Preparation, Antioxidant Activities and Bioactive Components of Kombucha Beverages from Golden-Flower Tea (Camellia petelotii) and Honeysuckle-Flower Tea (Lonicera japonica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Activation of Kombucha Starter Culture
2.3. Kombucha Preparation Based on Golden-Flower Tea and Honeysuckle-Flower Tea
2.4. Measurement of Antioxidant Capacity and TPC
2.5. Determination of the Contents of Bioactive Components by HPLC-PDA
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
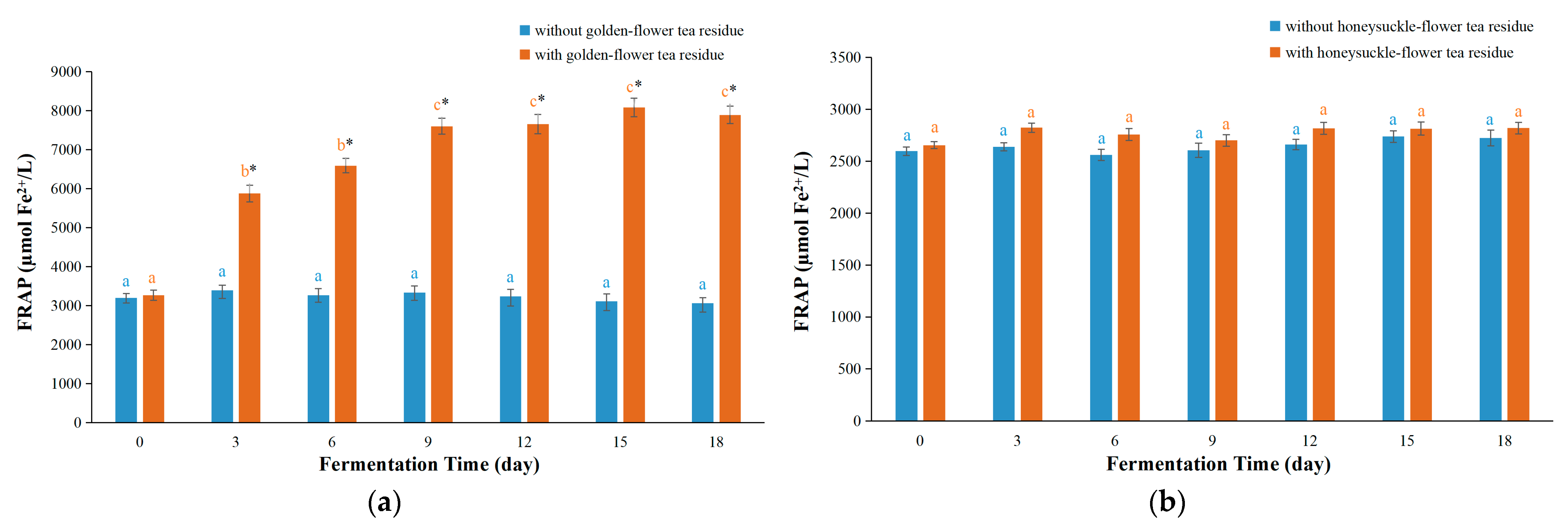
3.1. FRAP Values
3.2. TEAC Values
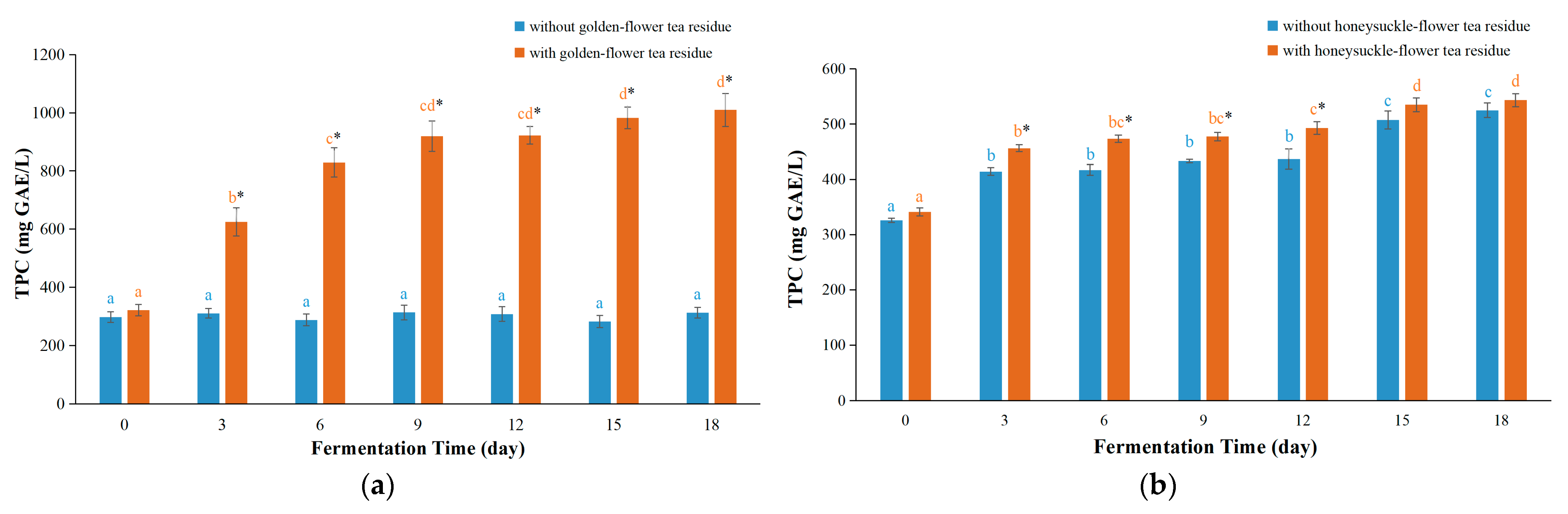
3.3. TPC Values
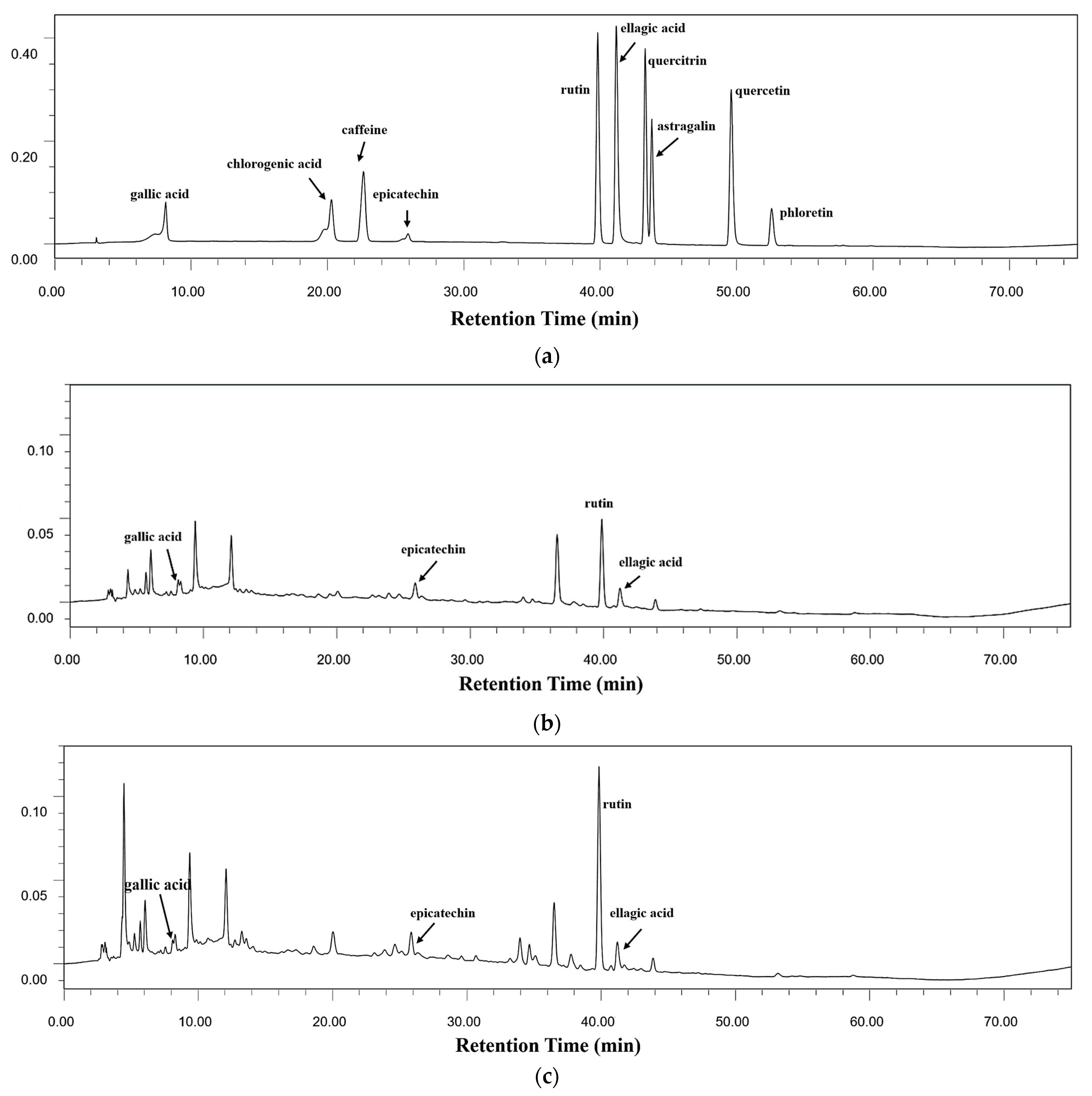
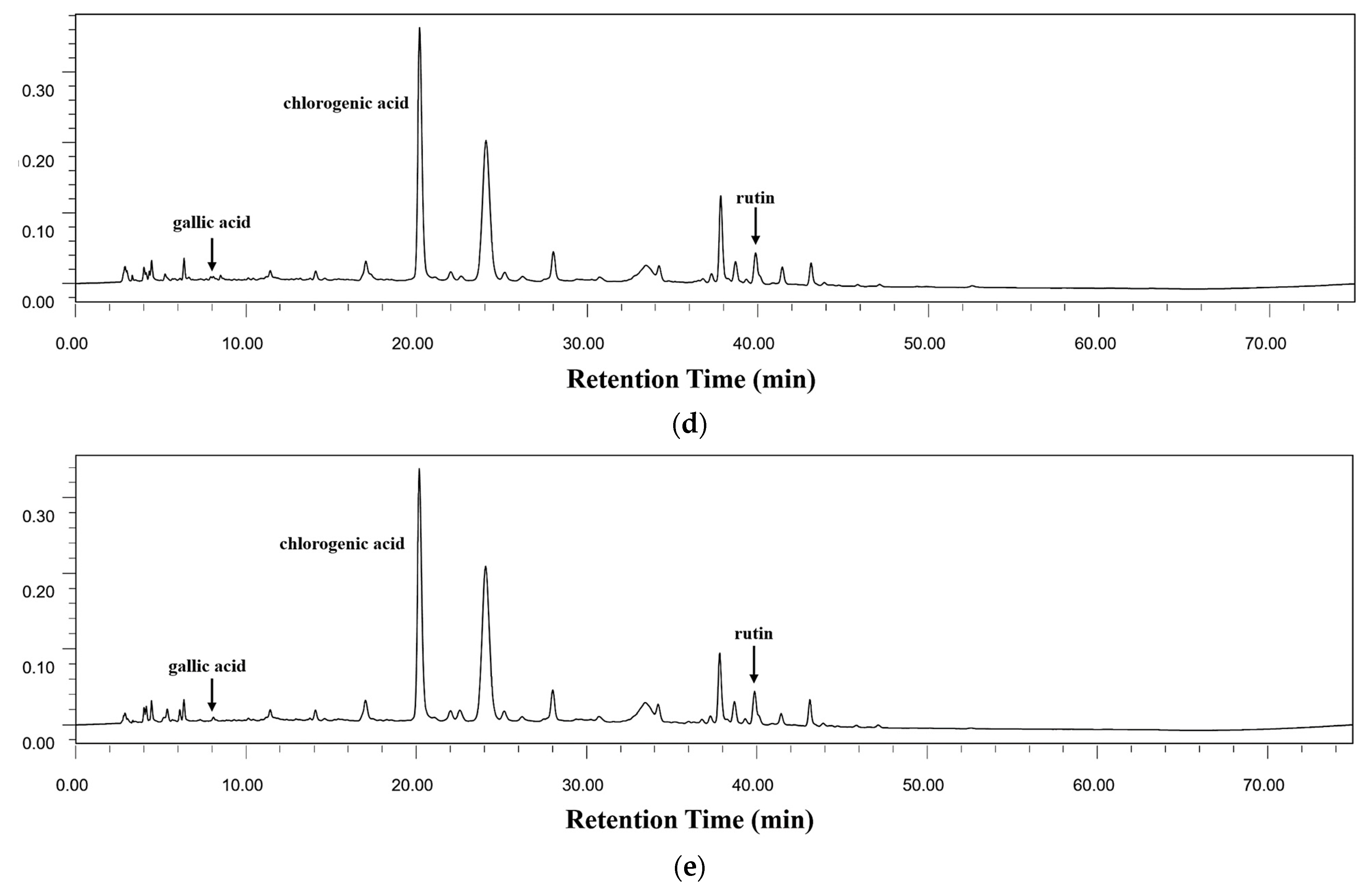
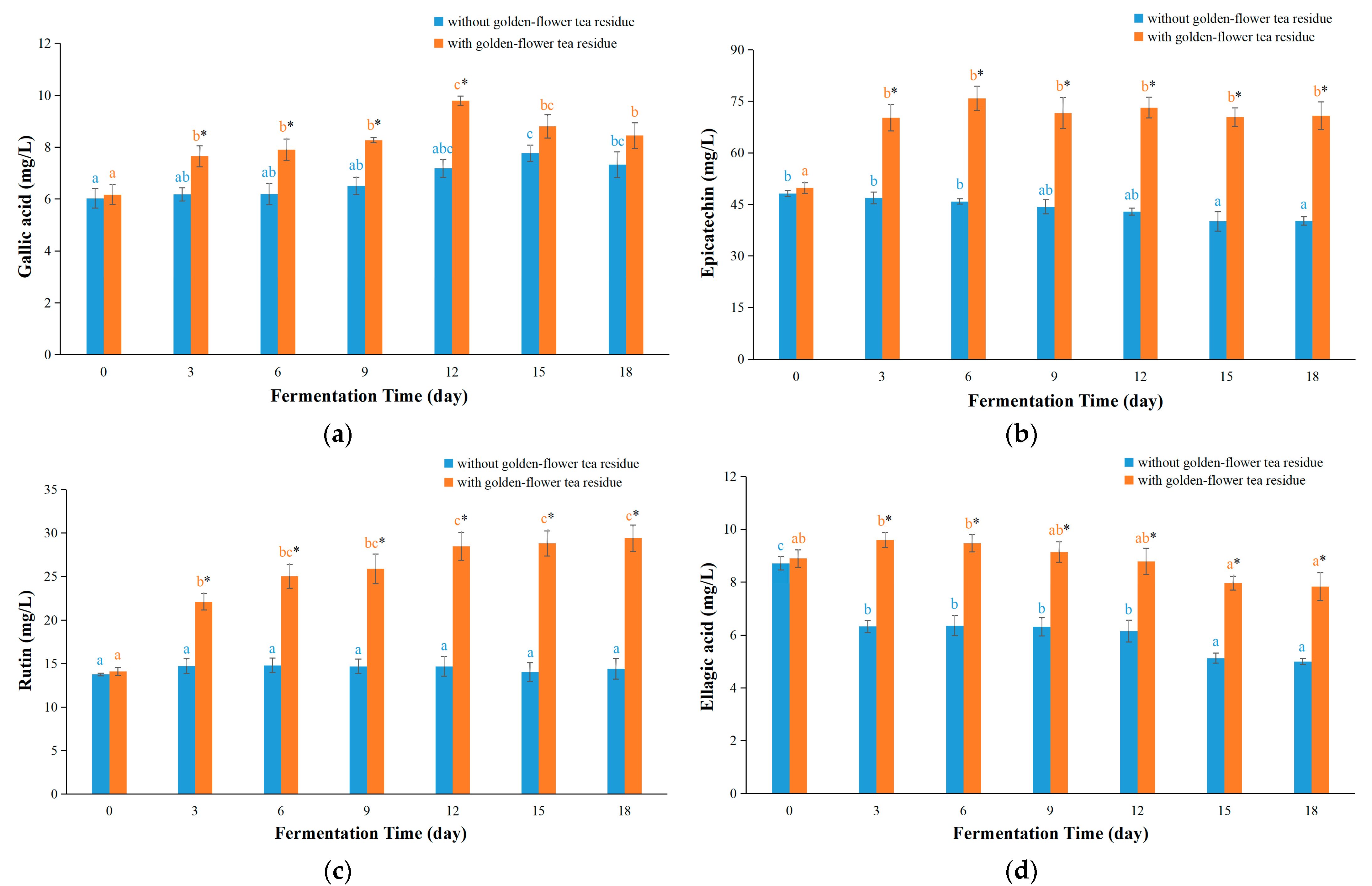
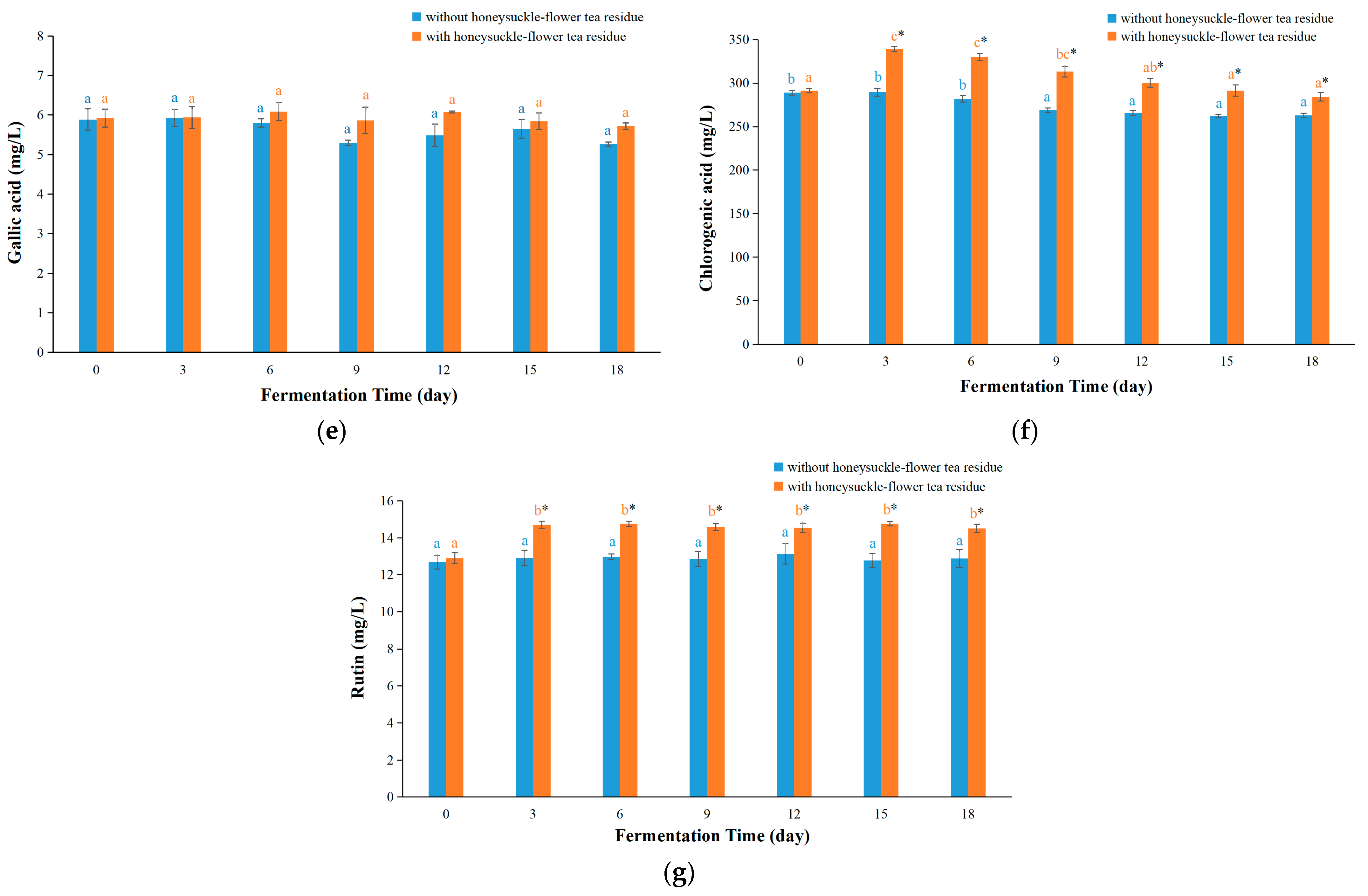
3.4. Contents of Bioactive Components
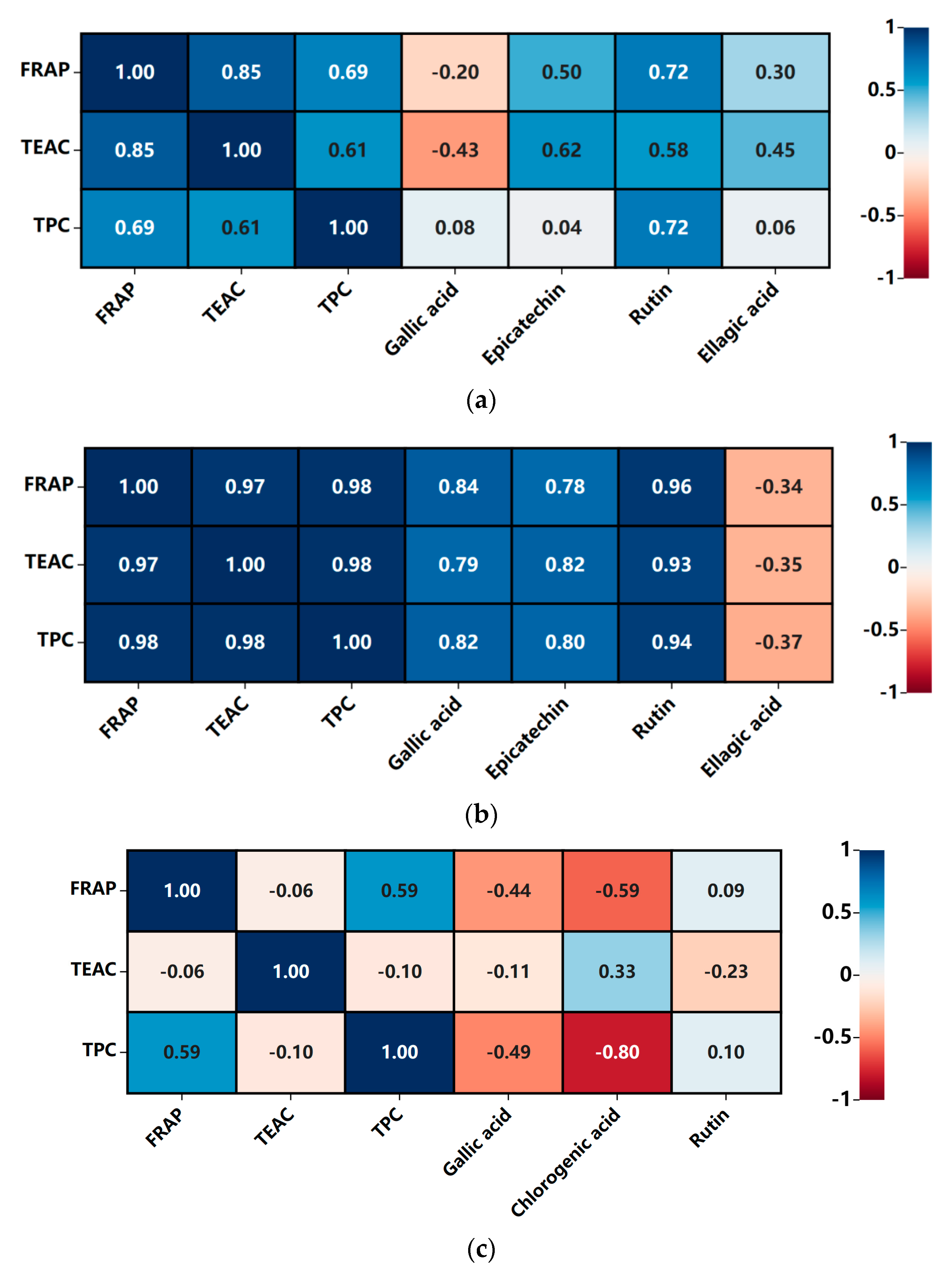
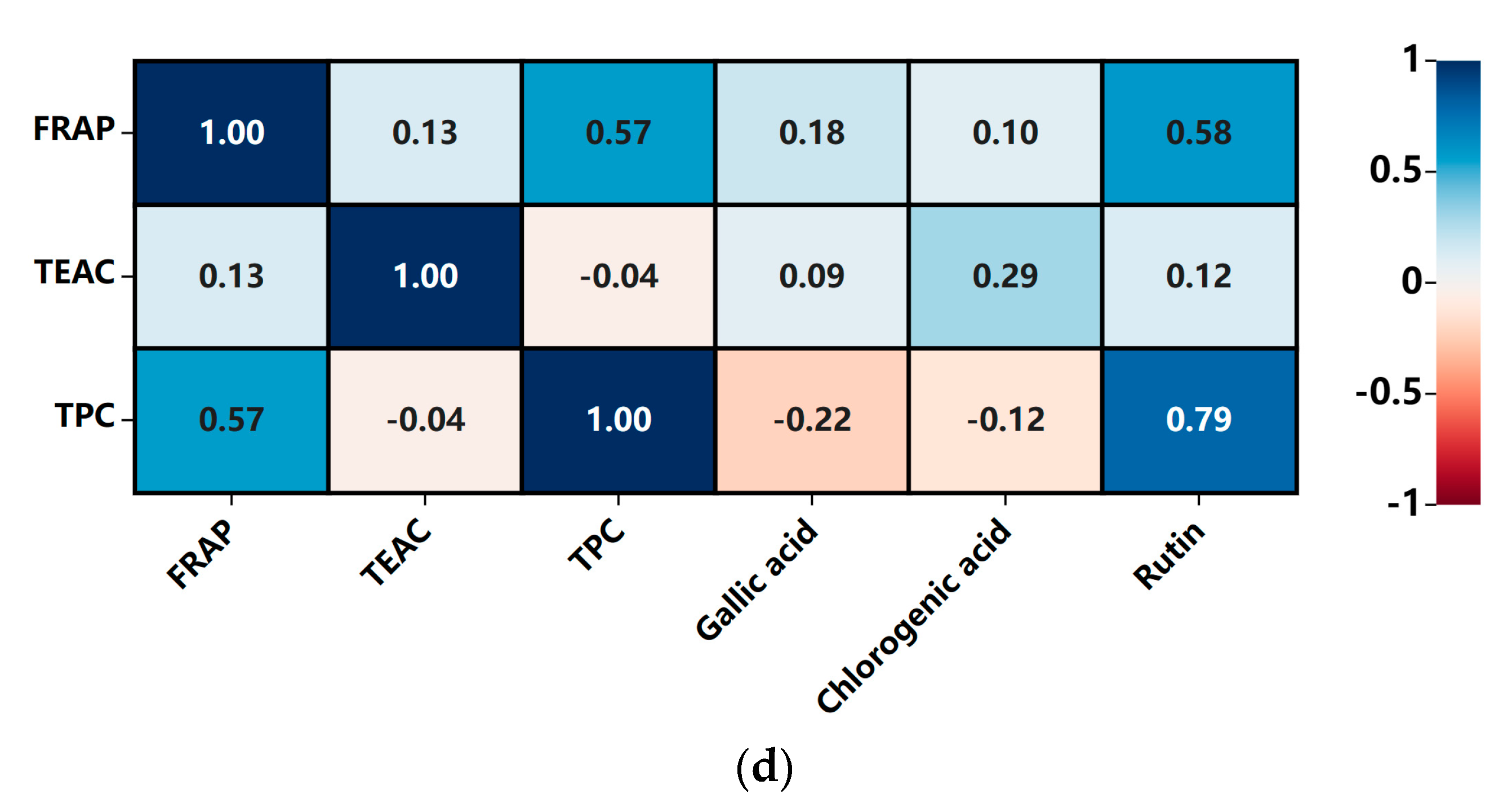
3.5. Correlations Analysis between the Antioxidant Capacities and Bioactive Components
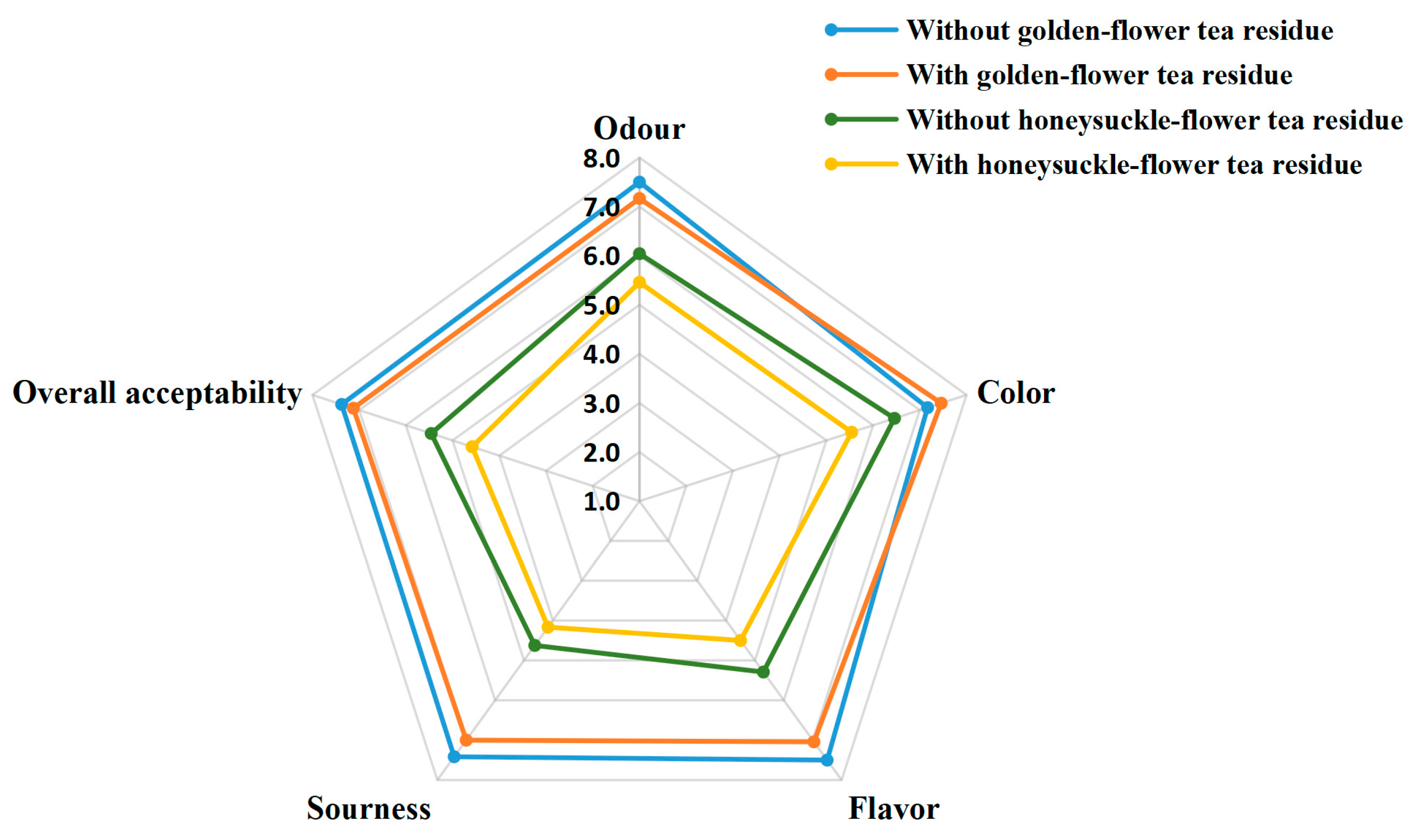
3.6. Sensory Evaluation
