Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of TMD Products
2.2. Ovariectomy Procedure
2.3. Diet Preparation
2.4. Experimental Design
2.5. Glucose Metabolism Measurement
2.6. Memory Function Measurement
2.7. BMD Measurement by Dual-Energy X-ray Absorptiometry (DEXA) and Sample Collection
2.8. Micro-Computed Tomography (CT) of the Femur
2.9. Biochemical Assay
2.10. Gut Microbiota in the TMD and Feces Evaluated by NGS
2.11. Statistical Analysis
3. Results
3.1. The Characteristics of Dried TMD with Different Amounts of B. subtilis and Biogenic Amines
3.2. Energy and Glucose Metabolism
3.3. OGTT and IPITT
3.4. Memory Functions and Depression
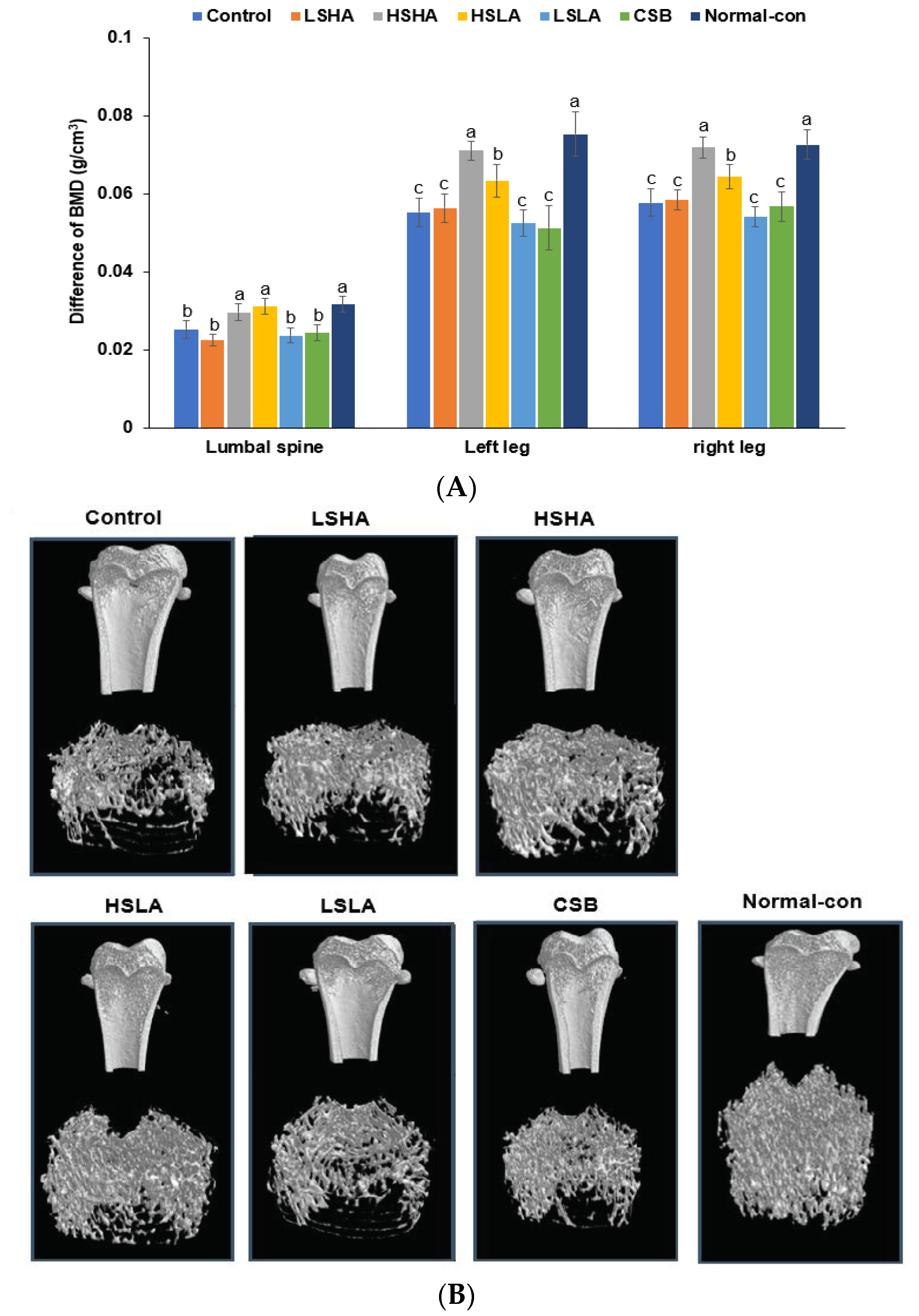
3.5. BMD by DEXA and Micro-CT
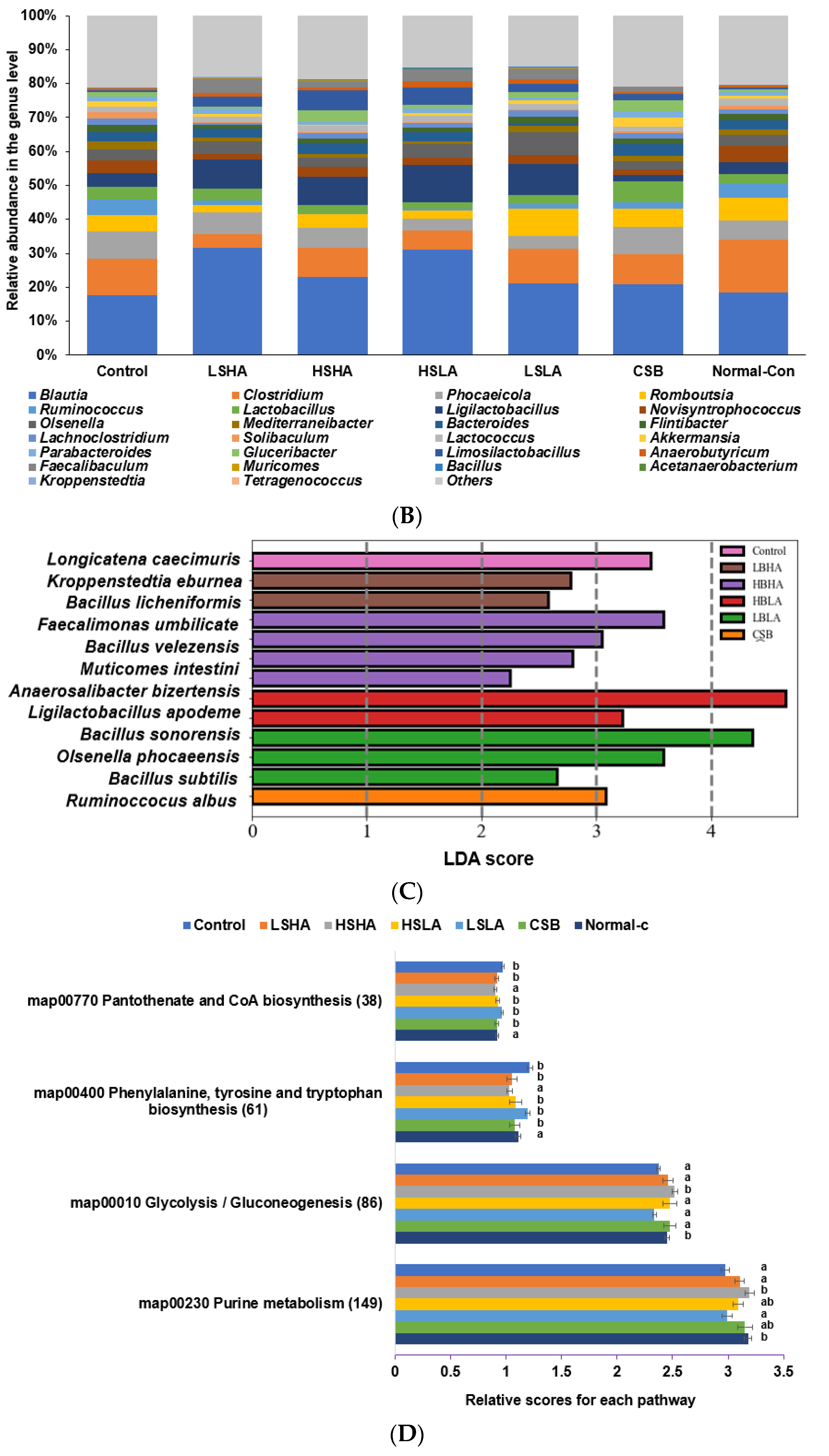
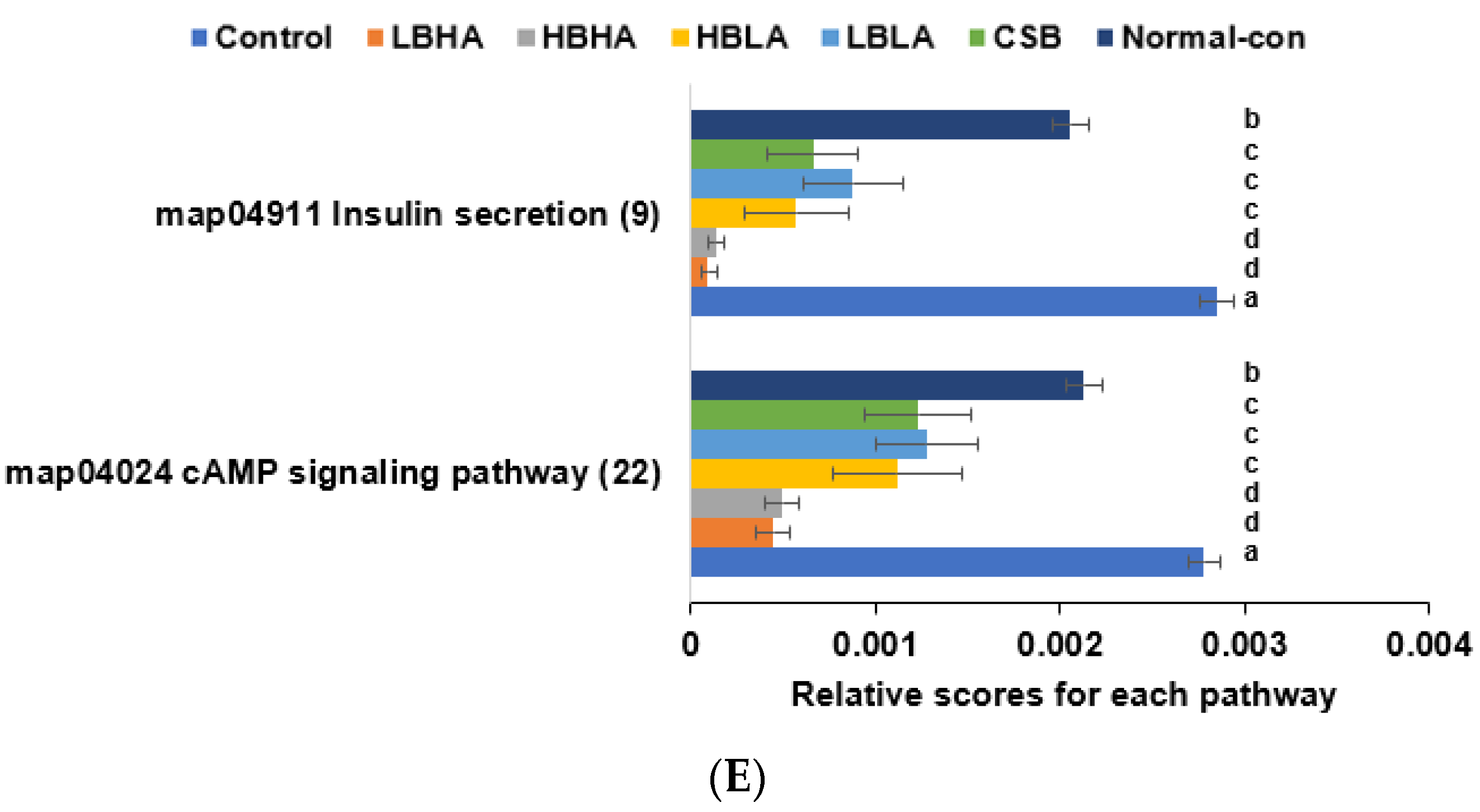
3.6. SCFA in the Blood from the Portal Vein and Fecal Bacteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kronenberg, F. Menopausal Hot Flashes: A Review of Physiology and Biosociocultural Perspective on Methods of Assessment 1,2. J. Nutr. 2010, 140, 1380S–1385S. [Google Scholar] [CrossRef]
- Haines, C.J.; Xing, S.-M.; Park, K.-H.; Holinka, C.F.; Ausmanas, M.K. Prevalence of menopausal symptoms in different ethnic groups of Asian women and responsiveness to therapy with three doses of conjugated estrogens/medroxyprogesterone acetate: The Pan-Asia menopause (PAM) study. Maturitas 2005, 52, 264–276. [Google Scholar] [CrossRef]
- Alemany, M. Estrogens and the regulation of glucose metabolism. World J. Diab. 2021, 12, 1622–1654. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, K.C.R.; Laurindo, C.P.; Machado, U.F. Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation. Cells 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hong, N.; Shim, J.-S.; Rhee, Y.; Kim, H.C. Association of Insulin Resistance with Lower Bone Volume and Strength Index of the Proximal Femur in Nondiabetic Postmenopausal Women. J. Bone Metab. 