Biochar and Nitrogen Fertilizer Change the Quality of Waxy and Non-Waxy Broomcorn Millet (Panicum miliaceum L.) Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broomcorn Millet Samples
2.2. Isolation and Purification of Broomcorn Millet Starch
2.3. Morphological Observation of Broomcorn Millet Starch
2.4. Granule Particle Size of Broomcorn Millet Starch
2.5. Determination of the Starch Content
2.6. Distribution of the Chain Length of Amylopectin
2.7. Structure of Broomcorn Millet Starch
2.8. Solubility and Swelling
2.9. Determination of Gel Consistency and Pasting Properties
2.10. Thermal Properties
2.11. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy (SEM) of Starch
3.2. Distribution of Starch Granule Sizes
3.3. Starch Content
3.4. Distribution of the Chain Lengths of Amylopectin
3.5. ATR–FTIR Analysis of Starch
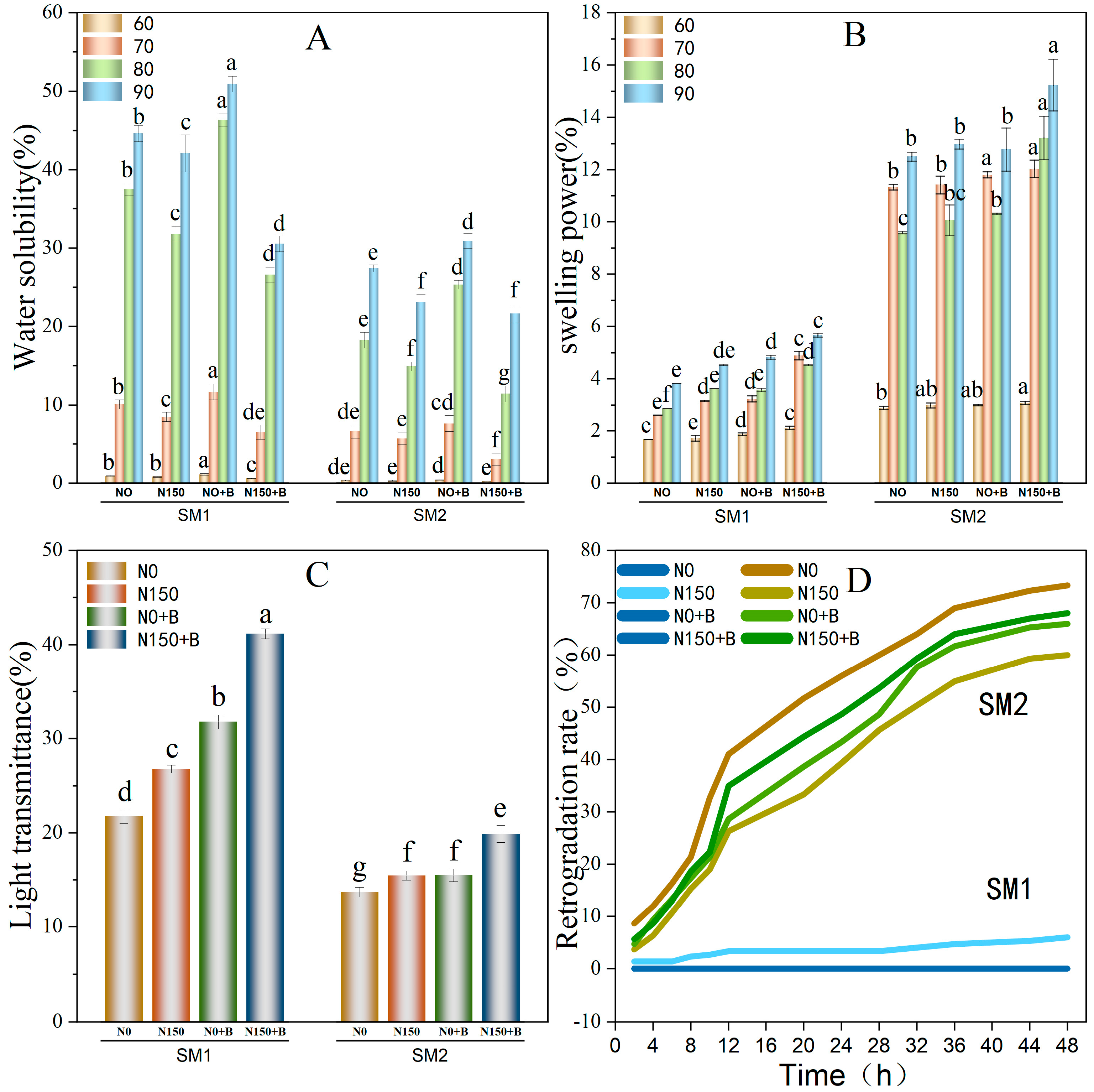
3.6. Swelling Power and the Water Solubility of Starch
3.7. Light Transmittance of Starch
3.8. Retrogradation
3.9. Gel Consistency of Starch
3.10. Pasting Properties
3.11. Differential Scanning Calorimetry (DSC)
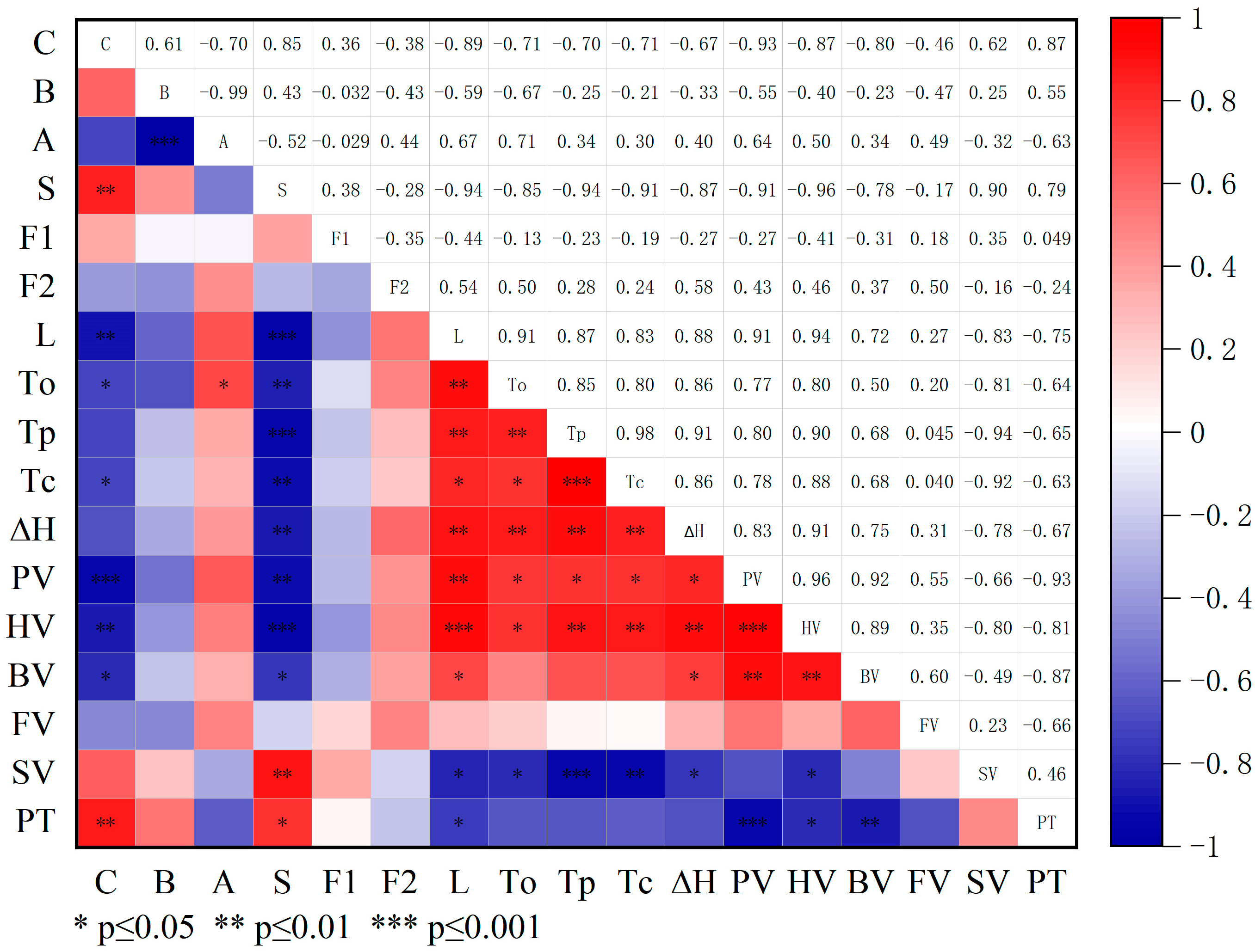
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Zhang, J.; Liu, K.-b.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X.J. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Gao, Y.; Yang, Q.; Dong, K.; Liu, T.; Feng, B. Comparative analysis of drought-responsive physiological and transcriptome in broomcorn millet (Panicum miliaceum L.) genotypes with contrasting drought tolerance. Ind. Crops Prod. 2022, 177, 114498. [Google Scholar] [CrossRef]
- Kalinova, J.; Moudry, J. Content and quality of protein in proso millet (Panicum miliaceum L.) varieties. Plant Foods Hum. Nutr. 2006, 61, 43–47. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, W.; Li, J.; Gong, X.; Feng, B. Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy). Molecules 2019, 24, 1743. [Google Scholar] [CrossRef]
- Bangar, S.P.; Ashogbon, A.O.; Dhull, S.B.; Thirumdas, R.; Kumar, M.; Hasan, M.; Chaudhary, V.; Pathem, S. Proso-millet starch: Properties, functionality, and applications. Int. J. Biol. Macromol. 2021, 190, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, S.; Zeng, L.; Han, W.; Ran, X. Study on physicochemical properties of purple waxy wheat starch. Int. J. Food Prop. 2021, 24, 471–481. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, C.; Zhou, G.; Huo, Z. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2017, 63, 525–532. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Zhang, M.; Zhu, G.; Lv, D.; Wang, Y.; Zhu, D.; Yan, Y. Comparative phosphoproteomic analysis under high-nitrogen fertilizer reveals central phosphoproteins promoting wheat grain starch and protein synthesis. Front. Plant Sci. 2017, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Karaman, K.; Kardes, Y.M.; Kale, H. Phytic acid content and starch properties of maize (Zea mays L.): Effects of irrigation process and nitrogen fertilizer. Food Chem. 2019, 283, 375–380. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, Q.; Li, E.; Yuan, L.; Wang, W.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J.; Gu, J. Effects of nitrogen fertilizer on structure and physicochemical properties of ‘super’rice starch. Carbohydr. Polym. 2020, 239, 116237. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hao, H.; Lei, H.; Ge, Y.; Shi, H.; Song, Y. Farm size, risk aversion and overuse of fertilizer: The heterogeneity of large-scale and small-scale wheat farmers in Northern China. Land 2021, 10, 111. [Google Scholar] [CrossRef]
- Shamrukh, M.; Akib, S. Trendline and monthly variations of nitrate in water supply wells in upper Egypt. Eng 2021, 2, 43–53. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Bucur, D.; Walkiewicz, A. Interaction of biochar with chemical, green and biological nitrogen fertilizers on nitrogen use efficiency indices. Agronomy 2022, 12, 2106. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H.J. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.J.B. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. J. Environ. Manag. 2022, 4, 36. [Google Scholar] [CrossRef]
- Ali, I.; Iqbal, A.; Ullah, S.; Muhammad, I.; Yuan, P.; Zhao, Q.; Yang, M.; Zhang, H.; Huang, M.; Liang, H. Effects of biochar amendment and nitrogen fertilizer on RVA profile and rice grain quality attributes. Foods 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Xu, X.; Wu, L.a.; Dai, G.; Zheng, W.; Xu, Z. Effect of biochar on rice starch properties and starch-related gene expression and enzyme activities. Sci. Rep. 2020, 10, 16917. [Google Scholar] [CrossRef]
- Cai, J.; Cai, C.; Man, J.; Zhou, W.; Wei, C. Structural and functional properties of C-type starches. Carbohydr. Polym. 2014, 101, 289–300. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Qu, Y.; Gao, X.; Liang, J.; Yang, P.; Feng, B. Comparison of physicochemical properties and cooking edibility of waxy and non-waxy proso millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278. [Google Scholar] [CrossRef]