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Martins Gonzalez, A.G.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha teamicrobiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food. Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha tea-A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Emiljanowicz, K.E.; Malinowska-Panczyk, E. Kombucha from alternative raw material—The review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative raw materials in kombucha production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Niziol-Lukaszewska, Z.; Bujak, T.; Zagorska-Dziok, M.; Wojciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Rahmani, R.; Beaufort, S.; Villarreal-Soto, S.A.; Taillandier, P.; Bouajila, J.; Debouba, M. Kombucha fermentation of African mustard (Brassica tournefortii) leaves: Chemical composition and bioactivity. Food Biosci. 2019, 30, 100414. [Google Scholar] [CrossRef]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Saimaiti, A.; Luo, M.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Gan, R.-Y.; Li, H.-B. Fermentation with tea residues enhances antioxidant activities and polyphenol contents in kombucha beverages. Antioxidants 2022, 11, 155. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.-Y.; Xiong, R.-G.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Luo, M.; Gan, R.-Y.; Li, H.-B. Antioxidant capacities and polyphenol contents of kombucha beverages based on vine tea and sweet tea. Antioxidants 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, X.; Li, M.; Ji, R.; Li, Z.; Wang, Y.; Guo, Y.; Liu, D.; Huang, B.; Du, H. Active fractions of golden-flowered tea (Camellia nitidissima Chi) inhibit epidermal growth factor receptor mutated non-small cell lung cancer via multiple pathways and targets in vitro and in vivo. Front. Nutr. 2022, 9, 1014414. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.X.; Chen, L.; Cui, W.Y.; Dong, Y.J.; Yuan, X.X.; Wang, L.; Liang, J.Y.; Zhao, S.C. Analysis of heavy metal content in edible honeysuckle (Lonicera japonica Thunb.) from China and health risk assessment. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2020, 55, 921–928. [Google Scholar] [CrossRef]
- He, D.; Li, X.; Sai, X.; Wang, L.; Li, S.; Xu, Y. Camellia nitidissima CW Chi: A review of botany, chemistry, and pharmacology. Phytochem. Rev. 2018, 17, 327–349. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Du, Z.; Xie, J.; Xia, L.; Hou, X.; Hao, E.; Deng, J. Proteome analysis of Camellia nitidissima Chi revealed its role in colon cancer through the apoptosis and ferroptosis pathway. Front. Oncol. 2021, 11, 727130. [Google Scholar] [CrossRef]