2018, 25, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Xu, J. Association between the Geriatric Nutritional Risk Index, bone mineral density and osteoporosis in type 2 diabetes patients. J. Diabetes Investig. 2020, 11, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and cognitive impairment: A narrative review of current knowledge. World J. Psychiatry 2021, 11, 412–428. [Google Scholar] [CrossRef]
- Willmann, C.; Brockmann, K.; Wagner, R.; Kullmann, S.; Preissl, H.; Schnauder, G.; Maetzler, W.; Gasser, T.; Berg, D.; Eschweiler, G.W.; et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res. Care 2020, 8, e001741. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Dyke, J.; Schelbaum, E.; Jett, S.; Loughlin, L.; Jang, G.; Rahman, A.; Hristov, H.; Pahlajani, S.; et al. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci. Rep. 2021, 11, 10867. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, E.S.; Kim, D.B.; Kang, S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E99–E109. [Google Scholar] [CrossRef]
- Carpenter, J.; Gass, M.L.; Maki, P.M.; Newton, K.M.; Pinkerton, J.V.; Taylor, M.; Utian, W.H.; Schnatz, P.F.; Kaunitz, A.M.; Shapiro, M.; et al. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015, 22, 1155–1172. [Google Scholar]
- Jayusman, P.A.; Nasruddin, N.S.; Baharin, B.; Ibrahim, N.I.; Ahmad Hairi, H.; Shuid, A.N. Overview on postmenopausal osteoporosis and periodontitis: The therapeutic potential of phytoestrogens against alveolar bone loss. Front. Pharmacol. 2023, 14, 1120457. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Du, Y.; Oh, C.; No, J. Effects of Soy Foods in Postmenopausal Women: A Focus on Osteosarcopenia and Obesity. J. Obes. Metab. Syndr. 2020, 29, 180–187. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S.; Moon, B.R. Fermented soybeans, Chungkookjang, prevent hippocampal cell death and β-cell apoptosis by decreasing pro-inflammatory cytokines in gerbils with transient artery occlusion. Exp. Biol. Med. 2016, 241, 296–307. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H. Therapeutic effects of polyphenols in fermented soybean and black soybean products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, M.J.; Park, S.