, T3, T4, T5, T6, T7, T10, and T11 samples, accompanied by a mass loss of 37.2–50.3%; (ii) two exothermic effects at 139–185 °C/298–315 °C in the case of T9, T13, and T15 samples, accompanied by a mass loss of 43.1–59.6%; and (iii) three exothermic effects at 143–188 °C/211–217 °C/304–329 °C in the case of T8, T12, and T14 samples, accompanied by a mass loss of 43.1–59.6% [7]. The third stage of decomposition, corresponding to the degradation of cellulose, hemicellulose, and lignin, was generally noticeable through a single exothermic effect at 424–504 °C, followed by a mass loss of 32.5–52.6%. In the case of T2 and T3 samples, two close effects on the same intense peak at 424–444 °C/438–480 °C with a mass loss of 32.5–40.8% and three exothermic effects at 432 °C/477 °C/496 °C with a mass loss of 34.1% in the case of T13 sample (green tea), corresponding to the decomposition of hemicellulose, cellulose, and lignin, were noted [7,43]. The total mass losses were in the range of 94.4–100%. Hemicellulose, a compound that possesses a lower degree of polymerization, is a mixture of various polymerized monosaccharides (xylose, mannose, glucose, galactose, and arabinose). At the same time, cellulose is a high-molecular-weight compound consisting of a long linear chain of D-glucosyl groups. The crystalline structure of cellulose renders its thermal degradation more difficult than that of hemicellulose. In addition, on account of the complex composition, the degradation of lignin and macromolecular substances was also more strenuous than that of hemicellulose [43].
3.2. Cellulose, Hemicelluloses, and Lignin Content of Tea Samples
In all tea samples, cellulose, hemicelluloses, and lignin were detected. The cellulose, hemicelluloses, and lignin contents were 5.6–19.3%, 5.2–20.4%, and 11.9–26.8%, respectively. The cellulose (11.6%), hemicelluloses (20.4%), and lignin (20.8%) content determined for T6 were in line with the results obtained by other researchers. Furthermore, 7.06% cellulose, 20.64% hemicelluloses, and 20.04% lignin were also published by Cai et al. (2019) for mint tea [43]. The compositions of cellulose, hemicelluloses, and lignin vary depending on tea plant constituents (flowers, leaves, and branches). Lesage-Meesen et al. (2015) reported 22.4% cellulose, 12.6% hemicelluloses, and 23.6% lignin from the lavender flower; 19.9% cellulose, 3.6% hemicelluloses, and 17.1% lignin from lavender leaves; and 42.7% cellulose, 13.0% hemicelluloses, and 23.1% of lavender branches [44]. Generally, the cellulose, hemicellulose, and lignin content differs by function, season, plant component (flowers, branches, and leaves), and climatologic conditions. In addition, the main components of tea give it rigidity and strength. The highest lignin concentration was found in black tea leaves (26.8%), due to their high syringyl content. The content of cellulose varied in the following order: T1 (19.3%) > T3 (18.5%) > T13 (18.1%) > T7 (16.4%) > T5 (14.2%) > T9 (13.2%) > T11 (12.8%) > T6 (11.6%) >T4 (10.7%), T10 (10.5%), T8 (10.2%), T12 (10.1%). Lignin components are the essential compounds that contribute to plant growth, structure integrity, and water transport. Lignin is the second component in plants after cellulose and contains monolignol (coumaryl, coniferyl, and sinapyl alcohol) and lignan (dimers of monolignols) [45]. The content of lignin is generally higher in leaves than in flowers. The higher lignin content was found in T11 (26.9%), T7 (26.8%), and T1 (24.5%). The hemicellulose content is higher in T6 (20.4%) and T7 (18.6%) and smaller in T9 (5.2%). The presence of cellulose, hemicelluloses, and lignin was confirmed by thermogravimetric analysis. The content of lignin varied in the following order: T11 (26.9%) > T7 (26.8%) > T13 (24.5%) > T1 (22.6%) > T8 > T6 (20.8%) >T3 (20.68%) >T4 (20.3%) > T10 (17.9%) > T9 (17.2%) > T5 (15.9%) > T2 (15.6%) > T12 (11.9%). In the instant tea samples, T14 and T15, cellulose, hemicelluloses, and lignin content were not identified.