- He, D.; Xu, Y. Toxicological evaluation of Camellia euphlebia leaves aqueous extract using acute and subacute toxicity studies in mice and genotoxicity studies. Evid.-Based Complement. Altern. Med. 2022, 2022, 7889199. [Google Scholar] [CrossRef]
- Tsoi, B.; Gao, C.; Yan, S.; Du, Q.; Yu, H.; Li, P.; Deng, J.; Shen, J. Camellia nitidissima Chi extract promotes adult hippocampal neurogenesis and attenuates chronic corticosterone-induced depressive behaviours through regulating Akt/GSK3 beta/CREB signaling pathway. J. Funct. Foods 2022, 95, 105199. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Han, X.; Su, L.; Ge, L.; Chen, Q.; Yang, K.; Mo, Q. Triterpenes and saponins from leaves of Camellia nitidissima, and cytotoxic activities against Bel-7402 and SMMC-7721 human liver cancer cells. Rec. Nat. Prod. 2022, 16, 550–558. [Google Scholar] [CrossRef]
- Liao, W.F.; Hu, X.D.; Du, Z.Y.; Wang, P.P.; Ding, K. A homogalacturonan from Lonicera japonica Thunb. disrupts angiogenesis via epidermal growth factor receptor and Delta-like 4 associated signaling. Glycoconj. J. 2022, 39, 725–735. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, W.A.; He, Z.B.; Li, S.; Liu, J. Network pharmacology-based strategy for exploring the pharmacological mechanism of honeysuckle (Lonicer japonica Thunb.) against Newcastle disease. Evid.-Based Complement. Altern. Med. 2022, 2022, 9265094. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Q.; Shi, L.P.; Cao, W.F.; Chen, Z.W.; Zuo, B.; Hu, J.Y. Add-on effect of honeysuckle in the treatment of coronavirus disease 2019: A systematic review and meta-analysis. Front. Pharmacol. 2021, 12, 708636. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.Y.; Xie, Y.L.; Wang, Y.Y.; Zhu, J.H.; Li, M.J.; Liu, X.H.; Zhao, D.B. Variation in contents of active components and antibacterial activity in different parts of Lonicera japonica Thunb. Asian Biomed. 2020, 14, 19–26. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, S.; Liu, Q.; Zhang, Y.Q. Spectrum-effect relationship study between HPLC fingerprints and antioxidant of honeysuckle extract. Biomed. Chromatogr. 2019, 33, e4583. [Google Scholar] [CrossRef]
- Zhou, X.N.; Lu, Q.Q.; Kang, X.Z.; Tian, G.; Ming, D.G.; Yang, J.L. Protective role of a new polysaccharide extracted from Lonicera japonica Thunb in mice with ulcerative colitis induced by dextran sulphate sodium. BioMed Res. Int. 2021, 2021, 8878633. [Google Scholar] [CrossRef]
- Shimamura, Y.; Shibata, M.; Sato, M.; Nagai, R.; Yang, P.; Shiokawa, K.I.; Kikuchi, H.; Masuda, S. Anti-hyperglycemic activity and inhibition of advanced glycation end products by Lonicera japonica Thunb. in streptozotocin-induced diabetic rats. Food Sci. Technol. Res. 2020, 26, 825–835. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Li, B.-Y.; Mao, Q.-Q.; Gan, R.-Y.; Cao, S.-Y.; Xu, X.-Y.; Luo, M.; Li, H.-Y.; Li, H.-B. Protective effects of tea extracts against alcoholic fatty liver disease in mice via modulating cytochrome P450 2E1 expression and ameliorating oxidative damage. Food Sci. Nutr. 2021, 9, 5626–5640. [Google Scholar] [CrossRef]
- Ivanisova, E.; Menhartova, K.; Terentjeva, M.; Harangozo, L.u.; Kantor, A.; Kacaniova, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol.-Mysore 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Fejer, J.; Grul’ova, D.; Eliasova, A.; Kron, I. Seasonal variability of Juniperus communis L. berry ethanol extracts: 2. In vitro ferric reducing ability of plasma (FRAP) assay. Molecules 2022, 27, 9027. [Google Scholar] [CrossRef]