-H.; Song, E.-J.; Nam, Y.-D.; Ahn, J.; Jang, Y.-J.; Ha, T.-Y.; Jung, C.H. Bioavailability of Isoflavone Metabolites After Korean Fermented Soybean Paste (Doenjang) Ingestion in Estrogen-Deficient Rats. J. Food Sci. 2018, 83, 2212–2221. [Google Scholar] [CrossRef]
- Heng, Y.; Kim, M.J.; Yang, H.-J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Ryu, M.S.; Yang, H.J.; Wu, X.H.; Jeong, D.Y.; Park, S.M. Bacterial Distribution, Biogenic Amine Contents, and Functionalities of Traditionally Made Doenjang, a Long-Term Fermented Soybean Food, from Different Areas of Korea. Microorganisms 2021, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Seo, B.Y.; Choi, H.S. Biogenic Amine Degradation by Bacillus Species Isolated from Traditional Fermented Soybean Food and Detection of Decarboxylase-Related Genes. J. Microbiol. Biotechnol. 2015, 25, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.H.; Park, Y.K.; Jin, Y.H.; Lee, J.H.; Hwang, H.J. Bacterial Production and Control of Biogenic Amines in Asian Fermented Soybean Foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef]
- Cha, Y.S.; Park, Y.; Lee, M.; Chae, S.W.; Park, K.; Kim, Y.; Lee, H.S. Doenjang, a Korean fermented soy food, exerts antiobesity and antioxidative activities in overweight subjects with the PPAR-γ2 C1431T polymorphism: 12-week, double-blind randomized clinical trial. J. Med. Food 2014, 17, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Ross, P.; Stanton, C. Beneficial Microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, M.; Jeong, S.; Yang, H.; Jeong, D. Characteristic analysis and production of short-ripened Korean traditional soy sauce added with rice bran. J. Korean Soc. Food Sci. Nutr. 2014, 43, 550–556. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838s–841s. [Google Scholar] [CrossRef]