3.3. HS-SPME GC-MS Analysis of Volatile Organic Compounds
Headspace-solid phase microextraction (HS-SPME) is regularly employed for extracting volatile aroma compounds and consists of isolating volatile substances in the headspace of the sample vial via a polymer fiber, followed by their detection using gas chromatography-mass spectrometry (GC-MS) [6]. The present study determines 66 volatile compounds that can be divided into eight classes within the tea samples, including 29 terpenes, 12 hydrocarbons, 9 esters, 7 aldehydes, 5 ketones, 2 alcohols, 1 furan, and 1 thiofuran (Table 1). The content and proportion of these groups varied in the studied tea samples; they were either diminished or newly generated, possibly due to the release of volatile compounds from the Maillard reaction, carotenoids or lipid degradation, and glycoside hydrolysis during the tea manufacturing process [2]. The drying method and temperature had a great effect on the relative content of volatile components and influenced the formation of aroma types [46]. The aroma of tea can be influenced by the plant cultivar, environment, and cultivation, as well as the processing technology [46].

Table 1.
Retention time, volatile organic compounds, formula, group, odor type, and content (%) identified by HS-SPME GC-MS for T1–T15 tea samples. Data are expressed as a mean ± standard deviation (n = 3).
Terpenes display the highest proportion (73.0%) in tea samples, inducing fruity, sweet, lemony, pine-like, minty, woody, balsamic, citrusy, floral, aromatic, green-like, or herbaceous [47]. Terpenoids, and mainly the C10 and C15 members within this family, generally impact the flavor profiles of most fruits as well as the scent of flowers. Moreover, the synthetic variations and derivatives of natural terpenes and terpenoids can expand upon the aroma varieties used in perfumery and as flavorings [48]. Limonene was predominant in all tea samples, although its amount varied among them as follows: T15 (97.8%) > T14 (61.2%) > T13 (23.8%). Limonene has ‘lemon’, ‘sweet orange peel’, and ‘licorice’ odors, which play an essential role in the unique aroma of tea [49]. A higher m-cymene content was detected in sample T7. Eucalyptol was the principal group of volatiles identified within the tea samples, although the amounts varied between samples as follows: T8 (62.8%) > T1 (43.2%) > T6 (27.6%) > T13 (17.3%). Eucalyptol, mainly found in sample T8, is utilized as a flavoring and fragrance ingredient due to its fresh and pleasant smell, spicy taste, and cooling effect [50]. Linalool is a monoterpene alcohol that accounts for around 70% of floral fragrances and is employed as a flavoring and air scent aside from its antibacterial and insecticidal properties [51]. 4-Terpineol displaying ‘pepper’, ‘woody’, and ‘earthy’ odors was also detected as one of the odor-active compounds in tea samples [40]. Linalool contributes to the sweet, tender, and fresh floral aroma with a specific smell of bergamot, which was the main contribution to the aroma characteristics of tea [52]. Carvone provided ‘mint’, ‘basil’, and ‘fennel’ odors, which were detected as one of the odor-active compounds of tea [49].
Hydrocarbons (10.6%) in tea samples induce fruity, camphoraceous, sweet, lemony, pine-like, minty, woody, resinous, balsamic, plastic, roasted, citrusy, floral, aromatic, green, chemical, herbaceous, clove, pepper, or spicy odors [47]. The literature studies should demonstrate that tea leaves accumulate aromatic hydrocarbons through airborne deposition in the environment and during treatment stages, such as drying by burning wood or coal [53].
Esters (6.0%) present in tea samples induced fruity (apple, pear, or cherry, among others), floral, herbaceous, sweet, refreshing, green, grassy, bergamot, lavender, and minty odors. Among these, ethyl salicylate can be described as having notes of ‘wintergreen’ and ‘mint’, and it was revealed as being an odor-active compound in the T9 sample [49].
Aldehydes (5.2%) are obtained through the oxidative degradation of amino acids during their interaction with sugars at high temperatures or through the interaction of amino acids and polyphenols in the presence of polyphenol oxidase [2,54]. Benzaldehyde, the singular odor-active aromatic aldehyde with the highest content in sample T4, is characterized by a caramel-like or roasted odor and commonly exists as the glycosidically bound form in tea [2]. The aromas conferred by aldehydes are green, fruity, fatty, grassy, oily, very strong, harsh, and lemony [52]. 2-Hexenal, providing ‘apple’ and ‘green’ odors, contributed to rich, fresh fruit and green leaf fragrance; n-heptanal provided ‘pungent’ and ‘unpleasant’ odors [49,52].
In all tea samples, ketones (4.6%) are associated with fruity, spicy, cinnamon, banana, mushroom, camphor, cedar leaf, mint, and bitter aromas [47]. Ketones are commonly derived from the degradation of carotenoids/unsaturated fatty acids and the hydrolysis of their glycoside precursors [54]. 7-Octen-2-one, described as possessing ‘fatty’, ‘fruity’, and ‘mushroom’ odors, was identified as the primary aroma compound degraded during the post-fermentation of tea [49]. 2-Heptanone contributed to ‘stale’ and ‘cabbage’ odors, but little contributed to the entire aroma formation of tea because of its high threshold and low presence [49].