- Han, M.; Dang, K.; Wang, J.; Gao, L.; Wang, H.; Ivanistau, A.; Yang, Q.; Feng, B. New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches. Molecules 2021, 26, 4283. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-y.; Woo, K.S.; Chung, H.-J. Starch characteristics of cowpea and mungbean cultivars grown in Korea. Food Chem. 2018, 263, 104–111. [Google Scholar] [CrossRef]
- Chemutai, L.; Musyoki, M.; Kioko, W.; Mwenda, N.; Muriira, K.; Piero, N. Physicochemical characterization of selected rice (Oryza sativa L.) genotypes based on gel consistency and alkali digestion. Biochem. Anal. Biochem. 2016, 5, 285. [Google Scholar] [CrossRef]
- Chao, G.; Gao, J.; Liu, R.; Wang, L.; Li, C.; Wang, Y.; Qu, Y.; Feng, B. Starch physicochemical properties of waxy proso millet (Panicum miliaceum L.). Starch-Stärke 2014, 66, 1005–1012. [Google Scholar] [CrossRef]
- Bechthel, D. Size distribution of starch granules isolated from hard red winter and soft red winter wheats. Starch-Stärke 1993, 70, 238–240. [Google Scholar]
- Duan, W.; Zhang, H.; Xie, B.; Wang, B.; Zhang, L. Impacts of nitrogen fertilization rate on the root yield, starch yield and starch physicochemical properties of the sweet potato cultivar Jishu 25. PLoS ONE 2019, 14, e0221351. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, Y.; Wang, M.; Jane, J.-l.; Du, S.-k. Physicochemical properties and in vitro digestibility of legume starches. Food Hydrocoll. 2017, 63, 249–255. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.; Farhat, I.; Mitchell, J. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Pu, H.; Yang, Q.; Chen, Z.; Zhu, Z. Cold-water solubility, oil-adsorption and enzymolysis properties of amorphous granular starches. Food Hydrocoll. 2021, 117, 106669. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, pasting, and thermal properties of native corn starch–mung bean protein isolate composites. Gels 2022, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.; Greenwood, C.; Muir, D. Studies on starches of high amylose content. Part 17. A review of current concepts. Starch-Stärke 1974, 26, 289–300. [Google Scholar] [CrossRef]
- Zhu, D.; Fang, C.; Qian, Z.; Guo, B.; Huo, Z. Differences in starch structure, physicochemical properties and texture characteristics in superior and inferior grains of rice varieties with different amylose contents. Food Hydrocoll. 2021, 110, 106170. [Google Scholar] [CrossRef]