- Zhang, T.; Kim, M.J.; Kim, M.J.; Wu, X.; Yang, H.J.; Yuan, H.; Huang, S.; Yoon, S.M.; Kim, K.N.; Park, S. Long-Term Effect of Porcine Brain Enzyme Hydrolysate Intake on Scopolamine-Induced Memory Impairment in Rats. Int. J. Mol. Sci. 2022, 23, 3361. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, K.; Xiao, Y.; Li, X.; Mo, S.; Yuan, Y.; Wang, P.; Yang, L.; Zhang, R.; Zhu, X. High-salt diet accelerates bone loss accompanied by activation of ion channels related to kidney and bone tissue in ovariectomized rats. Ecotoxicol. Environ. Saf. 2022, 244, 114024. [Google Scholar] [CrossRef]
- Park, S.; Yuan, H.; Zhang, T.; Wu, X.; Huang, S.K.; Cho, S.M. Long-term silk peptide intake promotes skeletal muscle mass, reduces inflammation, and modulates gut microbiota in middle-aged female rats. Biomed. Pharmacother. 2021, 137, 111415. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2021, 60, 1907–1919. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, Y.; Jeong, S.J.; Ryu, M.S.; Wu, X.; Yang, H.J.; Li, C.; Jeong, D.Y.; Park, S. Improvement of Estrogen Deficiency Symptoms by the Intake of Long-Term Fermented Soybeans Rich in Bacillus Species through Modulating Gut Microbiota in Estrogen-Deficient Rats. Foods 2023, 12, 1143. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Jeong, D.Y.; Kim, D.S.; Park, S. Chungkookjang with High Contents of Poly-γ-Glutamic Acid Improves Insulin Sensitizing Activity in Adipocytes and Neuronal Cells. Nutrients 2018, 10, 1588. [Google Scholar] [CrossRef]
- Sitges-Serra, A.; Girvent, M.; Pereira, J.A.; Jimeno, J.; Nogués, X.; Cano, F.J.; Sancho, J.J. Bone mineral density in menopausal women with primary hyperparathyroidism before and after parathyroidectomy. World J. Surg. 2004, 28, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J. Med. Food 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Słupski, W.; Jawień, P.; Nowak, B. Botanicals in Postmenopausal Osteoporosis. Nutrients 2021, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Iki, M.; Morita, A.; Kajita, E.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J. Nutr. 2006, 136, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.Y.; Youn, Y.N.; Koo, M.; Kim, H.J.; Choi, S.Y. Influence of water-soluble extracts of long-term fermented Doenjang on bone metabolism bioactivity and breast cancer suppression. Food Sci. Biotechnol. 2016, 25, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef]