The lowest content of alcohol (0.2%) accounts for cooling, camphoraceous, fresh pine, ozone, citrus, floral, green, peppermint, woody, earthy, and sweet odors [2].
Various compounds with higher content in different teas were described as having fruity or floral aromas, according to Fenaroli’s Handbook of Flavor Ingredients [47]. All these volatile compounds are classified into nine odor classes (floral, fruity, green, sweet, chemical, woody, citrus, roasted, and alcohol) (Table 1, Figure 2). The most volatile compounds are present in St. John’s wort tea (T10). Generally, fruity (35.1%) and floral (23.9%) aromas prevailed in the studied samples. The predominant fruity aroma was found in T1, T2, T3, T4, T5, T6, T8 (the most intense), T11, and T12. The predominant floral aroma was noted in T6 (the most intense), T7, and T10. The green aroma prevailed in the T sample, while the citrus aroma predominated in the T15, T14, and T13 samples. The flower aroma of α-pinene was found in all studied tea samples except for the T15 sample. Linalool is a kind of monoterpene alcohol that is rich in lavender, St. John’s wort, rose, thyme, chamomile, elder, linden, acacia, and blueberry teas and is associated with floral, citrus, and fruit flavors [4].
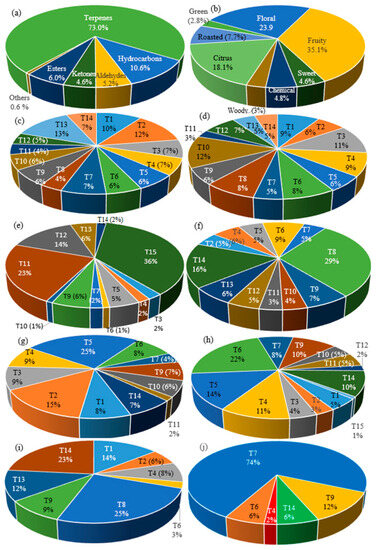
Figure 2.
Classification of volatile compounds identified in teas by chemical class (a) and aroma profile (b): floral (c), fruity (d), citrus (e), roasted (f), Chemical (g), sweet (h), woody (i), and green (j) aroma tea distribution.
3.4. FAMEs Content of Tea Samples
The FA contents of all tea-analyzed oils are presented in Table 2. SFAs, MUFAs, and PUFAs were identified in all oil tea extracts. The SFA classes include caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, eicosadienoic acid, tricosanoic acid, and lignoceric acid. The main SFAs present in all tea samples are palmitic acid (C16:0), followed by arachidic acid (C20:0) and stearic acid (C18:0). The MUFA included nine fatty acids: C14:1 (n-9), C15:1, C16:1 (n-7), C17:1, C18:1 (cis + trans) (n-9), C20:1 (n-9), C22:1 (n-9), and C24:1 (n-9). The peaks that overlap are isomers of oleic and linoleic acids. Moreover, cis-8,11,14-eicosatrienoic acid coelutes with heneicosanoic acid, and cis-4,7,10,13,16,19-docosa-hexanoic acid coelutes with nervonic acid.

Table 2.
Content of SFA, MUFA, and PUFA in oil tea samples (%).
The SFAs, MUFAs, PUFAs, and omega-3 and omega-6 content in the tea oil are presented in Figure 3. The MUFA component found to be most abundant is oleic acid, and its content varied in the following order: T3 > T9, T2 > T8 > T1 > T4 > T11 > T7 > T5 > T10 > T6 > T6 > T13 > T12. The greatest content of linoleic acid C18:2 (cis + trans) (n-9) was found in T5 (11.5%), followed by T8 (10.14%) and T2 (10.07%). The consumption of foods with a high content of PUFA has a positive influence on cardiovascular disease. The acacia tea samples contain the highest content of omega-6 (53.0%), while the lowest amount was identified in the Saint John’s Wort sample (11.0%). Acacia, Saint John’s Wort, rose, and yarrow are good sources of omega-6. The consumption of 3 g of omega-3 can reduce hypertension problems among all the acids [55]. Good sources of omega-3s are tea samples T6, T10, T2, T13, T3, and T1.
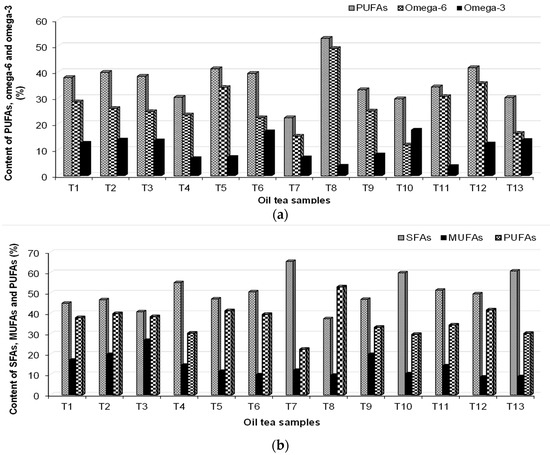
Figure 3.