- Lloyd, J.R. The A to B of starch granule formation in wheat endosperm. J. Exp. Bot. 2020, 71, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, J.; Gao, J.; Liu, J.; Yu, X.; Wang, Z.; Yang, X.; Ji, N. Comprehensive application of bio-char and nitrogen fertilizer in dry-land maize cultivation. Sci. Rep. 2022, 12, 13478. [Google Scholar] [CrossRef]
- Mo, Z.; Lei, S.; Ashraf, U.; Khan, I.; Li, Y.; Pan, S.; Duan, M.; Tian, H.; Tang, X. Silicon fertilization modulates 2-acetyl-1-pyrroline content, yield formation and grain quality of aromatic rice. J. Cereal Sci. 2017, 75, 17–24. [Google Scholar] [CrossRef]
- Kossmann, J.; Lloyd, J. Understanding and Influencing Starch Biochemistry. Crit. Rev. Plant Sci. 2010, 19, 171–226. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Q.; Xia, M.; Bai, W.; Wang, P.; Gao, X.; Li, J.; Feng, B.; Gao, J. Effects of nitrogen level on the physicochemical properties of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) starch. Int. J. Biol. Macromol. 2019, 129, 799–808. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, Y.; Liu, J.; Han, M.; Li, J.; Jin, F.; Feng, B.M. Transcriptome analysis reveals new insights in the starch biosynthesis of non-waxy and waxy broomcorn millet (Panicum miliaceum L.). Int. J. Biol. Macromol. 2023, 230, 123155. [Google Scholar] [CrossRef] [PubMed]
- Srikaeo, K.; Sangkhiaw, J. Technology, Effects of amylose and resistant starch on glycaemic index of rice noodles. LWT-Food Sci. Technol. 2014, 59, 1129–1135. [Google Scholar] [CrossRef]
- Li, C.; Zhou, D.; Fan, T.; Wang, M.; Zhu, M.; Ding, J.; Zhu, X.; Guo, W.; Shi, Y.-C. Structure and physicochemical properties of two waxy wheat starches. Food Chem. 2020, 318, 126492. [Google Scholar] [CrossRef]
- Li, C.; Gong, B. Insights into chain-length distributions of amylopectin and amylose molecules on the gelatinization property of rice starches. Int. J. Biol. Macromol. 2020, 155, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Javadian, N.; Mohammadi Nafchi, A.; Bolandi, M. The effects of dual modification on functional, microstructural, and thermal properties of tapioca starch. Food Sci. Nutr. 2021, 9, 5467–5476. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cheng, B.; Zhang, W.; Shu, Z.; Wang, P.; Zeng, X. Structural and functional characteristics of Japonica rice starches with different amylose contents. CyTA-J. Food 2021, 19, 532–540. [Google Scholar] [CrossRef]
- Jincai, T.; Chenxi, W.; Jiajun, L.; Shuangrong, D.; Yixin, W.; Xinhui, L.; Jiale, W.; Qinghua, Y.; Pengke, W.; Jinfeng, G. Effects of biochar coupled with chemical and organic fertilizer application on physicochemical properties and in vitro digestibility of common buckwheat (Fagopyrum esculentum Moench) starch. Int. J. Biol. Macromol. 2023, 246, 125591. [Google Scholar]