- Pinheiro, F.A.; Okumura, L.L.; Silva, A.F.S.; Silva, J.G.; Ferreira, L.R.; Barcellos, E.S.; Fontes, E.A.F. Applicability of a voltammetric assay based on the electroreduction of oxygen to evaluate the antioxidant capacity of pequi (Caryocar brasiliense Camb.) pulp. J. Braz. Chem. Soc. 2018, 29, 1653–1662. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.; Rangel, A. Automatic method for the determination of Folin-Ciocalteu reducing capacity in food products. J. Agric. Food Chem. 2006, 54, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, J.-M.; Mao, Q.-Q.; Shang, A.; Li, B.-Y.; Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Wei, X.-L.; Gan, R.-Y.; et al. Effects of Tannase and Ultrasound Treatment on the Bioactive Compounds and Antioxidant Activity of Green Tea Extract. Antioxidants 2019, 8, 362. [Google Scholar] [CrossRef]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-X.; Xiong, R.-G.; Cheng, J.; Xu, X.-Y.; Tang, G.-Y.; Huang, S.-Y.; Zhou, D.-D.; Saimaiti, A.; Gan, R.-Y.; Li, H.-B. Preparation, Antioxidant Activities and Bioactive Components of Kombucha Beverages from Golden-Flower Tea (Camellia petelotii) and Honeysuckle-Flower Tea (Lonicera japonica). Foods 2023, 12, 3010. https://doi.org/10.3390/foods12163010