The content of (a) SFAs, MUFAs, and PUFAs and (b) PUFAs, omega-6, and omega-3 in the analyzed oil tea samples.
The content of fatty acids reported in peppermint (T6) is in good agreement with the obtained results of Maffei et al. (1992), e.g., the FA fatty acids dominate in palmitate (16:0), linoleate (18:2), and linolenate (18:3) [56]. Eicosapentaenoic acid (EPA, C20:5, n-3) was found in small quantities in T5 and T10.
A high quantity of C20:2 (n-6) (PUFA) was found in T12 (24.04%). The 22:6 (n-3) PUFA was found only in thyme (T4) (6.5%) and T2 oils (6.0%). Moreover, thyme oil contains 20:4 (n-6) PUFA. The highest PUFA content was found in acacia (T8) (53%). Myristoleic acid (C14:1) was not identified in samples of acacia and linden oil.
3.5. Mineral Compositions and the Content of Total Polyphenols
The studied tea samples are abundant in major elements (Na, K, Ca, Mg, and P), which play major roles in both the growth and development of tea trees and the nutritional value of tea. The content of major and trace elements and polyphenols in the tea samples is highlighted in Table 3. Appreciable amounts of Na, K, Ca, Mg, and P were observed in all samples, except in the instant tea samples (T14 and T15). The major element content was reduced in the following order: K > Ca > Mg > P > Na, while the trace element content decreased in the Fe > Zn > Cu sequence. Excepting the instant tea samples, the lowest mineral and nitrogen content was determined for the T1 (lavender-flower) sample. A possible explanation for the chemical composition of different tea types could be reflected in the differences in varieties, growing soil, and manufacturing techniques that different tea leaves have been exposed to during their different phases of growth and processing [57]. Minerals play key roles in the body, from building strong bones to transmitting nerve impulses for a healthy and lengthy life [58]. Na, K, and Mg are involved in the body’s control, treatment, and management of various metabolic and heart disorders, while Ca is associated with healthy bones and teeth, although it plays an important role in maintaining extracellular fluids, blood clotting, and muscle contraction [59]. Phosphorus has a critical role in metabolism and growth, whereas Fe is a crucial element in living systems, being involved in the biosynthesis of hemoglobin in erythrocytes and the physiology of oxygen transportation [58]. In addition, nitrogen is one of the most beneficial nutritional elements for the tea plant, having a high impact on the overall quality of tea. The trace elements present within tea leaves form complexes with flavonols, tannins, catechols, and polyphenols [57]. Polyphenols present in tea are an order of phytochemicals possessing multiple phenolic groups, some of which are flavonoids and their glucosides, phenolic acids, and any other compounds containing phenolic hydroxyl groups (such as tyrosine). They are connected to health-promoting properties such as antidiabetic, antioxidant, anti-inflammatory, and anticarcinogenic. Catechins are revealed to reach a level of about 60–70% of tea polyphenols within the fresh tea leaves [20].

Table 3.
Minerals, nitrogen, and content of polyphenols for studied tea samples expressed as averages ± standard deviation (n = 3).
3.6. Multivariate Analysis
Hierarchical clustering (dendrogram) of the HCA of the tea samples is highlighted in Figure 4 and was realized by considering all the variables used for tea characterization. According to cluster analysis, two main clusters can be observed. The first group contains T1, T10, T13, T2, T5, T6, T7, T8, T11, and T9, and the second group contains T3, T4, T12, T14, and T15. The first cluster was further separated into three subclusters, and the second cluster was divided into two subclusters. The chemical composition of lavender is similar to that of Saint John’s Wort and green leaves, and the chemical composition is similar for chamomile, Yarrow, mint, and black.
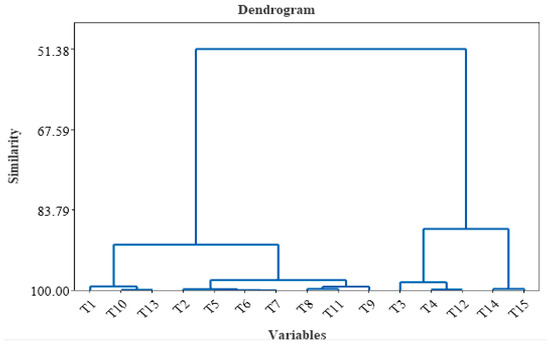
Figure 4.