- Marchant, I.C.; Chabert, S.; Martínez-Pinto, J.; Sotomayor-Zárate, R.; Ramírez-Barrantes, R.; Acevedo, L.; Córdova, C.; Olivero, P. Estrogen, Cognitive Performance, and Functional Imaging Studies: What Are We Missing About Neuroprotection? Front. Cell Neurosci. 2022, 16, 866122. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Jeong, S.Y.; Zhang, T.; Wu, X.; Qiu, J.Y.; Park, S. Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ischemic stroke injury and post-stroke hyperglycemia. Food Res. Int. 2020, 128, 108769. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-Rich Chungkookjang, Short-Term Fermented Soybeans: Prevents Memory Impairment by Modulating Brain Insulin Sensitivity, Neuro-Inflammation, and the Gut-Microbiome-Brain Axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Zhang, T.; Ryu, M.S.; Wu, X.; Yang, H.J.; Jeong, S.J.; Seo, J.W.; Jeong, D.Y.; Park, S. Alleviation of Neuronal Cell Death and Memory Deficit with Chungkookjang Made with Bacillus amyloliquefaciens and Bacillus subtilis Potentially through Promoting Gut-Brain Axis in Artery-Occluded Gerbils. Foods 2021, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, S.; Liu, D.; Qian, H.; Zhang, D.; Liu, Q. The differences between fecal microbiota and intestinal fluid microbiota in colon polyps: An observational study. Medicine 2021, 100, e28028. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Rasimus-Sahari, S.; Teplova, V.V.; Andersson, M.A.; Mikkola, R.; Kankkunen, P.; Matikainen, S.; Gahmberg, C.G.; Andersson, L.C.; Salkinoja-Salonen, M. The peptide toxin amylosin of Bacillus amyloliquefaciens from moisture-damaged buildings is immunotoxic, induces potassium efflux from mammalian cells, and has antimicrobial activity. Appl. Environ. Microbiol. 2015, 81, 2939–2949. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef] [PubMed]