Wu S-X, Xiong R-G, Cheng J, Xu X-Y, Tang G-Y, Huang S-Y, Zhou D-D, Saimaiti A, Gan R-Y, Li H-B. Preparation, Antioxidant Activities and Bioactive Components of Kombucha Beverages from Golden-Flower Tea (Camellia petelotii) and Honeysuckle-Flower Tea (Lonicera japonica). Foods. 2023; 12(16):3010. https://doi.org/10.3390/foods12163010
Chicago/Turabian StyleWu, Si-Xia, Ruo-Gu Xiong, Jin Cheng, Xiao-Yu Xu, Guo-Yi Tang, Si-Yu Huang, Dan-Dan Zhou, Adila Saimaiti, Ren-You Gan, and Hua-Bin Li. 2023. "Preparation, Antioxidant Activities and Bioactive Components of Kombucha Beverages from Golden-Flower Tea (Camellia petelotii) and Honeysuckle-Flower Tea (Lonicera japonica)" Foods 12, no. 16: 3010. https://doi.org/10.3390/foods12163010
APA StyleWu, S.-X., Xiong, R.-G., Cheng, J., Xu, X.-Y., Tang, G.-Y., Huang, S.-Y., Zhou, D.-D., Saimaiti, A., Gan, R.-Y., & Li, H.-B. (2023). Preparation, Antioxidant Activities and Bioactive Components of Kombucha Beverages from Golden-Flower Tea (Camellia petelotii) and Honeysuckle-Flower Tea (Lonicera japonica). Foods, 12(16), 3010. https://doi.org/10.3390/foods12163010