Hierarchical clustering (dendrogram) of different tea samples T1–T15 (including all variable analysis).
The HCA group of tea samples based on a set of variables like metals, polyphenols, carbohydrates (cellulose and hemicelluloses), and lignin is presented in Figure 5. Two clusters were formed, the first cluster containing Na, K, P, N, Cu, Mg, and Zn, and the second cluster containing Ca, Fe, polyphenols, hemicellulose, cellulose, and lignin. Hemicelluloses are cell wall polysaccharide fractions from tea samples and contain heteropolysaccharides, and polyphenols can be bound by polysaccharides by an adsorption system [60]. According to Liu (2017), the association between polyphenols and cellulose was possible due to hydrogen bonding linkage formation [61]. The polyphenols can bind to the cellulose surface; this process was accelerated in water. In addition, the metal content, particularly Ca, contributes to the growth and development of the tea plant, and Fe is required in a small amount and is used by the plant as a nutrient and for reproduction.
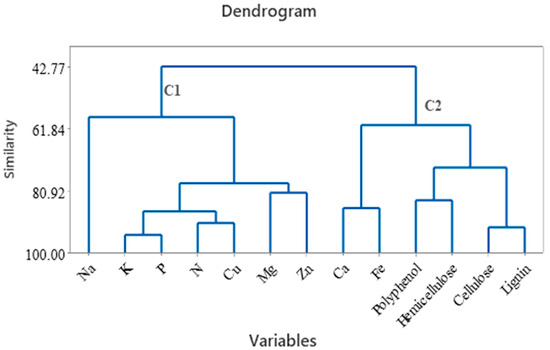
Figure 5.
Hierarchical clustering (dendrogram) of metals, cellulose, hemicellulose, lignin, and polyphenols from tea samples.
The other macronutrients, like nitrogen, potassium, and phosphorus, contribute to the tea plant’s growth. The HCA of different tea samples (Figure 6), considering the fatty acids and volatile compounds, obtained the following correlations: T6 correlated with T13 due to the high C18:3 (n-3) found in both samples and the correlation between T4, T11, T12, T6, and T13 due to abundant hydrocarbons correlated with PUFA.
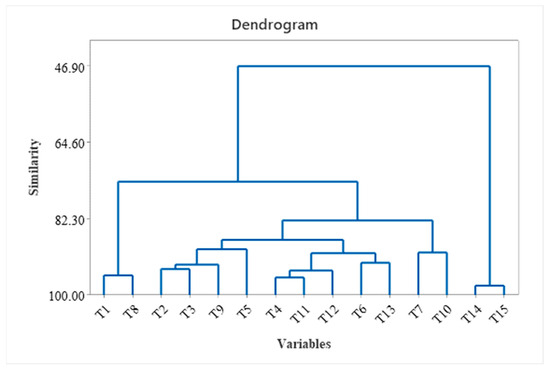
Figure 6.
Hierarchical clustering (dendrogram) of different tea samples T1–T15 (fatty acids and volatile compounds).
4. Conclusions
The present work examines a comparative analysis of fatty acids, volatile compounds, and minerals and presents an evaluation of the thermal behavior among different types of tea (leaves, flowers, and instant) samples. The volatile compound and aroma description profiles were also identified. The decomposition of the studied tea samples took place in three stages: moisture evaporation at 39–75 °C, followed by a mass loss of 1.7–13.5%; decomposition of volatile compounds and fatty acids at 139–329 °C, characterized by 1–3 exothermic effects and attended by a mass loss of 37.2–57%; and degradation of cellulose, hemicellulose, and lignin at 424–504 °C, characterized by 1–3 exothermic effects and accompanied by a mass loss of 32.5–52.6%. The total mass losses were in the range of 94.4–100%. The predominant aromas were fruity in T1, T2, T3, T4, T5, T6, T8 (most intense), T11, and T12 samples; floral in T6 (most intense), T7, and T10 samples; and green in the T9 sample, while the citrus aroma predominated in the T15, T14, and T13 samples. A total of 66 volatile compounds were separated into eight classes within the tea samples. This includes 29 terpenes, 12 hydrocarbons, 9 esters, 7 aldehydes, 5 ketones, 2 alcohols, 1 furan, and 1 thiofuran. The major element content decreased in the order (mg/kg): K (29.3–30,400) > Ca (1251–19,504) > Mg (11.9–3345) > P (286–5545) > Na (24.8–669), while the trace element content decreased in the order Fe (10.9–3912) > Zn (4.30–41.7) > Cu (1.10–7.70). In all flower and leaf teas, SFAs, MUFAs, and PUFAs were identified. A high content of omega-6 was quantified in acacia, Saint John’s Wort, rose, and yarrow, while omega-3 was found in mint, Saint John’s Wort, green, blueberry, and lavender samples. Some minerals (Fe, Cu, and Zn) were not identified in instant tea samples, and a low quantity of polyphenols and volatile compounds was obtained. Overall, it can be said that instant tea has fewer nutritional properties than brewed tea.