- Afsharmanesh, H.; Perez-Garcia, A.; Zeriouh, H.; Ahmadzadeh, M.; Romero, D. Aflatoxin degradation by Bacillus subtilis UTB1 is based on production of an oxidoreductase involved in bacilysin biosynthesis. Food Control 2018, 94, 48–55. [Google Scholar] [CrossRef]
- Chen, G.; Fang, Q.A.; Liao, Z.; Xu, C.; Liang, Z.; Liu, T.; Zhong, Q.; Wang, L.; Fang, X.; Wang, J. Detoxification of Aflatoxin B1 by a Potential Probiotic Bacillus amyloliquefaciens WF2020. Front. Microbiol. 2022, 13, 891091. [Google Scholar] [CrossRef]




| LSHA | HSHA | HSLA | LSLA | CSB | |
|---|---|---|---|---|---|
| Sodium (%) | 8.8 ± 0.1 a | 7.4 ± 0.2 c | 9.0 ± 0.1 a | 8.5 ± 0.2 b | - |
| Water (%) | 56.7 ± 0.37 b | 59.2 ± 0.32 a | 51.9 ± 0.33 c | 49.9 ± 0.36 d | |
| Histamine (ug/g) | 796 ± 1.4 b | 954 ± 0.6 a | 22.7 ± 0.2 d | 59.9 ± 0.4 c | - |
| Tyramine (ug/g) | 2629 ± 1.4 a | 1653 ± 1.4 b | 36.1 ± 1.4 d | 279 ± 1.7 c | - |
| Bacillus subtilis (%) | 15.0 ± 0.6 | 86.5 ± 2.3 | 92.0 ± 1.6 | 59.4 ± 1.5 | - |
| Bacillus licheniformis (%) | 35.1 ± 0.72 | 0.60 ± 0.0 | 0 ± 0 | 19.7 ± 0.12 | - |
| Weissella confuse (%) | 0.02 ± 0 | 4.3 ± 0.2 | 0.35 ± 0.01 | 0.01 ± 0 | - |
| Pediococcus acidilactici (%) | 0.16 ± 0 | 2.06 ± 0.07 | 0.05 ± 0 | 0.02 ± 0 | - |
| Bacillus coagulans (%) | 0.76 ± 0.02 | 0 ± 0 | 0 ± 0 | 0.03 ± 0 | - |
| Leuconostoc mesenteroides (%) | 0 ± 0 | 0.9 ± 0.04 | 0.01 ± 0 | 0.15 ± 0 | - |
| Leuconostoc citreum (%) | 0 ± 0 | 0.39 ± 0.01 | 0 ± 0 | 0.01 ± 0 | - |
| Staphylococcus aureus (%) | 0.09 ± 0 | 0 ± 0 | 0 ± 0 | 0.01 ± 0 | - |
| Acinetobacter baumannii (%) | 0.01 ± 0 | 0.04 ± 0 | 0 ± 0 | 0.01 ± 0 | - |
| Daidzein (ug/g) | 25.9 ± 0.05 b | 26.4 ± 0.62 b | 29.9 ± 0.48 a | 13.4 ± 0.31 c | 8.4 ± 0.11 d |
| Genistein (ug/g) | 43.8 ± 0.47 b | 42.6 ± 0.54 b | 47.2 ± 0.34 a | 26.3 ± 0.38 c | 9.2 ± 0.09 b |
| Glycitein (ug/g) | 6.7 ± 0.11 a | 6.3 ± 0.08 a | 6.8 ± 0.9 a | 1.8 ± 0.22 b | 3.5 ± 0.17 ab |
| Daidzin (ug/g) | - | - | - | 1.2 ± 0.09 | 35.4 ± 0.39 |
| Genistin (ug/g) | - | - | - | 2.5 ± 0.08 | 50.5 ± 0.58 |