- Singh, J.; McCarthy, O.J.; Singh, H.; Moughan, P.J.; Kaur, L. Morphological, thermal and rheological characterization of starch isolated from New Zealand Kamo Kamo (Cucurbita pepo) fruit–A novel source. Carbohydr. Polym. 2007, 67, 233–244. [Google Scholar] [CrossRef]
- Wang, Y.J. Study on Character and Starch Physico-Chemical Properties of Proso Millet; Northwest A: F University: Xianyang, China, 2012. [Google Scholar]
- Gao, L.; Bai, W.; Xia, M.; Wan, C.; Wang, M.; Wang, P.; Gao, X.; Gao, J. Diverse effects of nitrogen fertilizer on the structural, pasting, and thermal properties of common buckwheat starch. Int. J. Biol. Macromol. 2021, 179, 542–549. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Cao, X.; Sun, H.; Wang, C.; Ren, X.; Liu, H.; Zhang, Z.J. Effects of late-stage nitrogen fertilizer application on the starch structure and cooking quality of rice. J. Sci. Food Agric. 2018, 98, 2332–2340. [Google Scholar] [CrossRef]
- Hu, Y.; Cong, S.; Zhang, H. Comparison of the grain quality and starch physicochemical properties between japonica rice cultivars with different contents of amylose, as affected by nitrogen fertilization. Agriculture 2021, 11, 616. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Deng, X.; Pan, X.; Liao, P.; Xiong, Q.; Gao, H.; Wei, H.; Dai, Q.; Zeng, Y. Effects of biochar on rice yield, grain quality and starch viscosity attributes. J. Sci. Food Agric. 2023, 103, 5747–5753. [Google Scholar] [CrossRef]
- Gao, L.; Xia, M.; Wan, C.; Jia, Y.; Yang, L.; Wang, M.; Wang, P.; Yang, Q.; Yang, P.; Gao, X. Analysis of synthesis, accumulation and physicochemical properties of Tartary buckwheat starches affected by nitrogen fertilizer. Carbohydr. Polym. 2021, 273, 118570. [Google Scholar] [CrossRef] [PubMed]
- Simi, C.K.; Abraham, T.E. Physicochemical rheological and thermal properties of Njavara rice (Oryza sativa) starch. J. Agric. Food Chem. 2008, 56, 12105–12113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, M.; Zhou, Y.; Wu, Y.; Ouyang, J. Influence of ultrasound and microwave treatments on the structural and thermal properties of normal maize starch and potato starch: A comparative study. Food Chem. 2022, 377, 131990. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wan, C.; Wang, J.; Wang, P.; Gao, X.; Eeckhout, M.; Gao, J. Relationship between nitrogen fertilizer and structural, pasting and rheological properties on common buckwheat starch. Food Chem. 2022, 389, 132664. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.; Shahidi, F.; Majzoobi, M.; Koocheki, A.; Farahnaky, A. Structural, rheological, pasting and textural properties of granular cold water swelling maize starch: Effect of NaCl and CaCl2. Carbohydr. Polym. 2020, 242, 116406. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Animal 2020, 160, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
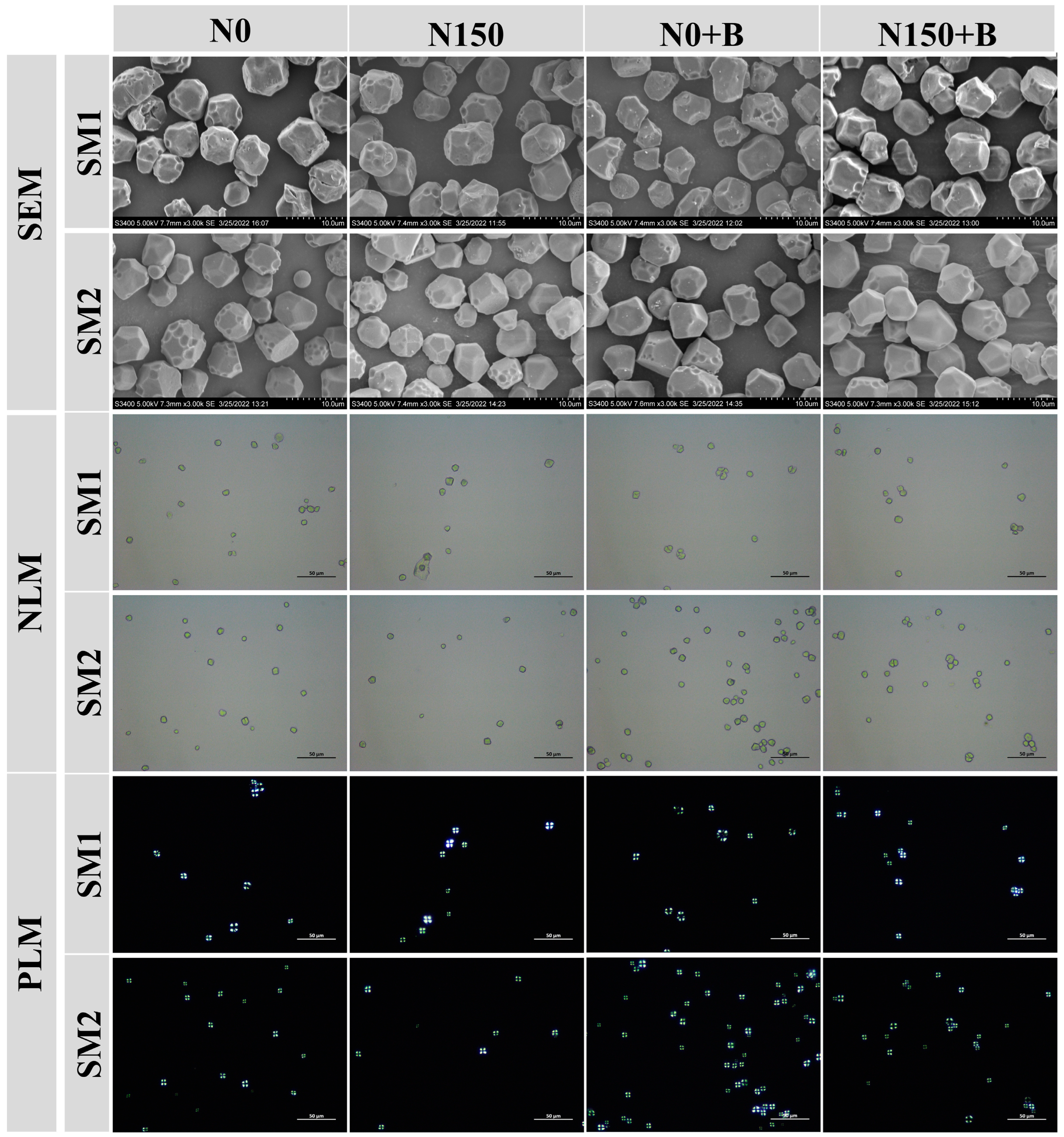
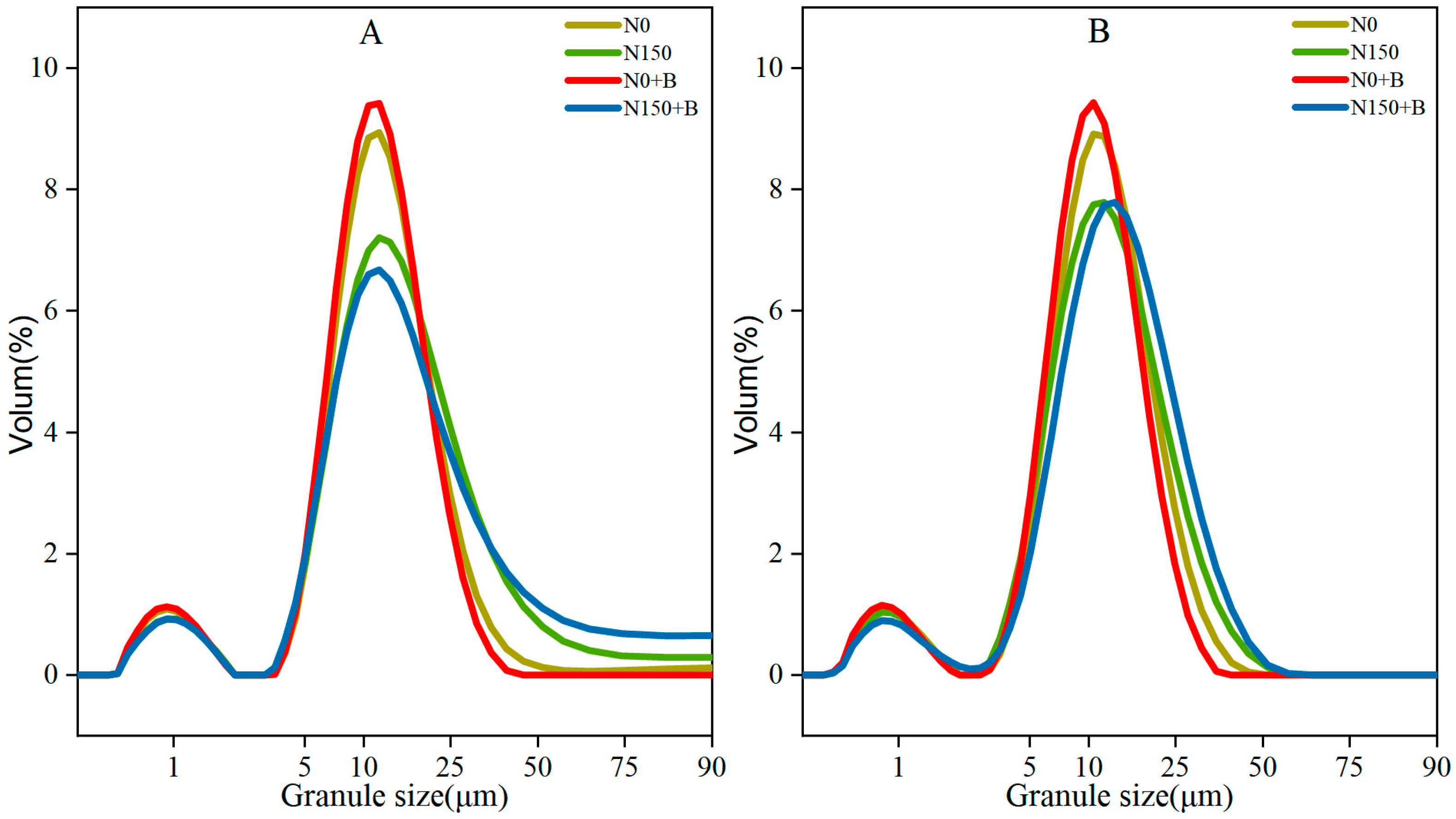
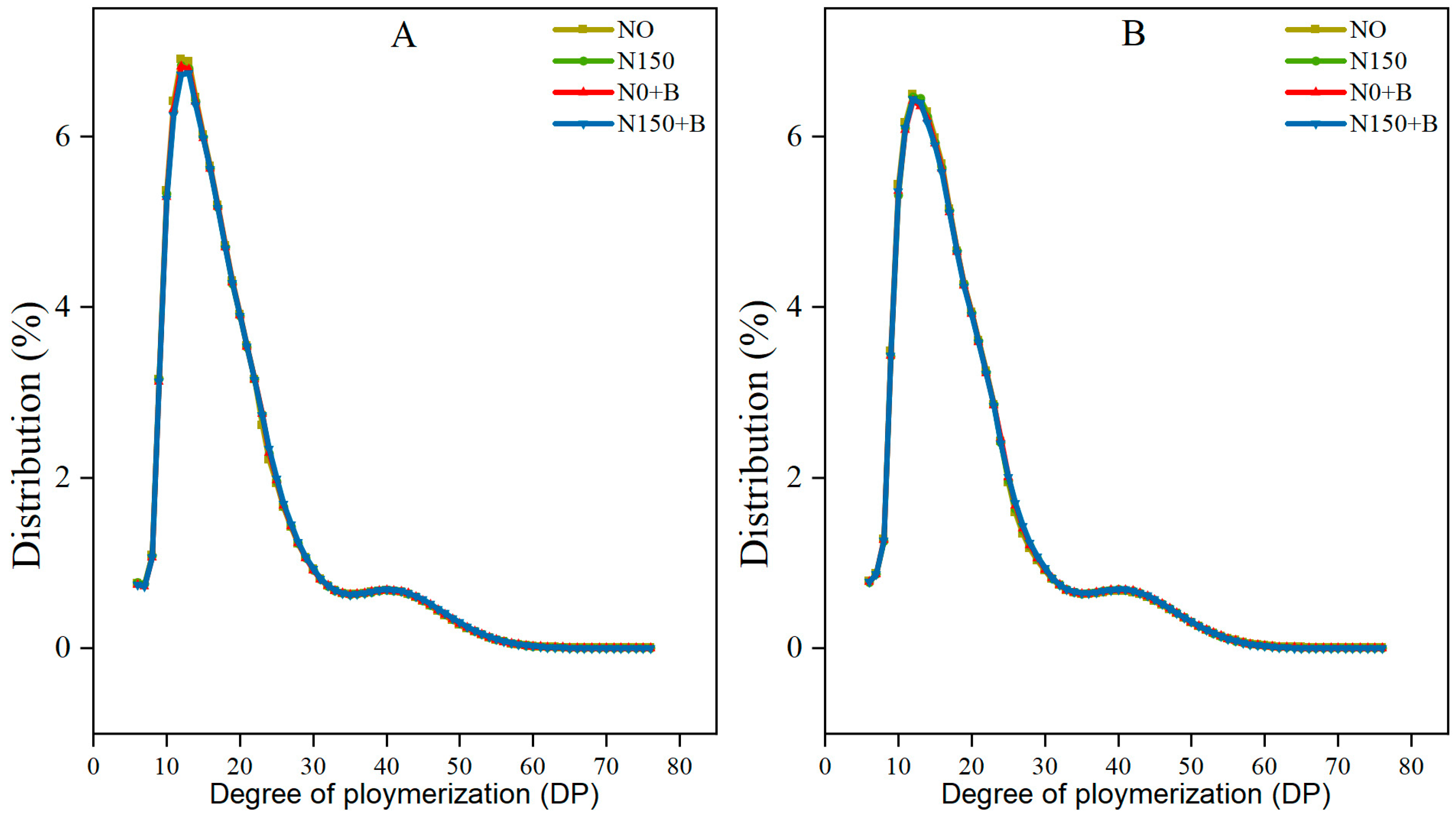
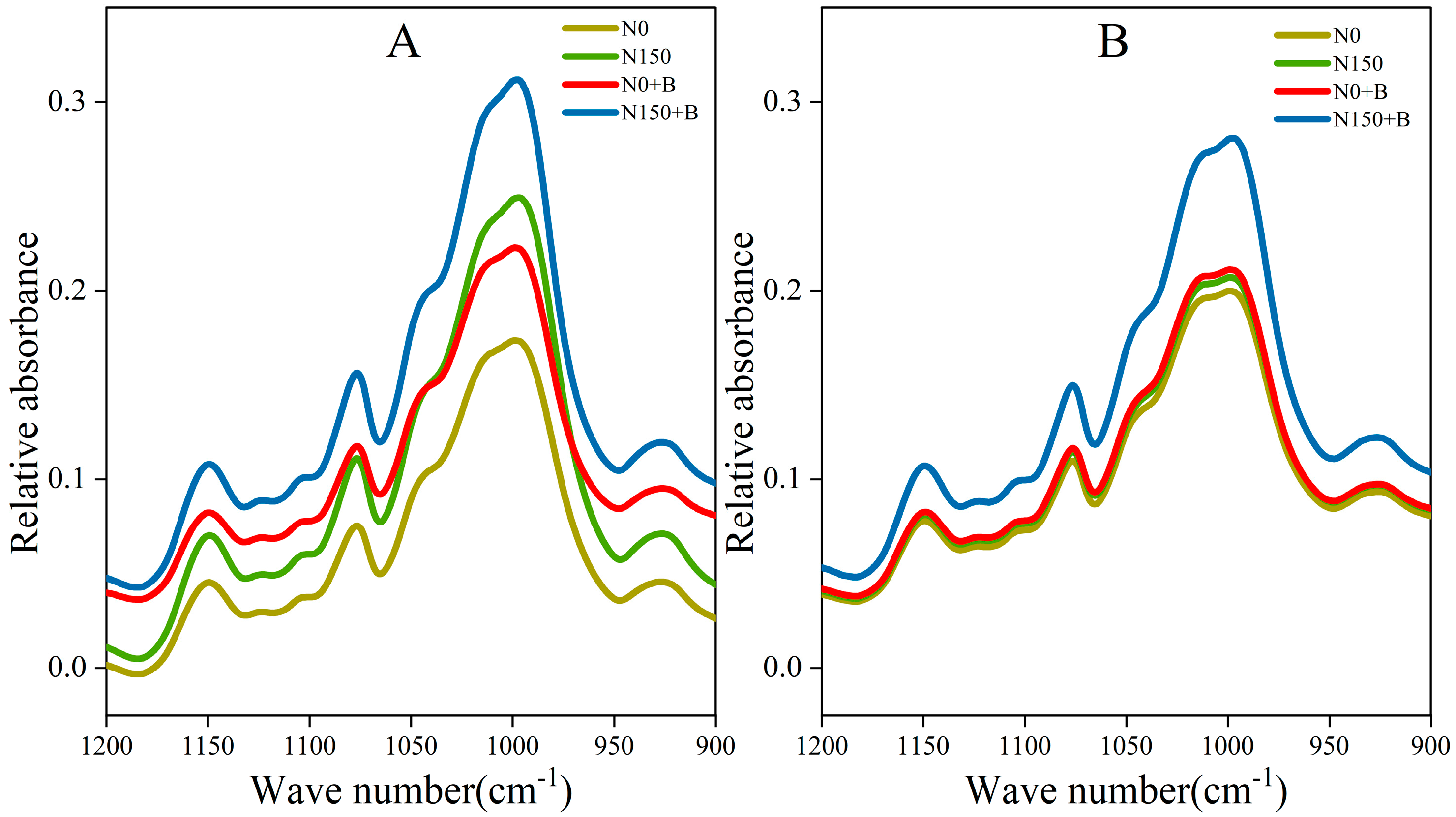
| Varieties | Treatments | Specific Measures |
|---|---|---|
| SM1 (Shanmi1) | N0 | No treatment |
| N150 | Nitrogen fertilizer (150 kg N ha−1) | |
| N0+B | Biochar (10 t hm−2) | |
| N150+B | Nitrogen fertilizer and biochar (150 kg N ha−1 and 10 t hm−2) | |
| SM2 (Shanmi2) | N0 | No treatment |
| N150 | Nitrogen fertilizer (150 kg N ha−1) | |
| N0+B | Biochar (10 t hm−2) | |
| N150+B | Nitrogen fertilizer and biochar (150 kg N ha−1 and 10 t hm−2) |
| Varieties | Treatments | Distribution of Starch Granules (%) | ||
|---|---|---|---|---|
| C (<5 μm) | B (5–15 μm) | A (>15 μm) | ||
| SM1 | N0 | 9.55 ± 0.17 de | 58.73 ± 1.54 b | 31.72 ± 1.70 c |
| N150 | 9.15 ± 0.32 e | 49.86 ± 2.74 c | 40.99 ± 3.07 b | |
| N0+B | 10.16 ± 0.34 c | 63.41 ± 2.40 a | 26.43 ± 2.73 d | |
| N150+B | 8.61 ± 0.30 f | 43.57 ± 1.78 d | 47.82 ± 2.07 a | |
| Average | 9.37% | 53.90% | 36.74% | |
| SM2 | N0 | 10.79 ± 0.17 b | 61.31 ± 1.23 ab | 27.90 ± 1.40 cd |
| N150 | 11.20 ± 0.41 ab | 58.19 ± 2.98 b | 30.61 ± 2.61 c | |
| N0+B | 11.39 ± 0.23 a | 63.70 ± 1.50 a | 24.91 ± 1.73 d | |
| N150+B | 9.86 ± 0.21 cd | 47.64 ± 1.88 c | 42.50 ± 2.08 b | |
| Average | 10.81% | 57.71% | 31.48% | |
| Analysis of Variance | Varieties | ** | ** | ** |
| Treatments | ** | ** | ** | |
| Varieties × Treatments | ns | * | * | |
| Varieties | Treatments | Total Starch Content (%) | Amylose Content (%) | Amylopectin Content (%) |
|---|---|---|---|---|
| SM1 | N0 | 62.39 ± 1.41 e | 4.00 ± 1.33 c | 96.00 ± 1.33 a |
| N150 | 65.22 ± 0.82 d | 3.48 ± 0.83 c | 96.52 ± 0.83 a | |
| N0+B | 63.12 ± 0.68 e | 3.37 ± 1.00 c | 96.63 ± 1.00 a | |
| N150+B | 66.53 ± 2.19 d | 2.97 ± 0.70 c | 97.03 ± 0.70 a | |
| SM2 | N0 | 69.18 ± 0.59 c | 33.18 ± 2.03 a | 66.82 ± 2.03 c |
| N150 | 71.13 ± 0.68 b | 30.51 ± 1.26 b | 69.49 ± 1.26 b | |
| N0+B | 71.39 ± 0.57 ab | 30.47 ± 0.62 b | 69.53 ± 0.62 b | |
| N150+B | 73.19 ± 0.97 a | 28.65 ± 1.79 b | 71.35 ± 1.79 b | |