Author Contributions
Study design, T.D. and L.S.; methodology, T.D., O.C. and L.S.; software, T.D., O.C. and L.S.; data validation, T.D., O.C. and L.S.; formal analysis, M.H.K., M.D., O.C. and M.D.; investigation, T.D. and L.S.; resources, T.D., M.H.K., M.D., O.C. and L.S.; data curation, T.D. and L.S.; writing—original draft devising, T.D., O.C. and L.S.; writing—review and editing, T.D., O.C. and L.S.; visualization, T.D.; supervision, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data discussed within this study is available from the corresponding author upon request.
Acknowledgments
This work was carried out through the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. PN 23 05, and the Ministry of Research, Innovation, and Digitization through Program 1—Development of the National Research and Development System, Subprogram 1.2—Institutional Performance—Projects that Finance the RDI Excellence, contract no. 18PFE/30 December 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomes, D.A.S.; Alves, J.P.S.; Silva, E.G.P.; Novaes, C.G.; Silva, D.S.; Aguiar, R.M.; Araújo, S.A.; Santos, A.C.L.; Bezerra, M.A. Evaluation of metal content in tea samples commercialized in sachets using multivariate data analysis techniques. Microchem. J. 2019, 151, 104248. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.-T.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef]
- Bora, P.; Bora, L.C. Microbial antagonists and botanicals mediated disease management in tea, Camellia sinensis (L.) O. Kuntze. An overview. Crop Prot. 2021, 148, 105711. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, B.; Xu, C.; Wang, J.; Wang, Z.; Huang, Y.; Ma, C. Impact of enzymatic fermentation on taste, chemical compositions and in vitro antioxidant activities in Chinese teas using E-tongue, HPLC and amino acid analyzer. LWT-Food Sci. Technol. 2022, 163, 113549. [Google Scholar] [CrossRef]
- Li, M.; Feng, Z.; Wang, F.; Chen, J.; Fan, J.; Wang, J.; Liu, Z.; Yin, J. Effects of brewing water on the volatile composition of tea infusions. Food Chem. 2023, 429, 136971. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Huang, J.; Huang, J.; Wu, W.; Tong, T.; Shujuan, L.; Zhou, L.; Liu, Z.; Zhang, S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT-Food Sci. Technol. 2022, 164, 113656. [Google Scholar] [CrossRef]
- Tian, L.; Shen, B.; Xu, H.; Li, F.; Wang, Y.; Singh, S. Thermal behavior of waste tea pyrolysis by TG-FTIR analysis. Energy 2016, 103, 533–542. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, Y.; Li, M.Y.; Mai, Y.H.; Guo, H.; Wadood, S.A.; Raza, A.; Wang, Y.; Zhang, J.Y.; Li, H.B.; et al. The chemical, sensory, and volatile characteristics of instant sweet tea (Lithocarpus litseifolius) using electronic nose and GC-MS-based metabolomics analysis. LWT 2022, 163, 113518. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and multivariate data analysis. J. Chrom. A 2013, 1313, 245–252. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, L.; Zhou, S.; Hong, Y.; Zhang, X.; Chen, H. Analysis of differences in the accumulation of tea compounds under various processing techniques, geographical origins, and harvesting seasons. Food Chem. 2023, 430, 137000. [Google Scholar] [CrossRef] [PubMed]
- Piyasena, K.G.N.P.; Hettiarachchi, L.S.K. Comparison of tea quality parameters of conventionally and organically grown tea, and effects of fertilizer on tea quality: A mini-review. Food Chem. Adv. 2023, 3, 100399. [Google Scholar] [CrossRef]
- Laza, D. Îndreptar profilactic și terapeutic de medicină naturistă. Păzitorul Adevărului 2000, 294. [Google Scholar]
- Cittan, M.; Altuntaş, E.; Ҫelik, A. Evaluation of antioxidant capacities and phenolic profiles in Tilia cordata fruit extracts: A comparative study to determine the efficiency of traditional hot water infusion method. Ind. Crops Prod. 2018, 122, 553–558. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Cui, J.; Li, H.; Hasan, B.; Xin, X. Chemical component and in vitro protective effects of Matricaria chamomilla (L.) against lipopolysaccharide insult. J. Ethnopharmacol. 2022, 296, 115471. [Google Scholar] [CrossRef] [PubMed]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Castillo, C.M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use. Phytochem. Pharmacol. Uses Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Milevskaya, V.V.; Prasad, S.; Temerdashev, Z.A. Extraction and chromatographic determination of phenolic compounds from medicinal herbs in the Lamiaceae and Hypericaceae families: A review. Microchem. J. 2019, 145, 1036–1049. [Google Scholar] [CrossRef]