| Glycitin (ug/g) | - | - | - | - | 8.7 ± 0.24 |
| Total isoflavonoid aglycans (ug/g) | 76.4 ± 1.87 b | 75.3 ± 1.11 b | 83.9 ± 0.73 b | 45.2 ± 0.56 c | 116 ± 0.82 a |
| Control | LSHA | HSHA | HSLA | LSLA | CSB | Normal-Con | |
|---|---|---|---|---|---|---|---|
| Weight gain (g/12 week) | 175 ± 12.1 a | 150 ± 12.7 b | 157 ± 10.1 b | 152 ± 12.1 b | 159 ± 14.2 b | 165 ± 16.3 ab | 117 ± 13.4 c |
| Visceral fat (g) | 11.1 ± 0.95 a | 10.8 ± 1.02 a | 7.73 ± 0.84 b | 7.35 ± 0.77 b | 5.92 ± 0.63 c | 7.15 ± 0.82 b | 5.75 ± 0.73 c |
| Food intake (g/day) | 13.6 ± 1.8 | 12.2 ± 1.3 | 12.6 ± 1.2 | 12.5 ± 1.4 | 13.0 ± 1.3 | 12.9 ± 1.4 | 11.6 ± 1.7 |
| Uterine weight (g) | 0.16 ± 0.02 b | 0.15 ± 0.02 b | 0.17 ± 0.03 b | 0.15 ± 0.02 b | 0.16 ± 0.02 b | 0.15 ± 0.02 b | 0.65 ± 0.03 a |
| Serum 17β-estradiol(pg/mL) | 1.56 ± 0.10 b | 1.52 ± 0.14 b | 1.61 ± 0.25 b | 1.49 ± 0.22 b | 1.53 ± 0.28 b | 1.42 ± 0.29 b | 7.47 ± 0.88 a |
| Fasting serum glucose (mg/dL) | 113 ± 4.47 a | 104 ± 5.58 ab | 103 ± 4.76 b | 102 ± 5.34 b | 109 ± 6.03 ab | 109 ± 7.46 ab | 95.3 ± 5.43 c |
| 2h-postprandial serum glucose (mg/dL) | 143 ± 7.23 a | 126 ± 6.75 b | 126 ± 6.55 b | 126 ± 7.56 b | 141 ± 7.32 a | 135 ± 7.44 ab | 125 ± 6.13 b |
| Fasting plasma insulin (ng/mL) | 1.56 ± 0.19 a | 1.11 ± 0.19 b | 1.04 ± 0.13 b | 0.83 ± 0.19 c | 1.49 ± 0.21 a | 0.93 ± 0.19 bc | 0.86 ± 0.10 c |
| HOMA-IR | 6.53 ± 0.53 a | 4.93 ± 0.53 b | 3.97 ± 0.34 c | 3.11 ± 0.44 c | 6.02 ± 0.59 a | 3.75 ± 0.51 c | 3.04 ± 0.26 c |
| Control | LSHA | HSHA | HSLA | LSLA | CSB | Normal-Con | |
|---|---|---|---|---|---|---|---|
| BMD (g/cm3) | 0.065 ± 0.016 d | 0.106 ± 0.013 c | 0.122 ± 0.016 c | 0.18 ± 0.047 b | 0.081 ± 0.019 d | 0.114 ± 0.001 c | 0.23 ± 0.016 a |
| BV/TV (%) | 11.4 ± 2.1 e | 15.9 ± 1 d | 17.9 ± 2.0 c | 23.8 ± 3.0 b | 13.5 ± 2.1 e | 17.1 ± 1.3 c | 30.6 ± 1.1 a |
| Tb.Th (mm) | 0.096 ± 0.003 c | 0.1 ± 0.005 b | 0.106 ± 0.002 a | 0.099 ± 0.001 b | 0.098 ± 0.004 b | 0.098 ± 0.003 b | 0.111 ± 0.003 a |