| Analysis of Variance | Varieties | ** | ** | ** |
| Treatments | ** | ** | ** | |
| Varieties × Treatments | ns | * | ns |
| Varieties | Treatments | Chain Length Distribution (%) | Average Degree of Polymerization (%) | |||
|---|---|---|---|---|---|---|
| DP 6–12 | DP 13–24 | DP 25–36 | DP ≥ 37 | |||
| SM1 | N0 | 24.50 ± 0.20 a | 54.51 ± 0.12 b | 12.06 ± 0.14 b | 8.93 ± 0.18 bc | 19.55 ± 0.08 b |
| N150 | 24.18 ± 0.08 c | 54.32 ± 0.09 c | 12.33 ± 0.11 ab | 9.17 ± 0.06 b | 19.66 ± 0.04 a | |
| N0+B | 24.21 ± 0.06 bc | 54.15 ± 0.04 d | 12.47 ± 0.04 a | 9.18 ± 0.06 b | 19.68 ± 0.03 a | |
| N150+B | 24.23 ± 0.04 bc | 53.92 ± 0.01 e | 12.28 ± 0.38 ab | 9.57 ± 0.33 a | 19.71 ± 0.01 a | |
| SM2 | N0 | 24.39 ± 0.05 ab | 54.64 ± 0.02 a | 12.29 ± 0.02 ab | 8.68 ± 0.05 c | 19.44 ± 0.02 c |
| N150 | 24.23 ± 0.06 bc | 54.54 ± 0.07 abc | 12.33 ± 0.05 ab | 8.90 ± 0.08 bc | 19.54 ± 0.04 b | |
| N0+B | 24.10 ± 0.10 c | 54.57 ± 0.03 ab | 12.40 ± 0.04 a | 8.93 ± 0.09 bc | 19.57 ± 0.04 b | |
| N150+B | 23.89 ± 0.11 d | 54.44 ± 0.04 c | 12.52 ± 0.04 a | 9.14 ± 0.11 b | 19.67 ± 0.05 a | |
| Analysis of Variance | Varieties | * | ** | ns | ** | ** |
| Treatments | ** | ** | ns | ** | ** | |
| Varieties × Treatments | * | ** | ns | ns | ns | |
| Varieties | Treatments | Gel Consistency (mm) | FT-IR Ratios | |
|---|---|---|---|---|
| 1045/1022 (cm−1) (R1) | 1022/995 (cm−1) (R2) | |||
| SM1 | N0 | 8.01 ± 0.17 c | 0.687 ± 0.001 d | 0.866 ± 0.019 ab |
| N150 | 10.63 ± 0.12 b | 0.691 ± 0.002 d | 0.803 ± 0.024 c | |
| N0+B | 11.28 ± 0.54 b | 0.752 ± 0.006 ab | 0.857 ± 0.013 b | |
| N150+B | 12.24 ± 0.82 a | 0.761 ± 0.012 a | 0.790 ± 0.053 c | |
| SM2 | N0 | 5.74 ± 0.22 d | 0.739 ± 0.010 c | 0.902 ± 0.007 a |
| N150 | 5.86 ± 0.08 d | 0.737 ± 0.008 c | 0.892 ± 0.014 ab | |
| N0+B | 6.02 ± 0.29 d | 0.740 ± 0.005 bc | 0.900 ± 0.010 ab | |
| N150+B | 6.36 ± 0.43 d | 0.741 ± 0.002 bc | 0.886 ± 0.008 ab | |
| Analysis of Variance | Varieties | ** | ** | ** |
| Treatments | ** | ** | ** | |
| Varieties × Treatments | ** | ** | ns | |
| Varieties | Treatments | PV (mPa s) | BV (mPa s) | FV (mPa s) | SV (mPa s) | PT (°C) |
|---|---|---|---|---|---|---|
| SM1 | N0 | 1656.67 ± 127.26 c | 901.67 ± 41.65 b | 841.67 ± 48.56 c | 124.00 ± 20.07 d | 79.17 ± 0.03 cd |
| N150 | 2101.33 ± 157.16 a | 1084.00 ± 73.18 a | 1189.67 ± 19.55 a | 207.67 ± 9.07 c | 78.38 ± 0.06 e | |
| N0+B | 1871.67 ± 47.54 b | 1106.00 ± 101.30 a | 1024.67 ± 43.00 b | 127.00 ± 16.70 d | 78.38 ± 0.03 e | |