- Al-Mijalli, H.S.; ELsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid. Based Complement. Altern. Med. 2022, 14, 9306251. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Xiao, J.; Ma, C.; Huang, Y. GC–MS and LC-MS/MS metabolomics revealed dynamic changes of volatile and non-volatile compounds during withering process of black tea. Food Chem. 2023, 410, 135396. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, Y.; Li, S.; Ziong, Y.; Li, Q.; Huang, J.; Liu, Z. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar]
- Na, C.; Ziwen, Z.; Yeyun, L.; Xianchen, Z. Exogenously applied Spd and Spm enhance drought tolerance in tea plants by increasing fatty acid desaturation and plasma membrane H+-ATPase activity. Plant Physiol. Biochem. 2022, 170, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.; Adole, P.S.; Vinod, K.V.; Pillai, A.A. A validated and optimized method for separation and quantification of total fatty acids by gas chromatography-ion trap mass spectrometry in human plasma. J. Chromatogr. B 2022, 1210, 123473. [Google Scholar] [CrossRef]
- Gómez-Coca, R.B.; Pérez-Camino, M.C.; Brereton, P.; Bendini, A.; Toschi, T.G.; Moreda, W. Fatty acid ethyl esters (FAEE) in virgin olive oil: A shorter and full validated approach as an alternative to the EU Official Method. Food Chem. 2022, 394, 133300. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Comparison and validation of 2 analytical methods for the determination of free fatty acids in dairy products by gas chromatography with flame ionization detection. J. Dairy Sci. 2016, 99, 5047–5063. [Google Scholar] [CrossRef]
- Pruksatrakul, T.; Phoopraintra, P.; Wilairat, P.; Chaiyen, P.; Chantiwas, R. Development of a sequential injection-liquid microextraction procedure with GC-FID for analysis of short-chain fatty acids in palm oil mill effluent. Talanta 2017, 165, 612–618. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xue, J.; Wang, H. Isolation and lipidomics characterization of fatty acids and phospholipids in shrimp waste through GC/FID and HILIC-QTrap/MS. J. Food Compos. Anal. 2021, 95, 103668. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Phar. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef]
- Guo, L.; Chen, M.; Guo, Y.; Liu, Z. Variations in fatty acids affected their derivative volatiles during Tieguanyin tea processing. Foods 2022, 11, 1563. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies—A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; Mar Contreas, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Özugul, F.; Aksun, E.T.; Öztekin, R.; Lorenzo, J.M. Effect of lavender and lemon balm extracts on fatty acid profile, chemical quality parameters and sensory quality of vacuum packaged anchovy (Engraulis encrasicolus) fillets under refrigerated condition. LWT-Food Sci. Technol. 2017, 84, 529–535. [Google Scholar] [CrossRef]
- Dai, H.; Ji, S.; Zhou, X.; Wi, B.; Cheng, S.; Zhang, F.; Wang, S.; Zhou, Q. Postharvest effects of sodium nitroprusside treatment on membrane fatty acids of blueberry (vaccinium corymbosum, cv. Bluecrop) fruit. Sci. Hortic. 2021, 288, 110307. [Google Scholar] [CrossRef]
- Qi, C.; Liu, G.; Ping, Y.; Yang, K.; Tan, Q.; Zhang, Y.; Chen, G.; Huang, X.; Xu, D. A comprehensive review of nano-delivery system for tea polyphenols: Construction, applications, and challenges. Food Chem. X 2023, 17, 100571. [Google Scholar] [CrossRef] [PubMed]
- Tolra, R.; Martos, S.; Hajiboland, R.; Poschenrieder, C. Aluminium alters mineral composition and polyphenol metabolism in leaves of tea plants (Camellia sinensis). J. Inorg. Biochem. 2020, 204, 110956. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Shanmugam, A.; Khaled, J.M.; Chung, M.; Thiruvengadam, M. Investigating the impact of tea mosquito bug on the phytochemical profile and quality of Indian tea cultivars using HPLC and LC-MS-based metabolic profiling. Ind. Crops Prod. 2023, 204, 117278. [Google Scholar] [CrossRef]
- Konieczynski, P.; Viapiana, A.; Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and Erva-mate (Ilex paraguariensis A. St.-Hil.) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef]