| Tb.N (1/mm) | 1.18 ± 0.08 c | 1.6 ± 0.18 b | 1.63 ± 0.19 b | 2.39 ± 0.36 a | 1.38 ± 0.24 c | 1.74 ± 0.01 b | 2.71 ± 0.08 a |
| Tb.Sp (mm) | 1.12 ± 0.08 a | 0.77 ± 0.19 b | 0.81 ± 0.15 b | 0.49 ± 0.21 c | 1.02 ± 0.23 a | 0.72 ± 0.05 b | 0.4 ± 0.08 c |
| Control | LSHA | HSHA | HSLA | LSLA | CSB | Normal-Con | |
|---|---|---|---|---|---|---|---|
| PTH (ng/mL) | 43.9 ± 5.49 a | 38.8 ± 4.62 b | 28 ± 5.15 c | 32 ± 6.7 bc | 39.4 ± 5.03 ab | 31.4 ± 6.32 bc | 37.5 ± 3.89 b |
| RANKL (pg/mL) | 32.3 ± 3.43 a | 29.4 ± 3.15 b | 28.1 ± 3.52 b | 24.1 ± 3.12 c | 30.9 ± 3.73 ab | 27.9 ± 4.12 b | 22.4 ± 3.52 c |
| OPG (ng/mL) | 14.4 ± 0.95 c | 14.7 ± 0.97 c | 17.5 ± 0.83 b | 16.9 ± 0.95 b | 18.9 ± 1.17 a | 17.2 ± 0.94 b | 17.3 ± 0.91 b |
| Osteocalcin (ng/mL) | 1.59 ± 0.17 a | 1.40 ± 0.21 ab | 1.13 ± 0.18 b | 1.06 ± 0.17 b | 1.38 ± 0.15 ab | 1.05 ± 0.14 b | 0.69 ± 0.08 c |
| BALP (U/L) | 37.2 ± 4.58 a | 28.2 ± 4.72 b | 26.9 ± 3.68 b | 25.4 ± 5.09 b | 28 ± 5.01 b | 19.9 ± 4.18 c | 15.9 ± 3.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-J.; Zhang, T.; Yue, Y.; Jeong, S.-J.; Ryu, M.-S.; Wu, X.; Li, C.; Jeong, D.-Y.; Park, S. Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota. Foods 2023, 12, 2958. https://doi.org/10.3390/foods12152958
Yang H-J, Zhang T, Yue Y, Jeong S-J, Ryu M-S, Wu X, Li C, Jeong D-Y, Park S. Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota. Foods. 2023; 12(15):2958. https://doi.org/10.3390/foods12152958
Chicago/Turabian StyleYang, Hee-Jong, Ting Zhang, Yu Yue, Su-Ji Jeong, Myeong-Seon Ryu, Xuangao Wu, Chen Li, Do-Yeon Jeong, and Sunmin Park. 2023. "Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota" Foods 12, no. 15: 2958. https://doi.org/10.3390/foods12152958
APA StyleYang, H.-J., Zhang, T., Yue, Y., Jeong, S.-J., Ryu, M.-S., Wu, X., Li, C., Jeong, D.-Y., & Park, S. (2023). Protective Effect of Long-Term Fermented Soybeans with Abundant Bacillus subtilis on Glucose and Bone Metabolism and Memory Function in Ovariectomized Rats: Modulation of the Gut Microbiota. Foods, 12(15), 2958. https://doi.org/10.3390/foods12152958