| N150+B | 1879.33 ± 61.85 b | 767.67 ± 34.93 c | 1021.33 ± 82.40 b | 168.00 ± 11.53 cd | 78.13 ± 0.46 e | |
| SM2 | N0 | 970.67 ± 7.64 d | 502.33 ± 6.51 d | 916.00 ± 4.00 bc | 447.67 ± 9.01 b | 80.22 ± 0.51 a |
| N150 | 942.33 ± 15.14 d | 443.67 ± 3.05 d | 893.33 ± 11.23 bc | 394.67 ± 9.29 b | 79.48 ± 0.46 bc | |
| N0+B | 907.67 ± 29.26 d | 468.67 ± 14.98 d | 912.00 ± 34.65 bc | 473.00 ± 20.07 b | 79.97 ± 0.02 ab | |
| N150+B | 1496.67 ± 153.64 c | 913.67 ± 91.28 b | 1231.67 ± 178.40 a | 648.67 ± 116.33 a | 78.62 ± 0.55 de | |
| Analysis of variance | Varieties | ** | ** | ns | ** | ** |
| Treatments | ** | ** | ** | ** | ** | |
| Varieties × Treatments | ** | ** | ** | ** | ** |
| Varieties | Treatments | To (°C) | Tp (°C) | ∆H (J/g) |
|---|---|---|---|---|
| SM1 | N0 | 66.70 ± 0.36 bc | 71.68 ± 0.09 c | 15.94 ± 0.58 bc |
| N150 | 68.26 ± 0.34 a | 72.31 ± 0.22 b | 18.26 ± 1.14 a | |
| N0+B | 67.20 ± 0.27 b | 72.99 ± 0.13 a | 17.47 ± 0.46 ab | |
| N150+B | 68.27 ± 0.55 a | 72.07 ± 0.11 b | 16.25 ± 0.95 bc | |
| SM2 | N0 | 65.75 ± 0.06 cd | 69.82 ± 0.19 d | 14.02 ± 0.98 d |
| N150 | 66.31 ± 1.10b cd | 69.74 ± 0.24 d | 14.76 ± 0.47 cd | |
| N0+B | 66.11 ± 0.38 cd | 69.91 ± 0.15 d | 15.04 ± 0.87 cd | |
| N150+B | 65.42 ± 0.50 d | 68.94 ± 0.07 e | 14.25 ± 1.24 d | |
| Analysis of variance | Varieties | ** | ** | ** |
| Treatments | * | ** | ** | |
| Varieties × Treatments | * | ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Mukhamed, B.; Yang, Q.; Luo, Y.; Tian, L.; Yuan, Y.; Huang, Y.; Feng, B. Biochar and Nitrogen Fertilizer Change the Quality of Waxy and Non-Waxy Broomcorn Millet (Panicum miliaceum L.) Starch. Foods 2023, 12, 3009. https://doi.org/10.3390/foods12163009
Zhang M, Mukhamed B, Yang Q, Luo Y, Tian L, Yuan Y, Huang Y, Feng B. Biochar and Nitrogen Fertilizer Change the Quality of Waxy and Non-Waxy Broomcorn Millet (Panicum miliaceum L.) Starch. Foods. 2023; 12(16):3009. https://doi.org/10.3390/foods12163009
Chicago/Turabian StyleZhang, Miaomiao, Bauyrzhan Mukhamed, Qinghua Yang, Yan Luo, Lixin Tian, Yuhao Yuan, Yani Huang, and Baili Feng. 2023. "Biochar and Nitrogen Fertilizer Change the Quality of Waxy and Non-Waxy Broomcorn Millet (Panicum miliaceum L.) Starch" Foods 12, no. 16: 3009. https://doi.org/10.3390/foods12163009
APA StyleZhang, M., Mukhamed, B., Yang, Q., Luo, Y., Tian, L., Yuan, Y., Huang, Y., & Feng, B. (2023). Biochar and Nitrogen Fertilizer Change the Quality of Waxy and Non-Waxy Broomcorn Millet (Panicum miliaceum L.) Starch. Foods, 12(16), 3009. https://doi.org/10.3390/foods12163009