- Bai, F.; Chen, G.; Niu, H.; Zhu, H.; Huang, Y.; Zhao, M.; Hou, R.; Peng, C.; Li, H.; Wan, X.; et al. The types of brewing water affect tea infusion flavor by changing the tea mineral dissolution. Food Chem. X 2023, 18, 100681. [Google Scholar] [CrossRef]
- Wang, H.; Chen, K.; Cheng, J.; Jiang, L.; Yu, D.; Dai, Y.; Wang, L. Ultrasound-assisted three phase partitioning for the simultaneous extraction of oil, protein and polysaccharide from pumpkin seeds. LWT-Food Sci. Technol. 2021, 151, 112200. [Google Scholar] [CrossRef]
- Dippong, T.; Senila, L.; Muresan, L.E. Preparation and Characterization of the Composition of Volatile Compounds, Fatty Acids and Thermal Behavior of Paprika. Foods 2023, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Crafack, M.; Jespersen, L.; Jakobsen, M. The Microbiology of Cacao Fermentation. In Chocolate in Health and Nutrition; Humana Press: Totowa, NJ, USA, 2012; Volume 7, pp. 39–60. [Google Scholar]
- Cai, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Pyrolytic kinetics, reaction mechanisms and products of waste tea via TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 184, 436–447. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin:a review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Wang, L.; Wen, M.; Chen, F.; Luo, Z.; Yin, J.; Chen, Y.; Huang, H. High oxygen atmospheric packaging (HOAP) reduces H2O2 production by regulating the accumulation of oxidative stress-related proteins in Chinese flowering cabbage. Postharvest Biol. Technol. 2020, 165, 111183. [Google Scholar] [CrossRef]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT—Food Sci. Technol. 2022, 161, 113394. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Wu, X.; Zhang, Y.; He, Z.; Zhang, Y.; Zhang, X.; Li, Q.; Huang, J.; Liu, Z. Pu-erh tea unique aroma: Volatile components, evaluation methods and metabolic mechanism of key odor-active compounds. Trends Food Sci. Technol. 2022, 124, 25–37. [Google Scholar] [CrossRef]
- Zeraatpisheh, Z.; Vatanparast, J. Eucalyptol induces hyperexcitability and epileptiform activity in snail neurons by inhibiting potassium channels. Eur. J. Pharmacol. 2015, 764, 70–78. [Google Scholar] [CrossRef]
- Shen, S.; Wu, H.; Li, T.; Sun, H.; Wang, Y.; Ning, J. Formation of aroma characteristics driven by volatile components during long-term storage of an tea. Food Chem. 2023, 411, 135487. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; He, J.-J.; Zhou, Y.-Z.; Li, Y.-L.; Zhou, H.-J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar]
- Benson, N.; Ahmadu, O.H.F.; Olugbuyiro, J.A.O.; Anake, W.U.; Adedapo, A.E.; Olajire, A.A. Concentrations, sources and risk characterisation of polycyclic aromatic hydrocarbons (PAHs) in green, herbal and black tea products in Nigeria. J. Food Comp. Anal. 2018, 66, 13–22. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Chen, W.; Lan, X.; Zhan, S.; Sun, Y.; Su, W.; Lin, C.-C.; Ni, L. Comparative study of the volatile fingerprints of roasted and unroasted oolong tea by sensory profiling and HS-SPME-GC-MS. Curr. Res. Food Sci. 2023, 6, 100442. [Google Scholar] [CrossRef]
- Naini, A.E.; Keyvandarian, N.; Mortazavi, M.; Taheri, S.; Hosseini, S.M. Effect of Omega-3 fatty acids on blood pressure and serum lipids in continuous ambulatory peritoneal dialysis patients. J. Res. Pharm. Pract. 2015, 4, 135–141. [Google Scholar] [PubMed]
- Maffei, M.; Scannerini, S. Seasonal variations in fatty acids from non-polar lipids of developing peppermint leaves. Phytochemistry 1992, 31, 479–484. [Google Scholar] [CrossRef]
- Deka, H.; Barman, T.; Sarmah, P.P.; Devi, A.; Tamuly, P.; Karak, T. Impact of processing method on selected trace elements content of green tea: Does CTC green tea infusion possess risk towards human health? Food Chem. X 2021, 12, 100173. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Afuape, A.O.; Afolayan, A.J.; Buwa-Komoreng, L.V. Proximate, vitamins, minerals and anti-nutritive constituents of the leaf and stem of Helichrysum odoratissimum (L.) Sweet: A Folk Medicinal Plant in South Africa. Int. J. Plant Biol. 2022, 13, 463–472. [Google Scholar] [CrossRef]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef]
- Liu, D.; Martinez-Sanz, M.; Lopez-Sanchez, P.; Gilbert, E.P.; Gidley, M.J. The adsorption behavior of polyphenols on cellulose is affected by processing history. Food Hydrocoll. 2017, 63, 496–507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).